Erythrocyte Membrane-Coated Arsenic Trioxide-Loaded Sodium Alginate Nanoparticles for Tumor Therapy
Abstract
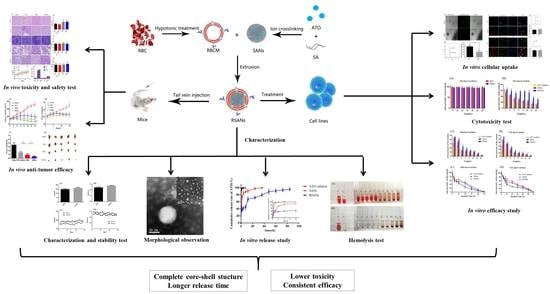
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cells and Animals
2.3. Preparation of SANs
2.4. Preparation of RBCM
2.5. Preparation of RSANs
2.6. Characterization and Stability Test
2.7. Morphological Observation
2.8. Drug Loading Capacity and In Vitro Release Study
2.9. Hemolysis Test
2.10. Macrophage Uptake Study
2.11. In Vitro Cellular Uptake
2.12. Cytotoxicity Test
2.13. In Vitro Efficacy Study
2.14. In Vivo Toxicity and Safety Test
2.15. In Vivo Anti-Tumor Studies
2.16. Statistics and Data Analysis
3. Results and Discussion
3.1. Characterization and Stability Test
3.2. Morphological Observation
3.3. In Vitro Release Study
3.4. Hemolysis Test
3.5. Macrophage Uptake Study
3.6. In Vitro Cellular Uptake
3.7. ATO Nanoparticles Cytotoxicity
3.8. In Vitro Efficacy Study
3.9. In Vivo Toxicity and Safety Test
3.10. In Vivo Anti-Tumor Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Evens, A.M.; Tallman, M.S.; Gartenhaus, R.B. The potential of arsenic trioxide in the treatment of malignant disease: Past, present, and future. Leuk. Res. 2004, 28, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Antman, K.H. Introduction: The history of arsenic trioxide in cancer therapy. Oncologist 2001, 6 (Suppl. 2), 1–2. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Vakili, M.R.; Li, X.F.; Lavasanifar, A.; Le, X.C. Polymeric micelles for GSH-triggered delivery of arsenic species to cancer cells. Biomaterials 2014, 35, 7088–7100. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.J.; Graham, B.; Dugo, E.; Berhaneselassie-Sumner, B.; Ndebele, K.; Tchounwou, P.B. Arsenic Trioxide Induces Apoptosis via Specific Signaling Pathways in HT-29 Colon Cancer Cells. J. Cancer Sci. Ther. 2017, 9, 298–306. [Google Scholar] [CrossRef]
- Akhtar, A.; Wang, S.X.; Ghali, L.; Bell, C.; Wen, X. Recent advances in arsenic trioxide encapsulated nanoparticles as drug delivery agents to solid cancers. J. Biomed. Res. 2017, 31, 177–188. [Google Scholar]
- Tchounwou, P.B.; Wilson, B.A.; Ishaque, A.B.; Schneider, J. Atrazine potentiation of arsenic trioxide-induced cytotoxicity and gene expression in human liver carcinoma cells (HepG2). Mol. Cell. Biochem. 2001, 222, 49–59. [Google Scholar] [CrossRef]
- Cai, B.; Wei, W.; Liu, A.; Jin, Z.; Lin, S. In vivo inhibitory effect of arsenic trioxide on transplanted tumor of pancreatic cancer BXPC-3 cells. Chin. Tradit. Herb. Drugs 2010, 41, 90–93. [Google Scholar]
- Murgo, A.J. Clinical Trials of Arsenic Trioxide in Hematologic and Solid Tumors: Overview of the National Cancer Institute Cooperative Research and Development Studies. Oncologist 2001, 6 (Suppl. 2), 22–28. [Google Scholar] [CrossRef]
- Kritharis, A.; Bradley, T.P.; Budman, D.R. The evolving use of arsenic in pharmacotherapy of malignant disease. Ann. Hematol. 2013, 92, 719–730. [Google Scholar] [CrossRef]
- Huang, A.H.; Han, S.P.; Lu, Y.P.; Ma, R.; Zheng, H.S.; Li, F.Z. Preparation and in vitro evaluation of arsenic trioxide glioma targeting drug delivery system loaded by PAMAM dendrimers co-modified with RGDyC and PEG. Chin. J. Tradit. Chin. Med. 2018, 43, 1618–1625. [Google Scholar]
- Fu, X.; Liang, Q.R.; Luo, R.G.; Li, Y.S.; Xiao, X.P.; Yu, L.L.; Shan, W.Z.; Fan, G.Q.; Tang, Q. An arsenic trioxide nanoparticle prodrug (ATONP) potentiates a therapeutic effect on an aggressive hepatocellular carcinoma model via enhancement of intratumoral arsenic accumulation and disturbance of the tumor microenvironment. J. Mater. Chem. B 2019, 7, 3088–3099. [Google Scholar] [CrossRef]
- Swindell, E.P.; Hankins, P.L.; Haimei, C.; Miodragovi, D.U.; O’Halloran, T.V. Anticancer activity of small-molecule and nanoparticulate arsenic(III) complexes. Inorg. Chem. 2013, 52, 12292–12304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.Y.; Chang, C.S.; Tang, J.L.; Tien, H.F.; Kuo, T.L.; Huang, S.F.; Yao, Y.T.; Chou, W.C.; Chung, C.Y.; Wang, C.H.; et al. Acute and chronic arsenic poisoning associated with treatment of acute promyelocytic leukaemia. Br. J. Haematol. 1998, 103, 1092–1095. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Ma, L.; Hu, N. Ailing No. I in Treating 62 Cases of Acute Promyelocytic Leukemia. Chin. J. Integr. Tradit. West. Med. 1999, 19, 473–476. [Google Scholar]
- Sarbari, A.; Sanjeeb, K.S. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 170–183. [Google Scholar]
- Sheldon, L.A. Inhibition of E2F1 Activity and Cell Cycle Progression by Arsenic via Retinoblastoma Protein. Cell Cycle 2017, 16, 2058–2072. [Google Scholar] [CrossRef] [Green Version]
- Jadhav, V.; Ray, P.; Sachdeva, G.; Bhatt, P. Biocompatible arsenic trioxide nanoparticles induce cell cycle arrest by p21 WAF1/CIP1 expression via epigenetic remodeling in LNCaP and PC3 cell lines. Life Sci. 2016, 148, 41–52. [Google Scholar] [CrossRef]
- Kopel, P.; Wawrzak, D.; Milosavljevic, V.; Moulick, A.; Vaculovicova, M.; Kizek, R. Nanotransporters for Anticancer Drug Delivery. Nanotechnol. Res. J. 2015, 2, 32–38. [Google Scholar]
- Wang, X.; Li, D.; Ghali, L.; Xia, R.; Munoz, L.P.; Garelick, H.; Bell, C.; Wen, X. Therapeutic Potential of Delivering Arsenic Trioxide into HPV-Infected Cervical Cancer Cells Using Liposomal Nanotechnology. Nanoscale Res. Lett. 2016, 11, 94. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Liu, Y.; Guo, M.; Fei, W.; Zheng, H.; Zhang, R.; Zhang, Y.; Wei, Y.; Zheng, G.; Li, F. pH-triggered sustained release of arsenic trioxide by polyacrylic acid capped mesoporous silica nanoparticles for solid tumor treatment in vitro and in vivo. J. Biomater. Appl. 2016, 31, 23. [Google Scholar] [CrossRef] [Green Version]
- Otto, D.P.; Otto, A.; de Villiers, M.M. Differences in physicochemical properties to consider in the design, evaluation and choice between microparticles and nanoparticles for drug delivery. Expert Opin. Drug Deliv. 2015, 12, 763–777. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Luo, Z.G.; He, X.W. Application Research of Sodium Alginate in Medical Engineering. Chin. Med. Equip. J. 2008, 29, 33–35. [Google Scholar]
- Zhang, X.H.; Cui, Y.D. Study on the Properties and Structure of Sodium Alginate Superabsorbent. Polym. Mater. Sci. Eng. 2006, 22, 91–94. [Google Scholar] [CrossRef]
- Zhong, J.J.; Wang, D.K.; Zhang, C.X.; Gao, H.; Zhang, X. Pharmaceutical significance of sodium alginate. Chin. J. New Drugs 2007, 16, 591–594. [Google Scholar]
- Li, Z.L.; Wang, J.; Chen, L.M.; Zhang, W.C.; Zhao, C.C. Preparation and properties of ceftriaxone chitosan-sodium alginate (calcium) microspheres. Chin. Antibiot. J. 2008, 33, 355–358. [Google Scholar]
- Daemi, H.; Barikani, M. Synthesis and characterization of calcium alginate nanoparticles, sodium homopolymannuronate salt and its calcium nanoparticles. Sci. Iran. 2012, 19, 2023–2028. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Bhanjana, G.; Sharma, A.; Sidhu, M.C.; Dilbaghi, N. Synthesis, characterization and on field evaluation of pesticide loaded sodium alginate nanoparticles. Carbohydr. Polym. 2014, 101, 1061–1067. [Google Scholar] [CrossRef]
- Sun, Y.; Su, J.; Liu, G.; Chen, J.; Zhang, X.; Zhang, R.; Jiang, M.; Qiu, M. Advances of blood cell-based drug delivery systems. Eur. J. Pharm. Sci. 2017, 96, 115–128. [Google Scholar] [CrossRef]
- Fang, R.H.; Hu, C.M.; Zhang, L. Nanoparticles disguised as red blood cells to evade the immune system. Expert Opin. Biol. Ther. 2012, 12, 385–389. [Google Scholar] [CrossRef]
- Tan, S.; Wu, T.; Zhang, D.; Zhang, Z. Cell or Cell Membrane-Based Drug Delivery Systems. Theranostics 2015, 5, 863–881. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.M.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H.; Zhang, L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. USA 2011, 27, 10980–10985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, J.H.; Sun, Q.; Meng, Q.; Yin, P.; Zhang, Z.; Zhang, H.Y.; Li, Y. Bioinspired Nanoparticles with NIR-Controlled Drug Release for Synergetic Chemophotothermal Therapy of Metastatic Breast Cancer. Adv. Funct. Mater. 2016, 26, 7495–7506. [Google Scholar] [CrossRef]
- Su, J.; Sun, H.; Meng, Q.; Yin, Q.; Tang, S.; Zhang, P.; Chen, Y.; Zhang, Z.; Yu, H.; Li, Y. Long Circulation Red-Blood-Cell-Mimetic Nanoparticles with Peptide-Enhanced Tumor Penetration for Simultaneously Inhibiting Growth and Lung Metastasis of Breast Cancer. Adv. Funct. Mater. 2016, 26, 1243–1252. [Google Scholar] [CrossRef]
- Su, J.; Sun, H.; Meng, Q.; Zhang, P.; Yin, Q.; Li, Y. Enhanced Blood Suspensibility and Laser-Activated Tumor-specific Drug Release of Theranostic Mesoporous Silica Nanoparticles by Functionalizing with Erythrocyte Membranes. Theranostics 2017, 7, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Hochmuth, R.M.; Mohandas, N.; Blackshear, P.L. Measurement of the elastic modulus for red cell membrane using a fluid mechanical technique. Biophys. J. 1973, 13, 747–762. [Google Scholar] [CrossRef] [Green Version]
- Oldenborg, P.A.; Zheleznyak, A.; Fang, Y.F.; Lagenaur, C.F.; Gresham, H.D.; Lindberg, F.P. Role of CD47 as a marker of self on red blood cells. Science 2000, 288, 2051–2054. [Google Scholar] [CrossRef]
- Weiwei, G.; Hu, C.M.J.; Fang, R.H.; Luk, B.T.; Jing, S.; Liangfang, Z. Surface functionalization of gold nanoparticles with red blood cell membranes. Adv. Mater. 2013, 25, 3549–3553. [Google Scholar]











| Tube No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2% RBC Suspension (mL) | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Saline (mL) | 0 | 2.5 | 2.4 | 2.3 | 2.2 | 2.1 | 2.0 | 1.9 | 1.8 | 1.7 | 1.6 | 1.5 |
| Ultrapure Water (mL) | 2.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Samples (mL) | 0 | 0 | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | 1.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lian, Y.; Wang, X.; Guo, P.; Li, Y.; Raza, F.; Su, J.; Qiu, M. Erythrocyte Membrane-Coated Arsenic Trioxide-Loaded Sodium Alginate Nanoparticles for Tumor Therapy. Pharmaceutics 2020, 12, 21. https://doi.org/10.3390/pharmaceutics12010021
Lian Y, Wang X, Guo P, Li Y, Raza F, Su J, Qiu M. Erythrocyte Membrane-Coated Arsenic Trioxide-Loaded Sodium Alginate Nanoparticles for Tumor Therapy. Pharmaceutics. 2020; 12(1):21. https://doi.org/10.3390/pharmaceutics12010021
Chicago/Turabian StyleLian, Yumei, Xuerui Wang, Pengcheng Guo, Yichen Li, Faisal Raza, Jing Su, and Mingfeng Qiu. 2020. "Erythrocyte Membrane-Coated Arsenic Trioxide-Loaded Sodium Alginate Nanoparticles for Tumor Therapy" Pharmaceutics 12, no. 1: 21. https://doi.org/10.3390/pharmaceutics12010021
APA StyleLian, Y., Wang, X., Guo, P., Li, Y., Raza, F., Su, J., & Qiu, M. (2020). Erythrocyte Membrane-Coated Arsenic Trioxide-Loaded Sodium Alginate Nanoparticles for Tumor Therapy. Pharmaceutics, 12(1), 21. https://doi.org/10.3390/pharmaceutics12010021