Optimization of Rifapentine-Loaded Lipid Nanoparticles Using a Quality-by-Design Strategy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Formulations
Nanoparticles Synthesis
2.2. Experimental Design
2.2.1. Box–Behnken Design
2.2.2. Optimization and Validation
2.3. NLC Characterization
2.3.1. Particle Size, Polydispersity, and Surface Charge
2.3.2. Drug Encapsulation Efficiency and Loading Capacity
2.3.3. Lyophilization
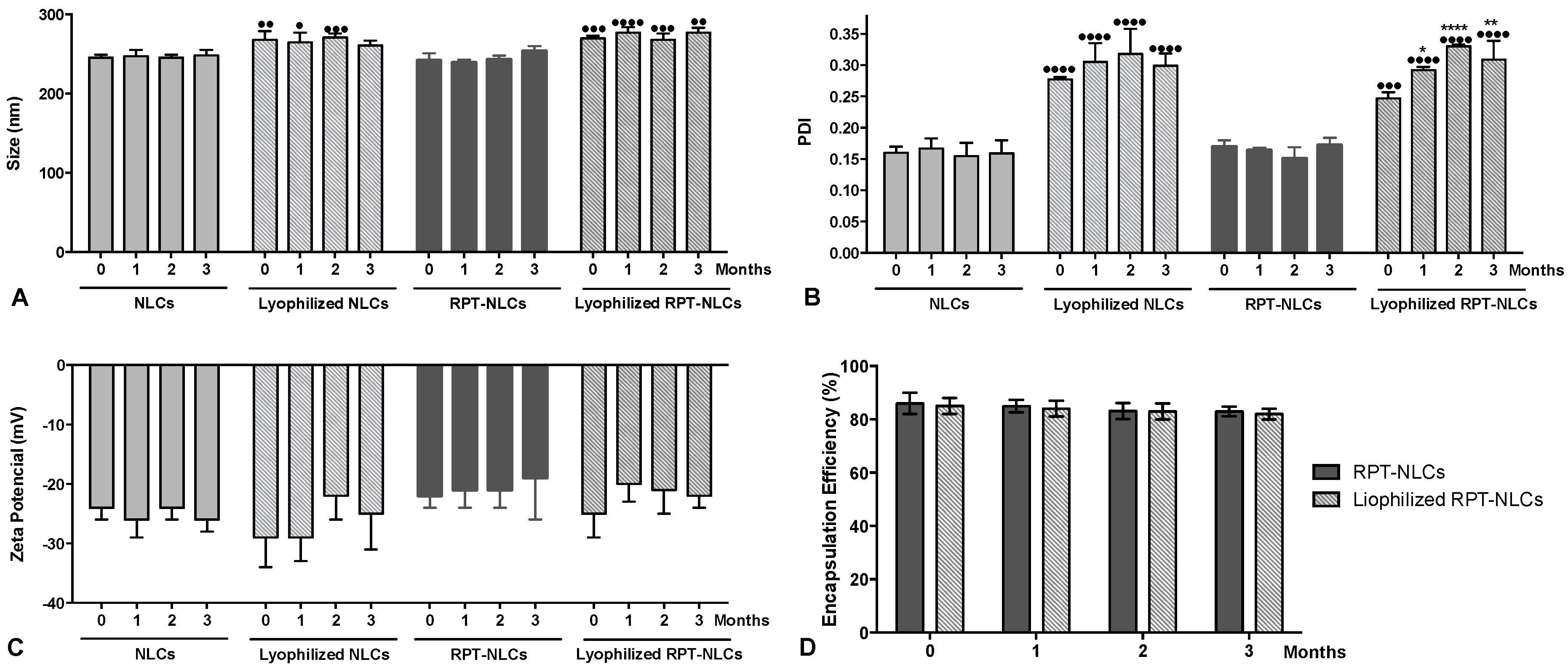
2.3.4. Storage Stability
2.3.5. Differential Scanning Calorimetry Analysis
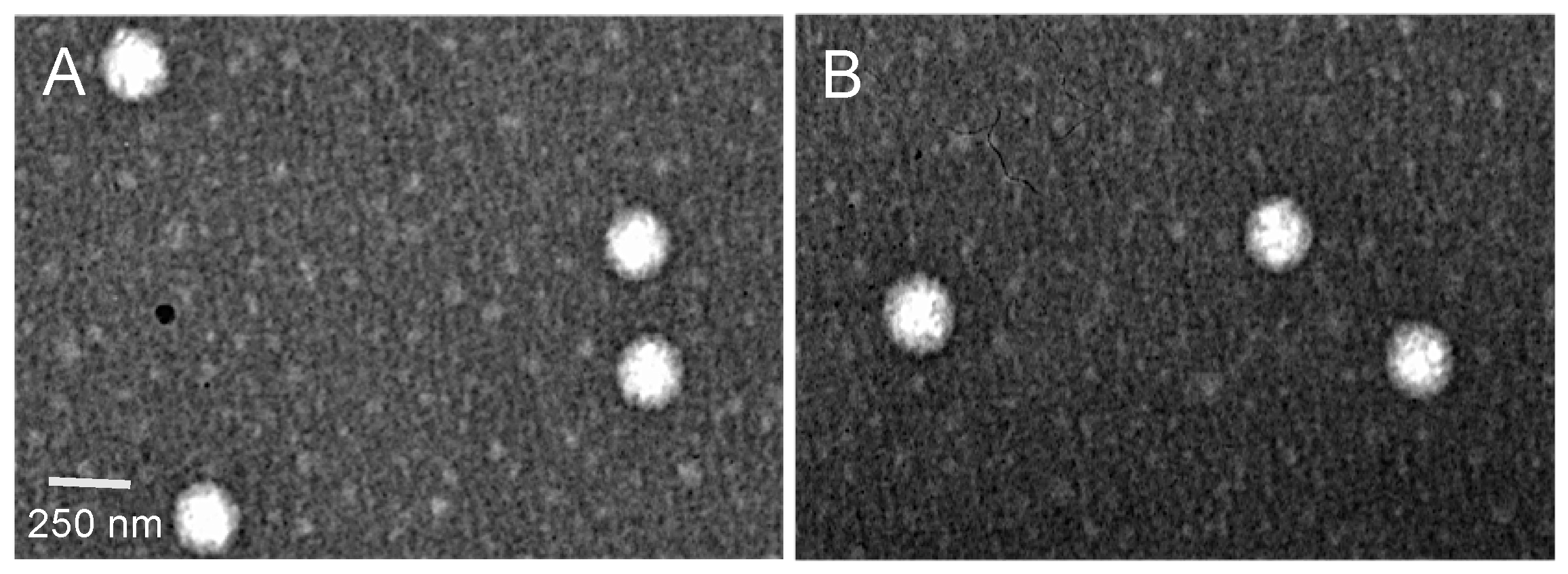
2.3.6. Transmission Electron Microscopy Analysis
2.4. Cell Culture Studies
2.4.1. Ethics Statement
2.4.2. Monocyte Isolation and Primary Human Macrophages Differentiation
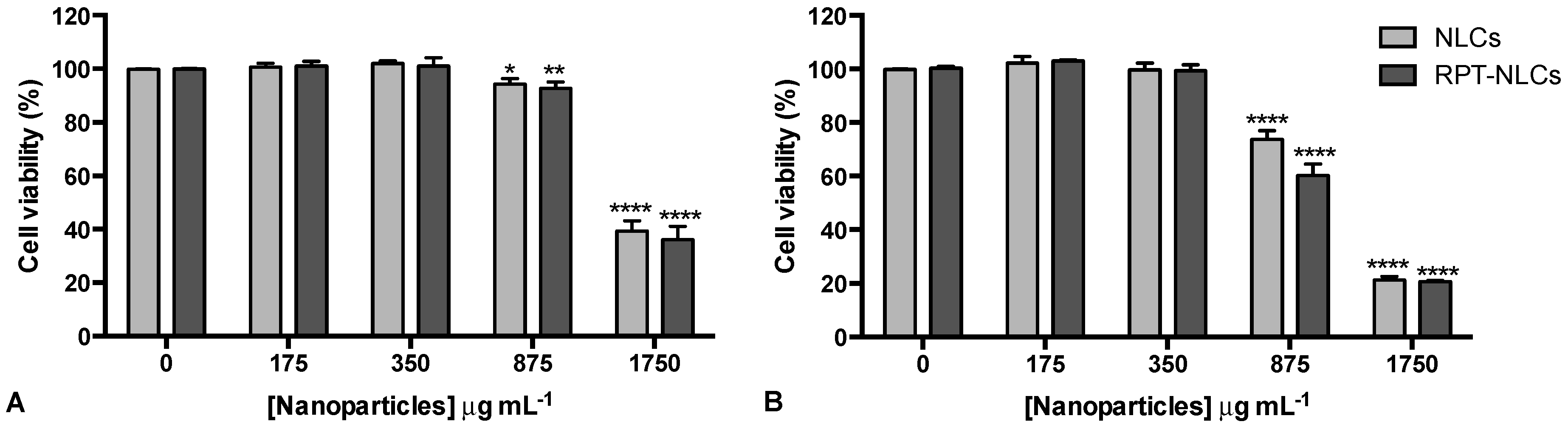
2.4.3. Cell Viability Assessment
2.5. Statistical Analysis
3. Results and Discussion
3.1. Selection of the Most Appropriate Lipid Composition
3.2. Validation of the Experimental Design
3.3. Characterization of the Optimized Nanoparticles
3.4. Physical Stability of Optimized Nanoparticles
3.5. Primary Human Macrophage Viability upon Nanoparticles Exposure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2019; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Magalhães, J.; Vieira, A.; Santos, S.G.; Pinheiro, M.; Reis, S. Oral administration of Nanoparticles-Based TB Drugs. In Multifunctional Systems for Combined Delivery, Biosensing and Diagnostics, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 307–326. ISBN 9780323527255. [Google Scholar]
- World Health Organization. Guidelines for Treatment of Drug-Susceptible Tuberculosis and Patient Care, 2017 Update; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Ahmad, S.; Mokaddas, E. Current status and future trends in the diagnosis and treatment of drug-susceptible and multidrug-resistant tuberculosis. J. Infect. Public Health 2014, 7, 75–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Global Tuberculosis Report 2017; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- World Health Organization. The End TB Strategy: Global Strategy and Targets for Tuberculosis Prevention, Care and Control after 2015; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Zhang, Y.; Post-Martens, K.; Denkin, S. New drug candidates and therapeutic targets for tuberculosis therapy. Drug Discov. Today 2006, 11, 21–27. [Google Scholar] [CrossRef]
- Zumla, A.; Chakaya, J.; Centis, R.; D’Ambrosio, L.; Mwaba, P.; Bates, M.; Kapata, N.; Nyirenda, T.; Chanda, D.; Mfinanga, S.; et al. Tuberculosis treatment and management-an update on treatment regimens, trials, new drugs, and adjunct therapies. Lancet Respir. Med. 2015, 3, 220–234. [Google Scholar] [CrossRef]
- Burman, W.J.; Gallicano, K.; Peloquin, C. Comparative pharmacokinetics and pharmacodynamics of the rifamycin antibacterials. Clin. Pharmacokinet. 2001, 40, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Munsiff, S.S.; Kambili, C.; Ahuja, S.D. Rifapentine for the treatment of pulmonary tuberculosis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2006, 43, 1468–1475. [Google Scholar] [CrossRef] [PubMed]
- Wagner, V.; Dullaart, A.; Bock, A.K.; Zweck, A. The emerging nanomedicine landscape. Nat. Biotechnol. 2006, 24, 1211–1217. [Google Scholar] [CrossRef]
- Zazo, H.; Colino, C.I.; Lanao, J.M. Current applications of nanoparticles in infectious diseases. J. Control. Release Off. J. Control. Release Soc. 2016, 224, 86–102. [Google Scholar] [CrossRef]
- Chaves, L.L.; Lima, S.; Vieira, A.C.C.; Ferreira, D.; Sarmento, B.; Reis, S. Overcoming clofazimine intrinsic toxicity: Statistical modelling and characterization of solid lipid nanoparticles. J. R. Soc. Interface 2018, 15. [Google Scholar] [CrossRef]
- Chaves, L.L.; Vieira, A.C.; Ferreira, D.; Sarmento, B.; Reis, S. Rational and precise development of amorphous polymeric systems with dapsone by response surface methodology. Int. J. Biol. Macromol. 2015, 81, 662–671. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, M.; Chaves, L.L.; Lima, S.A.; Reis, S. Optimization of nanostructured lipid carriers loaded with methotrexate: A tool for inflammatory and cancer therapy. Int. J. Pharm. 2015, 492, 65–72. [Google Scholar] [CrossRef]
- Granja, A.; Vieira, A.C.; Chaves, L.L.; Nunes, C.; Neves, A.R.; Pinheiro, M.; Reis, S. Folate-targeted nanostructured lipid carriers for enhanced oral delivery of epigallocatechin-3-gallate. Food Chem. 2017, 237, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.C.; Chaves, L.L.; Pinheiro, M.; Ferreira, D.; Sarmento, B.; Reis, S. Design and statistical modeling of mannose-decorated dapsone-containing nanoparticles as a strategy of targeting intestinal M-cells. Int. J. Nanomed. 2016, 11, 2601–2617. [Google Scholar] [CrossRef] [Green Version]
- Das Neves, J.; Sarmento, B. Precise engineering of dapivirine-loaded nanoparticles for the development of anti-HIV vaginal microbicides. Acta Biomater. 2015, 18, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, J.; Pinheiro, M.; Drasler, B.; Septiadi, D.; Petri-Fink, A.; Santos, S.G.; Rothen-Rutishauser, B.; Reis, S. Lipid nanoparticles biocompatibility and cellular uptake in a 3D human lung model. Nanomedicine (Lond. Engl.) 2019. [Google Scholar] [CrossRef] [Green Version]
- Neves, A.R.; Lucio, M.; Martins, S.; Lima, J.L.; Reis, S. Novel resveratrol nanodelivery systems based on lipid nanoparticles to enhance its oral bioavailability. Int. J. Nanomed. 2013, 8, 177–187. [Google Scholar] [CrossRef] [Green Version]
- Varshosaz, J.; Eskandari, S.; Tabbakhian, M. Freeze-drying of nanostructure lipid carriers by different carbohydrate polymers used as cryoprotectants. Carbohydr. Polym. 2012, 88, 1157–1163. [Google Scholar] [CrossRef]
- Vieira, A.C.; Magalhaes, J.; Rocha, S.; Cardoso, M.S.; Santos, S.G.; Borges, M.; Pinheiro, M.; Reis, S. Targeted macrophages delivery of rifampicin-loaded lipid nanoparticles to improve tuberculosis treatment. Nanomedicine 2017, 12, 2721–2736. [Google Scholar] [CrossRef]
- Oliveira, M.I.; Santos, S.G.; Oliveira, M.J.; Torres, A.L.; Barbosa, M.A. Chitosan drives anti-inflammatory macrophage polarisation and pro-inflammatory dendritic cell stimulation. Eur. Cells Mater. 2012, 24, 136–153. [Google Scholar] [CrossRef]
- Rezaie Shirmard, L.; Bahari Javan, N.; Khoshayand, M.R.; Kebriaee-Zadeh, A.; Dinarvand, R.; Dorkoosh, F.A. Nanoparticulate fingolimod delivery system based on biodegradable poly (3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV): Design, optimization, characterization and in-vitro evaluation. Pharm. Dev. Technol. 2017, 22, 860–870. [Google Scholar] [CrossRef]
- Ferreira, S.L.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandao, G.C.; da Silva, E.G.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef]
- Vivek, K.; Reddy, H.; Murthy, R.S. Investigations of the effect of the lipid matrix on drug entrapment, in vitro release, and physical stability of olanzapine-loaded solid lipid nanoparticles. AAPS PharmSciTech 2007, 8, E83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mor, N.; Simon, B.; Mezo, N.; Heifets, L. Comparison of activities of rifapentine and rifampin against Mycobacterium tuberculosis residing in human macrophages. Antimicrob. Agents Chemother. 1995, 39, 2073–2077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
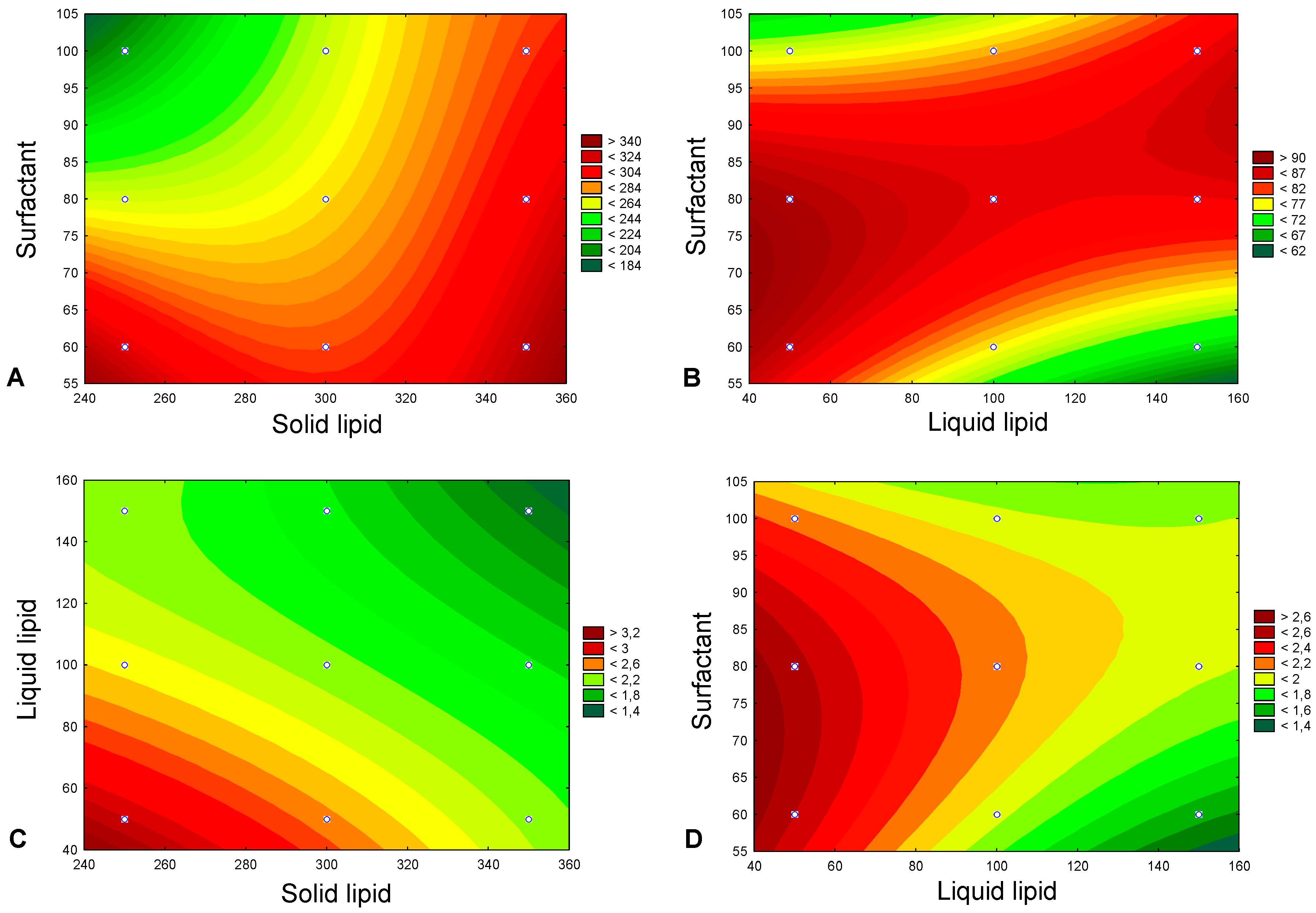
| Independent Variables | Coded Levels | ||
|---|---|---|---|
| Low Level (−1) | Medium Level (0) | High Level (1) | |
| X1 = solid lipid (mg) | 250 | 300 | 350 |
| X2 = liquid lipid (mg) | 50 | 100 | 150 |
| X3 = surfactant (mg) | 60 | 80 | 100 |
| Dependent variables | Criteria | ||
| Y1 = particle size (nm) Y2 = encapsulation efficiency (%) Y3 = loading capacity | Optimum (200 nm) | ||
| Maximum (100%) | |||
| Maximum | |||
| Sample | Independent Variables | Dependent Variables | ||||
|---|---|---|---|---|---|---|
| X1 (mg) | X2 (mg) | X3 (mg) | Y1 (nm) | Y2 (%) | Y3 | |
| 1 | 250 | 50 | 80 | 235 | 90.3 | 3.01 |
| 2 | 350 | 50 | 80 | 311 | 86.8 | 2.17 |
| 3 | 250 | 150 | 80 | 275 | 85.8 | 2.15 |
| 4 | 350 | 150 | 80 | 334 | 78.7 | 1.57 |
| 5 | 250 | 100 | 60 | 317 | 75.5 | 2.16 |
| 6 | 350 | 100 | 60 | 330 | 86.6 | 1.92 |
| 7 | 250 | 100 | 100 | 207 | 75.0 | 2.14 |
| 8 | 350 | 100 | 100 | 294 | 67.2 | 1.49 |
| 9 | 300 | 50 | 60 | 271 | 87.4 | 2.50 |
| 10 | 300 | 150 | 60 | 322 | 69.2 | 1.54 |
| 11 | 300 | 50 | 100 | 251 | 75.2 | 2.15 |
| 12 | 300 | 150 | 100 | 272 | 84.8 | 1.89 |
| 13 | 300 | 100 | 80 | 277 | 84.9 | 2.12 |
| 14 | 300 | 100 | 80 | 254 | 89.1 | 2.23 |
| 15 | 300 | 100 | 80 | 282 | 83.2 | 2.08 |
| Size-Y1 | EE-Y2 | LC-Y3 | ||||
|---|---|---|---|---|---|---|
| Coeff. | p-Value | Coeff. | p-Value | Coeff. | p-Value | |
| Intercept | 284.917 | 0.000 | 80.208 | 0.000 | 2.058 | 0.000 |
| X1 | 30.833 | 0.031 | −1.492 | 0.318 | −0.311 | 0.009 |
| X12 | −6.438 | 0.239 | 0.852 | 0.394 | 0.003 | 0.906 |
| X2 | 16.500 | 0.097 | −2.817 | 0.131 | −0.345 | 0.007 |
| X22 | −2.438 | 0.595 | −0.685 | 0.477 | −0.044 | 0.164 |
| X3 | −30.167 | 0.032 | −3.033 | 0.116 | −0.075 | 0.122 |
| X32 | −1.563 | 0.727 | 3.977 | 0.037 | 0.105 | 0.035 |
| X1X2 | −4.250 | 0.627 | −0.900 | 0.613 | 0.065 | 0.236 |
| X1X3 | 18.500 | 0.132 | −4.725 | 0.090 | −0.103 | 0.119 |
| X2X3 | −7.500 | 0.421 | 6.950 | 0.045 | 0.175 | 0.046 |
| R2 | 0.976 | 0.975 | 0.994 | |||
| Dependent Variables | Predicted Values | Experimental Values |
|---|---|---|
| Y1 = particle size (nm) | 235 | 242 ± 9 |
| Y2 = EE (%) | 90 | 86 ± 4 |
| Y3 = LC | 3.0 | 2.9 ± 0.1 |
| Samples | Diameter (nm) | PDI | ζ-Potential (mV) | EE (%) | LC |
|---|---|---|---|---|---|
| NLCs | 245 ± 4 | 0.16 ± 0.01 | –24 ± 2 | - | - |
| RPT-NLCs | 242 ± 9 | 0.17 ± 0.01 | –22 ± 2 | 86 ± 4 | 2.9 ± 0.1 |
| Samples | ΔH (Jg−1) | ΔTonset (°C) | Melting Point (°C) | ΔTend (°C) |
|---|---|---|---|---|
| NLCs | 93.7 | 58.7 | 60.3 | 61.8 |
| RPT-NLCs | 89.0 | 49.0 | 58.7 | 60.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magalhães, J.; L. Chaves, L.; C. Vieira, A.; G. Santos, S.; Pinheiro, M.; Reis, S. Optimization of Rifapentine-Loaded Lipid Nanoparticles Using a Quality-by-Design Strategy. Pharmaceutics 2020, 12, 75. https://doi.org/10.3390/pharmaceutics12010075
Magalhães J, L. Chaves L, C. Vieira A, G. Santos S, Pinheiro M, Reis S. Optimization of Rifapentine-Loaded Lipid Nanoparticles Using a Quality-by-Design Strategy. Pharmaceutics. 2020; 12(1):75. https://doi.org/10.3390/pharmaceutics12010075
Chicago/Turabian StyleMagalhães, Joana, Luise L. Chaves, Alexandre C. Vieira, Susana G. Santos, Marina Pinheiro, and Salette Reis. 2020. "Optimization of Rifapentine-Loaded Lipid Nanoparticles Using a Quality-by-Design Strategy" Pharmaceutics 12, no. 1: 75. https://doi.org/10.3390/pharmaceutics12010075
APA StyleMagalhães, J., L. Chaves, L., C. Vieira, A., G. Santos, S., Pinheiro, M., & Reis, S. (2020). Optimization of Rifapentine-Loaded Lipid Nanoparticles Using a Quality-by-Design Strategy. Pharmaceutics, 12(1), 75. https://doi.org/10.3390/pharmaceutics12010075








