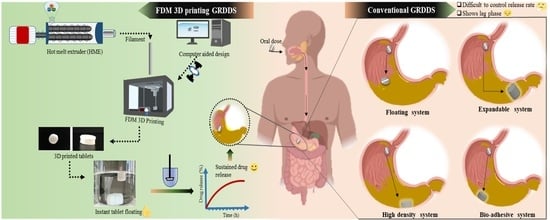
Fabrication of Intragastric Floating, Controlled Release 3D Printed Theophylline Tablets Using Hot-Melt Extrusion and Fused Deposition Modeling
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Preparation of THEO-Loaded Filaments
2.3. Fabrication of FDM 3DP Tablets
2.4. Physicochemical Characterization
2.4.1. Determination of Drug Loading in 3DP Tablets
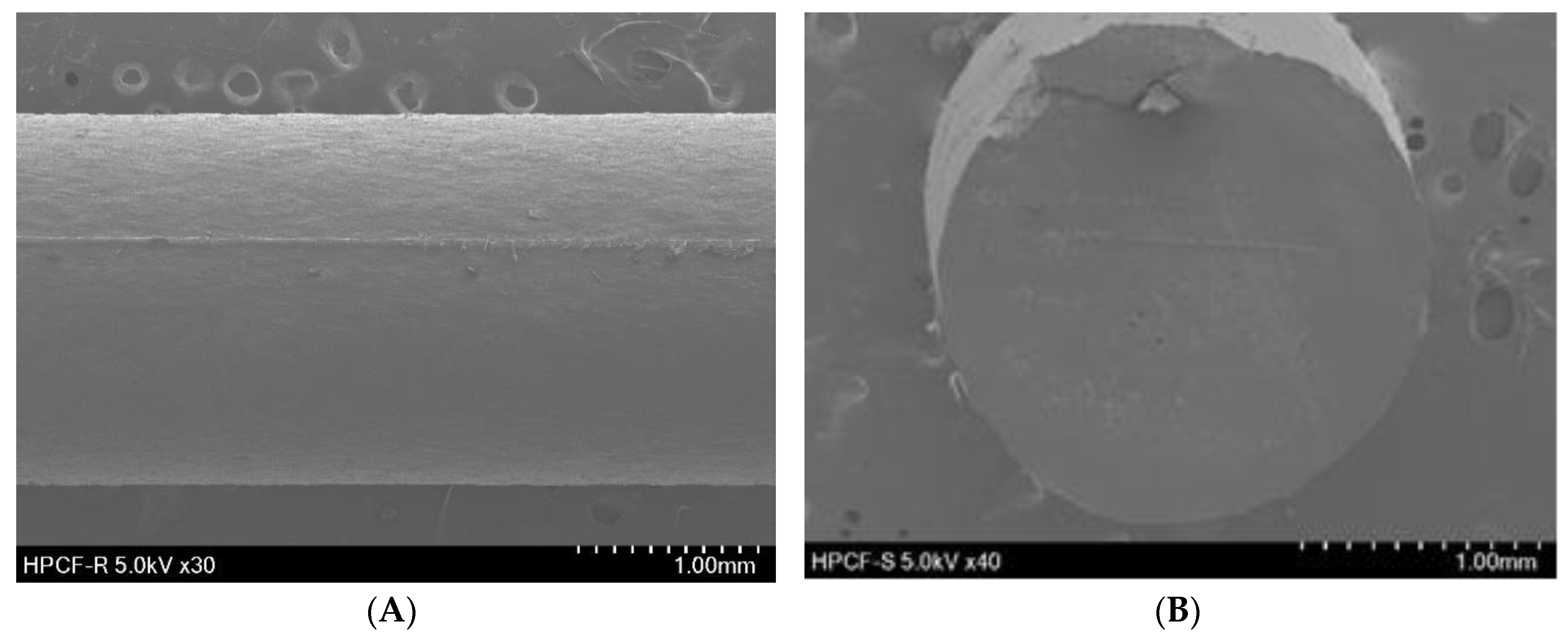
2.4.2. Scanning Electron Microscopy (SEM)
2.4.3. Differential Scanning Calorimetry (DSC)
2.4.4. Powder X-Ray Diffraction (PXRD)
2.5. In Vitro Dissolution and Floating Study
2.6. Dissolution Kinetics Studies
3. Results and Discussion
3.1. Preparation of Drug-Loaded THEO Filaments
3.2. 3DP of THEO Dosage Forms
3.3. Physicochemical State Characterization
3.3.1. Determination of Drug Loading
3.3.2. Scanning Electron Microscopy (SEM)
3.3.3. Differential Scanning Calorimetry (DSC)
3.3.4. Powder X-Ray Diffraction (PXRD)
3.4. In Vitro Floating and Dissolution Study
3.5. Dissolution Kinetic Studies
- The initial drug concentration in a matrix system is much higher than the drug solubility.
- Drug diffusion is one-dimensional because edge effects are insignificant.
- The thickness of the dosage form is much larger than the size of the suspended drug particles (macro or nanoparticles).
- The swelling or dissolution of the polymer carrier is negligible.
- The drug diffusion coefficient is constant.
- Perfect sink conditions are achieved in the release medium.
Zero-Order Drug Release
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Streubel, A.; Siepmann, J.; Bodmeier, R. Drug delivery to the upper small intestine window using gastroretentive technologies. Curr. Opin. Pharmacol. 2006, 6, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Shah, H.P.; Prajapati, S.T. Quality by design based development and optimization of novel gastroretentive floating osmotic capsules of clopidogrel bisulfate. J. Pharm. Investig. 2019, 49, 295–311. [Google Scholar] [CrossRef]
- Lamichhane, S.; Park, J.B.; Sohn, D.H.; Lee, S. Customized Novel Design of 3D Printed Pregabalin Tablets for Intra-Gastric Floating and Controlled Release Using Fused Deposition Modeling. Pharmaceutics 2019, 11, 564. [Google Scholar] [CrossRef] [PubMed]
- Chavanpatil, M.D.; Jain, P.; Chaudhari, S.; Shear, R.; Vavia, P.R. Novel sustained release, swellable and bioadhesive gastroretentive drug delivery system for ofloxacin. Int. J. Pharm. 2006, 316, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Klausner, E.A.; Lavy, E.; Friedman, M.; Hoffman, A. Expandable gastroretentive dosage forms. J. Control. Release 2003, 90, 143–162. [Google Scholar] [CrossRef]
- Li, Z.; Xu, H.; Li, S.; Li, Q.; Zhang, W.; Ye, T.; Yang, X.; Pan, W. A novel gastro-floating multiparticulate system for dipyridamole (DIP) based on a porous and low-density matrix core: In vitro and in vivo evaluation. Int. J. Pharm. 2014, 461, 540–548. [Google Scholar] [CrossRef]
- Pawar, V.K.; Kansal, S.; Asthana, S.; Chourasia, M.K. Industrial perspective of gastroretentive drug delivery systems: Physicochemical, biopharmaceutical, technological and regulatory consideration. Expert Opin. Drug Deliv. 2012, 9, 551–565. [Google Scholar] [CrossRef]
- Arora, S.; Ali, J.; Ahuja, A.; Khar, R.K.; Baboota, S. Floating drug delivery systems: A review. AAPS PharmSciTech 2005, 6, E372–E390. [Google Scholar] [CrossRef]
- Kim, S.; Hwang, K.M.; Park, Y.S.; Nguyen, T.T.; Park, E.S. Preparation and evaluation of non-effervescent gastroretentive tablets containing pregabalin for once-daily administration and dose proportional pharmacokinetics. Int. J. Pharm. 2018, 550, 160–169. [Google Scholar] [CrossRef]
- Park, B.J.; Choi, H.J.; Moon, S.J.; Kim, S.J.; Bajracharya, R.; Min, J.Y.; Han, H.K. Pharmaceutical applications of 3D printing technology: Current understanding and future perspectives. J. Pharm. Investig. 2019, 49, 575–585. [Google Scholar] [CrossRef]
- Guvendiren, M.; Molde, J.; Soares, R.M.D.; Kohn, J. Designing Biomaterials for 3D Printing. ACS Biomater. Sci. Eng. 2016, 2, 1679–1693. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, A.; Pumera, M. 3D-printing technologies for electrochemical applications. Chem. Soc. Rev. 2016, 45, 2740–2755. [Google Scholar] [CrossRef] [PubMed]
- Gross, B.C.; Erkal, J.L.; Lockwood, S.Y.; Chen, C.; Spence, D.M. Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. Anal. Chem. 2014, 86, 3240–3253. [Google Scholar] [CrossRef]
- Tan, D.; Maniruzzaman, M.; Nokhodchi, A. Advanced pharmaceutical applications of hot-melt extrusion coupled with fused deposition modelling (FDM) 3D printing for personalised drug delivery. Pharmaceutics 2018, 10, 203. [Google Scholar] [CrossRef] [PubMed]
- Tagami, T.; Fukushige, K.; Ogawa, E.; Hayashi, N.; Ozeki, T. 3D printing factors important for the fabrication of polyvinylalcohol filament-based tablets. Biol. Pharm. Bull. 2017, 40, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Kim, T.H.; Jeong, S.W.; Chung, S.E.; Kim, D.H.; Shin, B.S. Development of a gastroretentive delivery system for acyclovir by 3D printing technology and its in vivo pharmacokinetic evaluation in Beagle dogs. PLoS ONE 2019, 14, e0216875. [Google Scholar] [CrossRef]
- Charoenying, T.; Patrojanasophon, P.; Ngawhirunpat, T.; Rojanarata, T.; Akkaramongkolporn, P.; Opanasopit, P. Fabrication of floating capsule-in-3D-printed devices as gastro-retentive delivery systems of amoxicillin. J. Drug Deliv. Sci. Technol. 2020, 55, 101393. [Google Scholar] [CrossRef]
- Chai, X.; Chai, H.; Wang, X.; Yang, J.; Li, J.; Zhao, Y.; Cai, W.; Tao, T.; Xiang, X. Fused Deposition Modeling (FDM) 3D Printed Tablets for Intragastric Floating Delivery of Domperidone. Sci. Rep. 2017, 7, 2829. [Google Scholar] [CrossRef]
- Fu, J.; Yin, H.; Yu, X.; Xie, C.; Jiang, H.; Jin, Y.; Sheng, F. Combination of 3D printing technologies and compressed tablets for preparation of riboflavin floating tablet-in-device (TiD) systems. Int. J. Pharm. 2018, 549, 370–379. [Google Scholar] [CrossRef]
- Chen, D.; Xu, X.Y.; Li, R.; Zang, G.A.; Zhang, Y.; Wang, M.R.; Xiong, M.F.; Xu, J.R.; Wang, T.; Fu, H.; et al. Preparation and In vitro Evaluation of FDM 3D-Printed Ellipsoid-Shaped Gastric Floating Tablets with Low Infill Percentages. AAPS PharmSciTech 2020, 21, 6. [Google Scholar] [CrossRef]
- Dai, L.; Cheng, T.; Duan, C.; Zhao, W.; Zhang, W.; Zou, X.; Aspler, J.; Ni, Y. 3D printing using plant-derived cellulose and its derivatives: A review. Carbohydr. Polym. 2019, 203, 71–86. [Google Scholar] [CrossRef]
- Kempin, W.; Franz, C.; Koster, L.C.; Schneider, F.; Bogdahn, M.; Weitschies, W.; Seidlitz, A. Assessment of different polymers and drug loads for fused deposition modeling of drug loaded implants. Eur. J. Pharm. Biopharm. 2017, 115, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Feng, X.; Patil, H.; Tiwari, R.V.; Repka, M.A. Coupling 3D printing with hot-melt extrusion to produce controlled-release tablets. Int. J. Pharm. 2017, 519, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Kamel, S.; Ali, N.; Jahangir, K.; Shah, S.M.; El-Gendy, A.A. Pharmaceutical significance of cellulose: A review. Express Polym. Lett. 2008, 2, 758–778. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.; Quinn, M. Handbook of Pharmaceutical Excipients; Libros Digitales-Pharmaceutical Press: London, UK, 2009. [Google Scholar]
- Sarode, A.; Wang, P.; Cote, C.; Worthen, D.R. Low-viscosity hydroxypropylcellulose (HPC) grades SL and SSL: Versatile pharmaceutical polymers for dissolution enhancement, controlled release, and pharmaceutical processing. AAPS PharmSciTech 2013, 14, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Crowley, M.M.; Zhang, F.; Repka, M.A.; Thumma, S.; Upadhye, S.B.; Battu, S.K.; McGinity, J.W.; Martin, C. Pharmaceutical applications of hot-melt extrusion: Part I. Drug Dev. Ind. Pharm. 2007, 33, 909–926. [Google Scholar] [CrossRef]
- Goyanes, A.; Fina, F.; Martorana, A.; Sedough, D.; Gaisford, S.; Basit, A.W. Development of modified release 3D printed tablets (printlets) with pharmaceutical excipients using additive manufacturing. Int. J. Pharm. 2017, 527, 21–30. [Google Scholar] [CrossRef]
- Lamichhane, S.; Bashyal, S.; Keum, T.; Noh, G.; Seo, J.E.; Bastola, R.; Choi, J.; Sohn, D.H.; Lee, S. Complex formulations, simple techniques: Can 3D printing technology be the Midas touch in pharmaceutical industry? Asian J. Pharm. Sci. 2019, 14, 465–479. [Google Scholar] [CrossRef]
- Pietrzak, K.; Isreb, A.; Alhnan, M.A. A flexible-dose dispenser for immediate and extended release 3D printed tablets. Eur. J. Pharm. Biopharm. 2015, 96, 380–387. [Google Scholar] [CrossRef]
- Räsänen, E.; Rantanen, J.; Jørgensen, A.; Karjalainen, M.; Paakkari, T.; Yliruusi, J. Novel identification of pseudopolymorphic changes of theophylline during wet granulation using near infrared spectroscopy. J. Pharm. Sci. 2001, 90, 389–396. [Google Scholar] [CrossRef]
- Leuner, C.; Dressman, J. Improving drug solubility for oral delivery using solid dispersions. Eur. J. Pharm. Biopharm. 2000, 50, 47–60. [Google Scholar] [CrossRef]
- Tran, P.H.L.; Tran, H.T.T.; Lee, B.J. Modulation of microenvironmental pH and crystallinity of ionizable telmisartan using alkalizers in solid dispersions for controlled release. J. Control. Release 2008, 129, 59–65. [Google Scholar] [CrossRef]
- Klein, S. The use of biorelevant dissolution media to forecast the in vivo performance of a drug. AAPS J. 2010, 12, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, W.; Vo, A.Q.; Feng, X.; Ye, X.; Kim, D.W.; Repka, M.A. Hydroxypropyl methylcellulose-based controlled release dosage by melt extrusion and 3D printing: Structure and drug release correlation. Carbohydr. Polym. 2017, 177, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guan, X.; Cui, M.; Zhu, Z.; Chen, K.; Wen, H.; Jia, D.; Hou, J.; Xu, W.; Yang, X.; et al. Preparation and investigation of novel gastro-floating tablets with 3D extrusion-based printing. Int. J. Pharm. 2018, 535, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, M.L. (Ed.) 5-Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Sawston, UK, 2015; pp. 63–86. [Google Scholar]
- Higuchi, T. Rate of Release of Medicaments from Ointment Bases Containing Drugs in Suspension. J. Pharm. Sci. 1961, 50, 874–875. [Google Scholar] [CrossRef] [PubMed]
- Omidian, H.; Park, K. Introduction to Hydrogels. In Biomedical Applications of Hydrogels Handbook; Ottenbrite, R.M., Park, K., Okano, T., Eds.; Springer: New York, NY, USA, 2010; pp. 1–16. [Google Scholar]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2012, 64, 163–174. [Google Scholar] [CrossRef]
- Siepmann, J.; Göpferich, A. Mathematical modeling of bioerodible, polymeric drug delivery systems. Adv. Drug Deliv. Rev. 2001, 48, 229–247. [Google Scholar] [CrossRef]
- Peppas, N.A. Analysis of Fickian and non-Fickian drug release from polymers. Pharm. Acta Helv. 1985, 60, 110–111. [Google Scholar]
- Peppas, N.A.; Narasimhan, B. Mathematical models in drug delivery: How modeling has shaped the way we design new drug delivery systems. J. Control. Release 2014, 190, 75–81. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of solute release, III, Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]







| Formulation | Infill (%) | Shell (mm) | Diameter (X, mm) | Diameter (Y, mm) | Thickness (Z, mm) | Weight (mg) |
|---|---|---|---|---|---|---|
| T1 | 30 | 0.8 | 10.54 ± 0.15 | 10.30 ± 0.17 | 4.63 ± 0.02 | 353.71 ± 8.78 |
| T2 | 20 | 0.8 | 10.22 ± 0.04 | 10.25 ± 0.01 | 5.02 ± 0.37 | 326.30 ± 17.63 |
| T3 | 10 | 0.8 | 10.52 ± 0.11 | 10.12 ± 0.09 | 4.74 ± 0.13 | 297.30 ± 5.35 |
| T4 | 0 | 0.8 | 10.48 ± 0.40 | 10.41 ± 0.27 | 4.68 ± 0.04 | 273.70 ± 4.72 |
| T5 | 20 | 1.2 | 10.40 ± 0.25 | 10.57 ± 0.14 | 5.09 ± 0.03 | 401.96 ± 8.75 |
| T6 | 20 | 0.4 | 10.55 ± 0.14 | 10.46 ± 0.36 | 5.13 ± 0.03 | 322.95 ± 5.32 |
| T7 | 20 | 0 | 10.55 ± 0.13 | 10.40 ± 0.16 | 5.17 ± 0.11 | 266.24 ± 3.50 |
| Formulation | Ritger-Peppas Model | Linear Model | Peppas-Sahlin Model | |||||
|---|---|---|---|---|---|---|---|---|
| Exponent, n | R2 | Slope, k | R2 | k1 | k2 | m | R2 | |
| T1 | 1.11 | 0.9846 | 0.20 | 0.9876 | −0.92 | 0.41 | 0.45 | 0.9814 |
| T2 | 1.39 | 0.9927 | 0.19 | 0.9781 | −0.55 | 0.13 | 0.56 | 0.9837 |
| T3 | 0.89 | 0.9896 | 0.25 | 0.9519 | 0.80 | 0.20 | 0.52 | 1.0000 |
| T4 | 0.90 | 0.9998 | 0.26 | 0.9422 | 0.87 | 0.01 | 0.73 | 0.9997 |
| T5 | 0.73 | 0.9969 | 0.24 | 0.9708 | 1.20 | 0.02 | 0.63 | 0.9980 |
| T6 | 0.84 | 0.9868 | 0.23 | 0.9594 | 0.40 | 0.45 | 0.44 | 0.9998 |
| T7 | 0.79 | 0.9881 | 0.30 | 0.9043 | −3.35 | 3.03 | 0.32 | 0.9894 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giri, B.R.; Song, E.S.; Kwon, J.; Lee, J.-H.; Park, J.-B.; Kim, D.W. Fabrication of Intragastric Floating, Controlled Release 3D Printed Theophylline Tablets Using Hot-Melt Extrusion and Fused Deposition Modeling. Pharmaceutics 2020, 12, 77. https://doi.org/10.3390/pharmaceutics12010077
Giri BR, Song ES, Kwon J, Lee J-H, Park J-B, Kim DW. Fabrication of Intragastric Floating, Controlled Release 3D Printed Theophylline Tablets Using Hot-Melt Extrusion and Fused Deposition Modeling. Pharmaceutics. 2020; 12(1):77. https://doi.org/10.3390/pharmaceutics12010077
Chicago/Turabian StyleGiri, Bhupendra Raj, Eon Soo Song, Jaewook Kwon, Ju-Hyun Lee, Jun-Bom Park, and Dong Wuk Kim. 2020. "Fabrication of Intragastric Floating, Controlled Release 3D Printed Theophylline Tablets Using Hot-Melt Extrusion and Fused Deposition Modeling" Pharmaceutics 12, no. 1: 77. https://doi.org/10.3390/pharmaceutics12010077
APA StyleGiri, B. R., Song, E. S., Kwon, J., Lee, J.-H., Park, J.-B., & Kim, D. W. (2020). Fabrication of Intragastric Floating, Controlled Release 3D Printed Theophylline Tablets Using Hot-Melt Extrusion and Fused Deposition Modeling. Pharmaceutics, 12(1), 77. https://doi.org/10.3390/pharmaceutics12010077