Sulforaphane-Loaded Ultradeformable Vesicles as A Potential Natural Nanomedicine for the Treatment of Skin Cancer Diseases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Ethosomes®
2.3. Preparation of Transfersomes®
2.4. Physico-Chemical Characterization of Vesicle Formulations
2.5. Entrapment Efficacy of Ethosomes® and Transfersomes®
2.6. Sulforaphane Release Profiles
2.7. Percutaneous Permeation of Sulforaphane-Loaded Deformable Vesicles
2.8. Cell Cultures
2.9. Evaluation of In Vitro Anticancer Activity
2.10. HPLC Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Physico-Chemical and Technological Characterization of Ethosomes® and Transfersomes®
3.2. Evaluation of Sulforaphane Release and the Percutaneous Permeation Profile
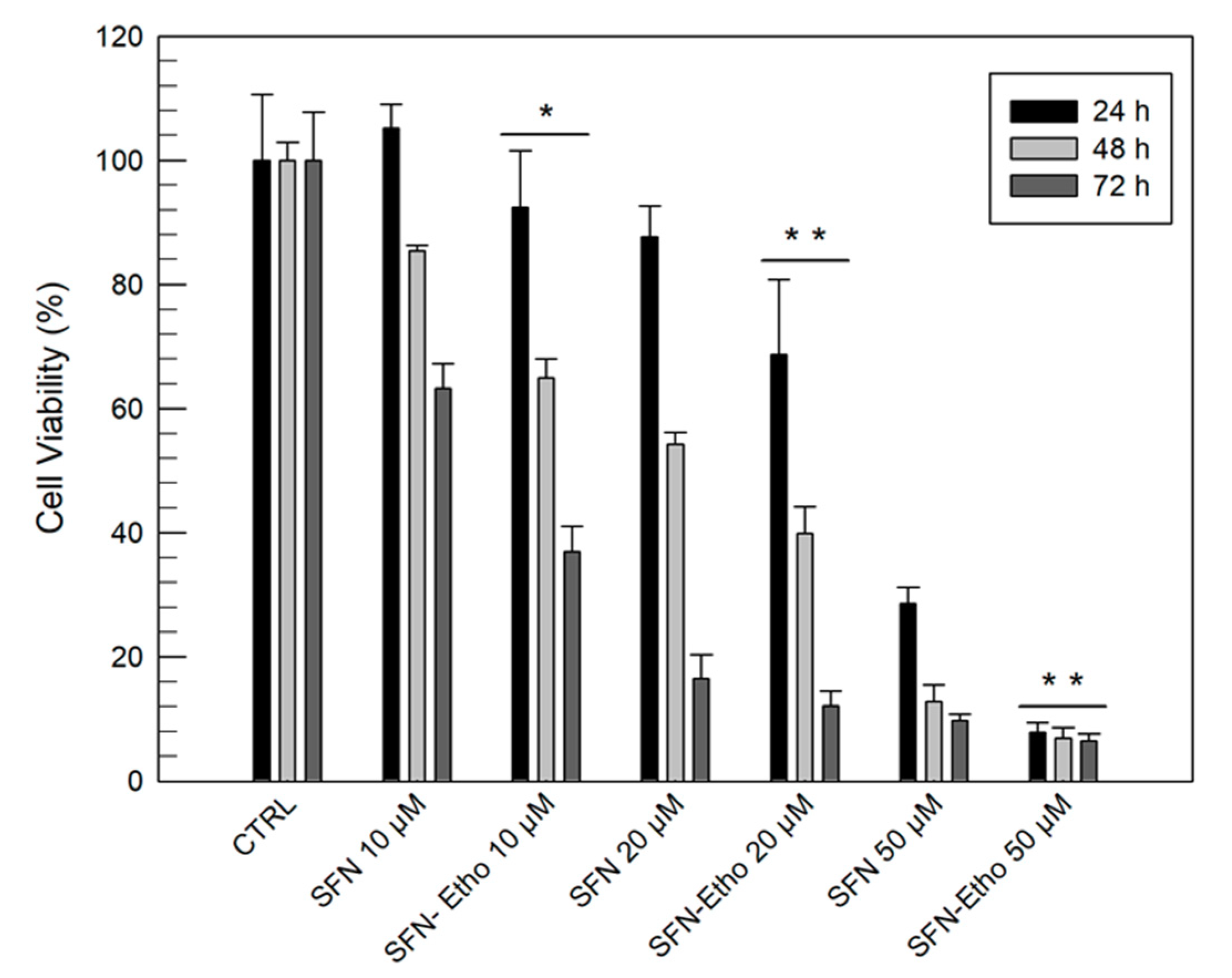
3.3. In Vitro Anticancer Activity of Sulforaphane and Sulforaphane-Loaded Ethosomes®
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ye Won, A.; Kyoung, A.J.; So-Youn, W.; Jihee Lee, K.; Young Hae, C. Sulforaphane exerts its anti- nflammatory effect against amyloid-b peptide via STAT-1 dephosphorylation and activation of Nrf2/HO-1 cascade in human THP-1 macrophages. Neurobiol. Aging 2016, 38, 1–10. [Google Scholar] [CrossRef]
- Carrasco-Pozo, C.; Tan, K.N.; Borges, K. Sulforaphane is anticonvulsant and improves mitochondrial function. J. Neurochem. 2015, 135, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Briones-Herrera, A.; Avila-Rojas, S.H.; Aparicio-Trejo, O.E.; Cristóbal, M.; León-Contreras, J.C.; Hernández-Pando, R.; Pinzón, E.; Pedraza-Chaverri, J.; Sánchez-Lozada, L.G.; Tapia, E. Sulforaphane prevents maleic acid-induced nephropathy by modulating renal hemodynamics, mitochondrial bioenergetics and oxidative stress. Food Chem. Toxicol. 2018, 115, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Beltrán, C.E.; Calderón-Oliver, M.; Tapia, E.; Medina-Campos, O.N.; Sánchez-González, D.J.; Martínez-Martínez, C.M.; Ortiz-Vega, K.M.; Franco, M.; Pedraza-Chaverri, J. Sulforaphane protects against cisplatin-induced nephrotoxicity. Toxicol. Lett. 2010, 192, 278–285. [Google Scholar] [CrossRef]
- Wang, W.; He, Y.; Yu, G.; Li, B.; Sexton, D.W.; Wileman, T.; Roberts, A.A.; Hamilton, C.J.; Liu, R.; Chao, Y.; et al. Sulforaphane Protects the Liver against CdSe Quantum Dot-Induced Cytotoxicity. PLoS ONE 2015, 10, e0138771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrasco-Pozo, C.; Tan, K.N.; Gotteland, M.; Borges, K. Sulforaphane Protects against High Cholesterol-Induced Mitochondrial Bioenergetics Impairments, Inflammation, and Oxidative Stress and Preserves Pancreatic β-Cells Function. Oxid. Med. Cell Longev. 2017, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, Y.; Chen, Q.; Sun, Y.P.; Wang, X.; Lv, L.; Zhang, L.P.; Liu, J.S.; Zhao, S.; Wang, X.L. Sulforaphane protection against the development of doxorubicin-induced chronic heart failure is associated with Nrf2 Upregulation. Cardiovasc Ther. 2017, 35, e12277. [Google Scholar] [CrossRef] [PubMed]
- Ferreira de Oliveira, J.M.; Costa, M.; Pedrosa, T.; Pinto, P.; Remedios, C.; Oliveira, H.; Pimentel, F.; Almeida, L.; Santos, C. Sulforaphane induces oxidative stress and death by p53-independent mechanism: implication of impaired glutathione recycling. PLoS ONE 2014, 9, e92980. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.A.; Murata, H.; Sakabe, T.; Sowa, Y.; Horie, N.; Nakanishi, R.; Sakai, T.; Kubo, T. Sulforaphane induces cell cycle arrest and apoptosis in murine osteosarcoma cells in vitro and inhibits tumor growth in vivo. Oncol. Rep. 2007, 180, 1263–1268. [Google Scholar] [CrossRef] [Green Version]
- Gianfredi, V.; Nucci, D.; Vannini, S.; Villarini, M.; Moretti, M. In vitro Biological Effects of Sulforaphane (SFN), Epigallocatechin-3-gallate (EGCG), and Curcumin on Breast Cancer Cells: A Systematic Review of the Literature. Nutr. Cancer 2017, 69, 969–978. [Google Scholar] [CrossRef]
- Beaver, J.H.; Kuintzle, L.M.; Buchanan, R.; Wiley, A.; Glasser, M.W.; Wong, S.T.; Johnson, C.P.; Chang, G.S.; Löhr, C.V.; Williams, D.E.; et al. Long noncoding RNAs and sulforaphane: a target for chemoprevention and suppression of prostate cancer. J. Nutr. Biochem. 2017, 42, 72–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, Y.; Zhou, Y.; Wu, S.; Hu, Y.; Lin, K.; Wang, Y.; Zheng, Z.; Wu, W. Sulforaphane Induced Apoptosis via Promotion of Mitochondrial Fusion and ERK1/2-Mediated 26S Proteasome Degradation of Novel Pro-survival Bim and Upregulation of Bax in Human Non-Small Cell Lung Cancer Cells. J. Cancer 2017, 8, 2456–2470. [Google Scholar] [CrossRef] [PubMed]
- Arcidiacono, P.; Ragonese, F.; Stabile, A.; Pistilli, A.; Kuligina, E.; Rende, M.; Bottoni, U.; Calvieri, S.; Crisanti, A.; Spaccapelo, R. Antitumor activity and expression profiles of genes induced by sulforaphane in human melanomacells. Eur. J. Nutr. 2018, 57, 2547–2569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franklin, S.J.; Dickinson, S.E.; Karlage, K.L.; Bowden, G.T.; Myrdal, P.B. Stability of sulforaphane for topical formulation. Drug Dev. Ind. Pharm. 2014, 40, 494–502. [Google Scholar] [CrossRef] [Green Version]
- Cosco, D.; Celia, C.; Cilurzo, F.; Trapasso, E.; Paolino, D. Colloidal carriers for the enhanced delivery through the skin. Expert Opin. Drug Deliv. 2008, 5, 737–755. [Google Scholar] [CrossRef]
- Touitou, E.; Dayan, N.; Bergelson, L.; Godin, B.; Eliaz, M. Ethosomes – novel vesicular carriers for enhanced delivery: characterization and skin penetration properties. J. Control. Release 2000, 65, 403–418. [Google Scholar] [CrossRef]
- Paolino, D.; Celia, C.; Trapasso, E.; Cilurzo, F.; Fresta, M. Paclitaxel-loaded ethosomes®: Potential treatment of squamous cell carcinoma, a malignant transformation of actinic keratosis. Eur. J. Pharm. Biopharm. 2012, 81, 102–112. [Google Scholar] [CrossRef]
- Paolino, D.; Lucania, G.; Mardente, D.; Alhaique, F.; Fresta, M. Ethosomes for skin delivery of ammonium glycyrrhizinate: in vitro percutaneous permeation through human skin and in vivo anti-inflammatory activity on human volunteers. J. Control. Release 2005, 106, 99–110. [Google Scholar] [CrossRef]
- Paolino, D.; Cosco, D.; Cilurzo, F.; Fresta, M. Innovative drug delivery systems for the administration of natural compounds. Curr. Bioactive Compd. 2007, 3, 262–277. [Google Scholar] [CrossRef]
- Cevc, G.; Gebauer, D.; Stieber, J.; Schatzlein, A.; Blume, G. Ultraflexible vesicles, Transfersomes, have an extremely low pore penetration resistance and transport therapeutic amounts of insulin across the intact mammalian skin. Biochim. Biophys Acta 1998, 1368, 201–215. [Google Scholar] [CrossRef] [Green Version]
- Di Francesco, M.; Primavera, R.; Fiorito, S.; Cristiano, M.C.; Taddeo, V.; Epifano, F.; Di Marzio, L.; Genovese, S.; Celia, C. Acronychiabaueri Analogue Derivative-Loaded Ultradeformable Vesicles: Physicochemical Characterization and Potential Applications. Planta Med. 2016, 83, 482–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, S. Lipid-based nano-delivery systems for skin delivery of drugs and bioactives. Front Pharmacol. 2015, 6, 219. [Google Scholar] [CrossRef] [PubMed]
- Gillet, A.; Compère, P.; Lecomte, F.; Hubert, P.; Ducat, E.; Evrard, B.; Piel, G. Liposome surface charge influence on skin penetration behavior. Int. J. Pharm. 2011, 411, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Celia, C.; Cilurzo, F.; Trapasso, E.; Cosco, D.; Fresta, M.; Paolino, D. Ethosomes® and transfersomes® containing linoleic acid: physicochemical and technological features of topical drug delivery carriers for the potential treatment of melasma disorders. Biomed. Microdevices 2012, 14, 119–130. [Google Scholar] [CrossRef]
- De Rose, R.F.; Cristiano, M.C.; Celano, M.; Maggisano, V.; Vero, A.; Lombardo, G.E.; Di Francesco, M.; Paolino, D.; Russo, D.; Cosco, D. PDE5 inhibitors-loaded nanovesicles: Physico-chemical properties and in vitro antiproliferative activity. Nanomaterials 2016, 6, E92. [Google Scholar] [CrossRef] [Green Version]
- Cristiano, M.C.; Cosco, D.; Celia, C.; Tudose, A.; Mare, R.; Paolino, D.; Fresta, M. Anticancer activity of all-trans retinoic acid-loaded liposomes on human thyroid carcinoma cells. Colloids Surf. B 2017, 150, 408–416. [Google Scholar] [CrossRef]
- Celia, C.; Trapasso, E.; Cosco, D.; Paolino, D.; Fresta, M. Turbiscan Lab® Expert analysis of the stability of ethosomes® and ultradeformable liposomes containing a bilayer fluidizing agent. Colloids Surf. B 2009, 72, 155–160. [Google Scholar] [CrossRef]
- Krishnaiah, Y.S.R.; Pavurala, N.; Yang, Y.; Manda, P.; Katragadda, U.; Yang, J.; Shah, R.; Fang, G.; Khan, M.A. In vitro drug transfer due to drug retention in human epidermis pretreated with application of marketed estradiol transdermal systems. AAPS PharmSciTech 2017, 18, 2131–2140. [Google Scholar] [CrossRef]
- Cilurzo, F.; Cristiano, M.C.; Di Marzio, L.; Cosco, D.; Carafa, M.; Ventura, C.A.; Fresta, M.; Paolino, D. Influence of the supramolecular micro-assembly of multiple emulsions on their biopharmaceutical features and in vivo therapeutic response. Curr. Drug Targets 2015, 16, 1612–1622. [Google Scholar] [CrossRef]
- Parry, G.E.; Dunn, P.; Shah, V.P.; Pershing, L.K. Acyclovir bioavailability in human skin. J. Investig. Dermatol. 1992, 98, 856–863. [Google Scholar] [CrossRef] [Green Version]
- Campas-Baypoli, O.N.; Sánchez-Machado, D.I.; Bueno-Solano, C.; Ramírez-Wong, B.; López-Cervantes, J. HPLC method validation for measurement of sulforaphane level in broccoli by-products. Biomed Chromatogr. 2010, 24, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Ogiso, T.; Yamaguchi, T.; Iwaki, M.; Tanino, T.; Miyake, Y. Effect of Positively and Negatively Charged Liposomes on Skin Permeation of Drugs. J. Drug Target 2001, 9, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Kohli, A.K.; Alpar, H.O. Potential use of nanoparticles for transcutaneous vaccine delivery: effect of particle size and charge. Int. J. Pharm. 2004, 275, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.L.; Adhikary, G.; Grun, D.; Kaetzel, D.M.; Eckert, R.L. The Ezh2 polycomb group protein drives an aggressive phenotype in melanoma cancer stem cells and is a target of diet derived sulforaphane. Mol. Carcinog. 2016, 55, 2024–2036. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.C.; Shih, T.Y; Kuo, C.L.; Ma, Y.S.; Yang, J.L.; Wu, P.P; Huang, Y.P.; Lai, K.C.; Chung, J.G. Sulforaphane induces cell death through G2/M phase arrest and triggers apoptosis in HCT 116 human colon cancer cells. Am. J. Chin. Med. 2016, 44, 1289–1310. [Google Scholar] [CrossRef]
- Godin, B.; Touitou, E. Mechanism of bacitracin permeation enhancement through the skin and cellular membranes from an ethosomal carrier. J. Control. Release 2004, 94, 365–379. [Google Scholar] [CrossRef]




| Vesicular Nanocarriers | Formulations | Composition | ||
|---|---|---|---|---|
| EtOH % (w/v) | PL90G®% (w/v) | SC % (w/v) | ||
| Ethosomes® | A | 30 | 1 | −− |
| B | 30 | 2 | −− | |
| C | 30 | 3 | −− | |
| D | 40 | 1 | −− | |
| E | 40 | 2 | −− | |
| F | 40 | 3 | −− | |
| G | 45 | 1 | −− | |
| H | 45 | 2 | −− | |
| I | 45 | 3 | −− | |
| Transfersomes® | J | −− | 88 | 12 |
| Formulations | Physico-Chemical Parameters | ||
|---|---|---|---|
| Mean Size (nm) | Polydispersity Index | Zeta Potential (mV) | |
| A | 147 ± 1 | 0.360 ± 0.052 | −12 ± 1 |
| B | 194 ± 6 | 0.128 ± 0.002 | −28 ± 1 |
| C | 270 ± 3 | 0.376 ± 0.067 | −20 ± 2 |
| D | 285 ± 4 | 0.104 ± 0.023 | −25 ± 1 |
| E | 216 ± 2 | 0.103 ± 0.003 | −26 ± 1 |
| F | 287 ± 3 | 0.299 ± 0.034 | −19 ± 2 |
| G | 102 ± 6 | 0.400 ± 0.087 | −21 ± 2 |
| H | 113 ± 3 | 0.280 ± 0.008 | −20 ± 1 |
| I | 220 ± 1 | 0.294 ± 0.056 | −17 ± 3 |
| J | 192 ± 2 | 0.202 ± 0.011 | −30 ± 1 |
| Formulations | Physico-Chemical Parameters | |||
|---|---|---|---|---|
| Mean Size (nm) | Polydispersity Index | Zeta Potential (mV) | EE(%) | |
| B | 329 ± 4 | 0.40 ± 0.02 | −28 ± 1 | 60.4 ± 5.1 |
| D | 407 ± 4 | 0.30 ± 0.03 | −26 ± 1 | 67.2 ± 4.1 |
| E | 227 ± 3 | 0.01 ± 0.01 | −26 ± 1 | 87.5 ± 2.5 |
| J | 195 ± 1 | 0.21 ± 0.02 | −30 ± 2 | 86.2 ± 2.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristiano, M.C.; Froiio, F.; Spaccapelo, R.; Mancuso, A.; Nisticò, S.P.; Udongo, B.P.; Fresta, M.; Paolino, D. Sulforaphane-Loaded Ultradeformable Vesicles as A Potential Natural Nanomedicine for the Treatment of Skin Cancer Diseases. Pharmaceutics 2020, 12, 6. https://doi.org/10.3390/pharmaceutics12010006
Cristiano MC, Froiio F, Spaccapelo R, Mancuso A, Nisticò SP, Udongo BP, Fresta M, Paolino D. Sulforaphane-Loaded Ultradeformable Vesicles as A Potential Natural Nanomedicine for the Treatment of Skin Cancer Diseases. Pharmaceutics. 2020; 12(1):6. https://doi.org/10.3390/pharmaceutics12010006
Chicago/Turabian StyleCristiano, Maria Chiara, Francesca Froiio, Roberta Spaccapelo, Antonia Mancuso, Steven P. Nisticò, Betty P. Udongo, Massimo Fresta, and Donatella Paolino. 2020. "Sulforaphane-Loaded Ultradeformable Vesicles as A Potential Natural Nanomedicine for the Treatment of Skin Cancer Diseases" Pharmaceutics 12, no. 1: 6. https://doi.org/10.3390/pharmaceutics12010006
APA StyleCristiano, M. C., Froiio, F., Spaccapelo, R., Mancuso, A., Nisticò, S. P., Udongo, B. P., Fresta, M., & Paolino, D. (2020). Sulforaphane-Loaded Ultradeformable Vesicles as A Potential Natural Nanomedicine for the Treatment of Skin Cancer Diseases. Pharmaceutics, 12(1), 6. https://doi.org/10.3390/pharmaceutics12010006