Carbohydrate Immune Adjuvants in Subunit Vaccines
Abstract
:1. Introduction
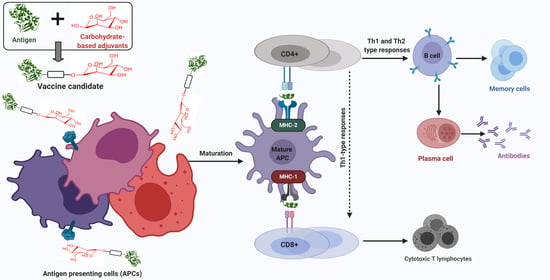
2. Immunostimulation
2.1. Innate and Adaptive Immunity
2.2. Mucosal and Systemic Immunity
3. Adjuvants
4. Carbohydrate-Based Adjuvants
4.1. Saccharides
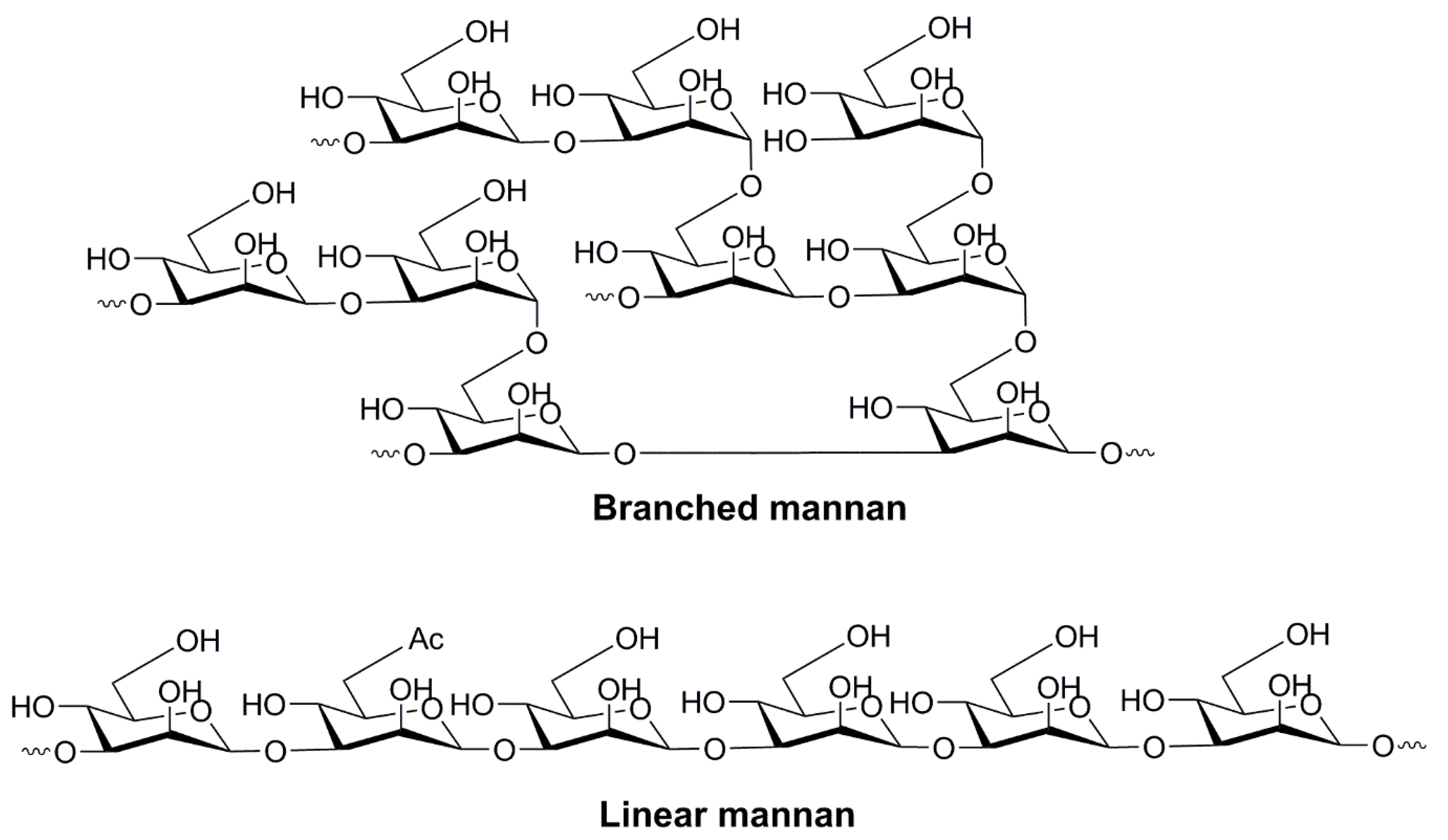
4.1.1. Mannose
4.1.2. Oligo- and Polysaccharides of Mannose
4.1.3. Glucan
4.1.4. Chitosan
4.1.5. Hyaluronic Acid
4.2. Saccharide Derivatives
4.2.1. Lipid A and its Derivatives
4.2.2. CAF01
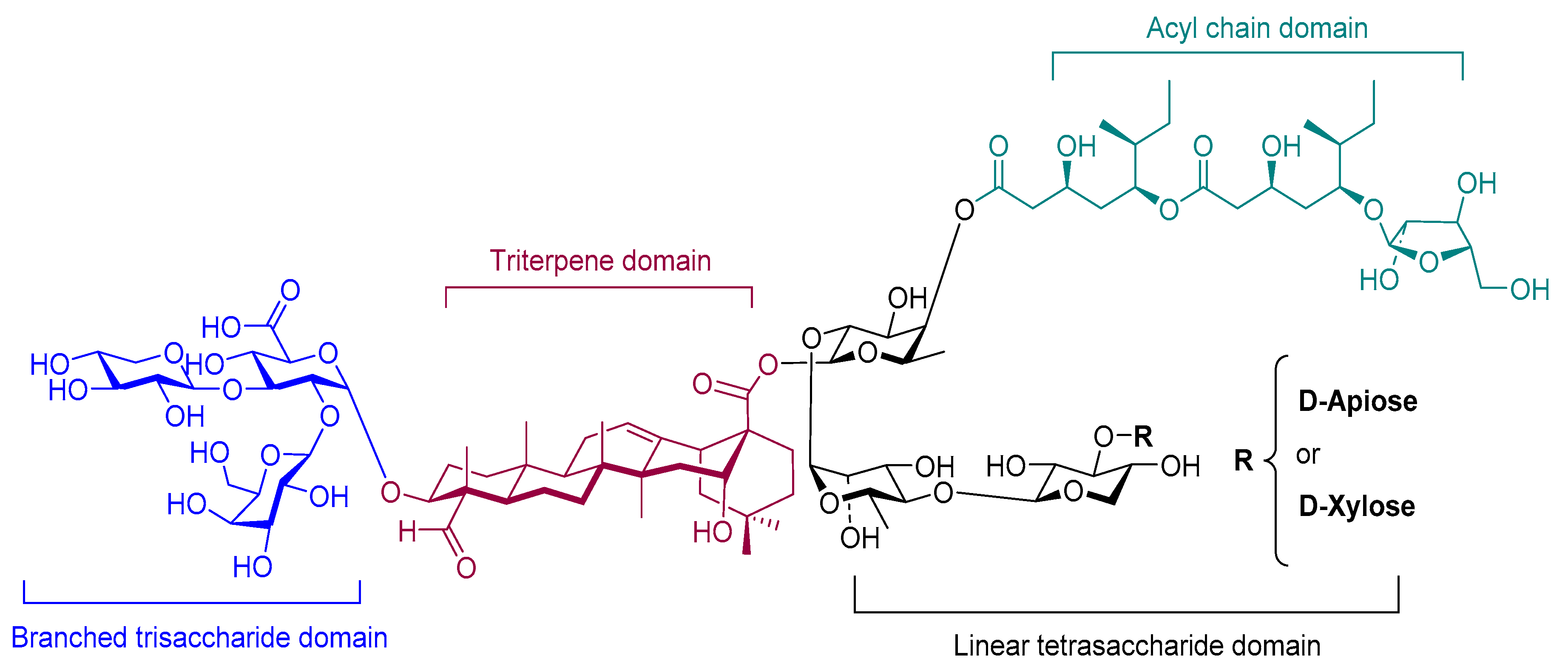
4.2.3. Saponin (QS-21)
4.2.4. α-Galactosylceramide
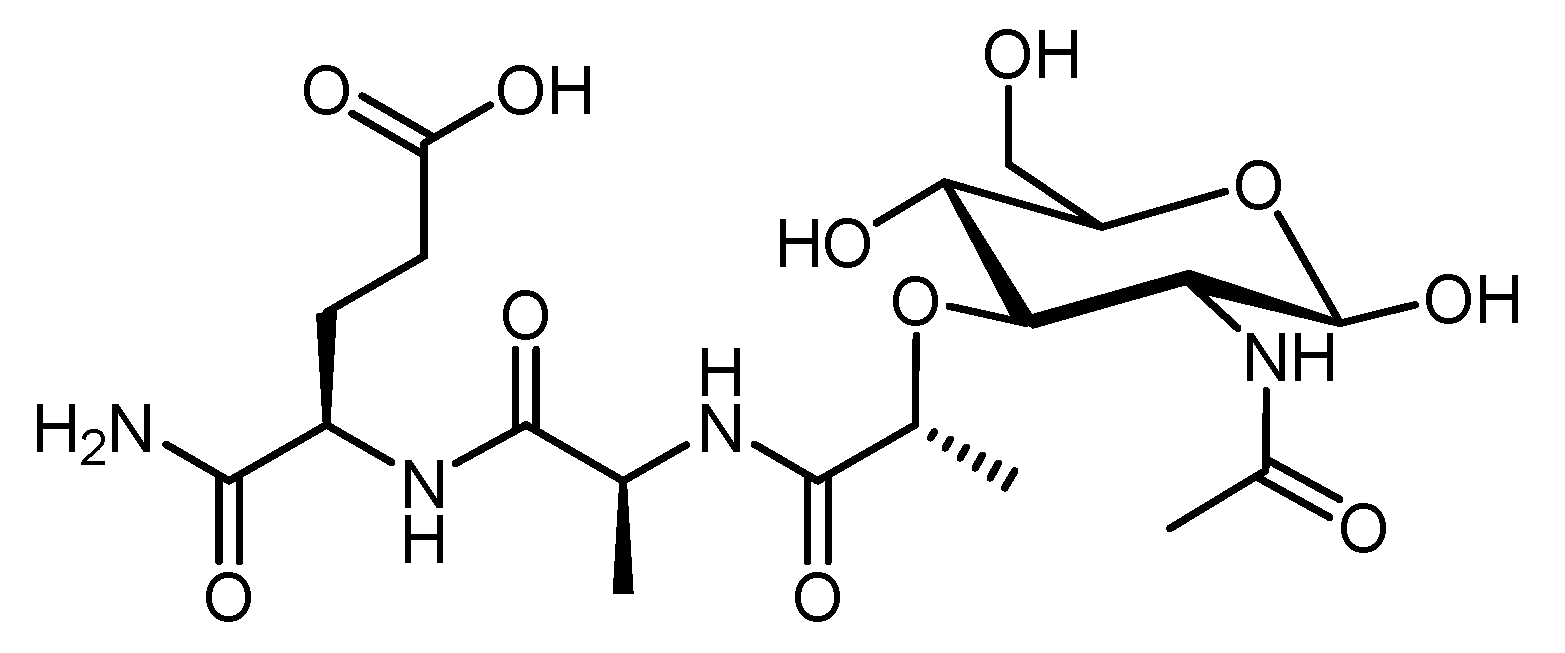
4.2.5. Muramyl Dipeptide (MDP)
5. Conclusions
Funding
Conflicts of Interest
References
- Vartak, A.; Sucheck, S. Recent advances in subunit vaccine carriers. Vaccines 2016, 4, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skwarczynski, M.; Toth, I. Peptide-based synthetic vaccines. Chem. Sci. 2016, 7, 842–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skwarczynski, M.; Zaman, M.; Toth, I. Lipo-peptides/saccharides for peptide vaccine delivery. In Handbook of Biologically Active Peptides, 2nd ed.; Kastin, A.J., Ed.; Academic Press: Boston, MA, USA, 2013; Volume 78, pp. 571–579. ISBN 978-0-1238-5095-9. [Google Scholar] [CrossRef]
- Bergeon, J.A.; Chan, Y.N.; Charles, B.G.; Toth, I. Oral absorption enhancement of dipeptide L-Glu-L-Trp-OH by lipid and glycosyl conjugation. J. Pept. Sci. 2008, 90, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, D.H.; Viac, J.; Werling, D.; Rème, C.A.; Gatto, H. Role of sugars in surface microbe–host interactions and immune reaction modulation. Vet. Dermatol. 2007, 18, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, S.; Lepenies, B. Glycans as vaccine antigens and adjuvants: Immunological considerations. In Carbohydrate-Based Vaccines; Lepenies, B., Ed.; Humana Press: New York, NY, USA, 2015; Volume 1331, pp. 11–26. ISBN 978-1-4939-2873-6. [Google Scholar] [CrossRef]
- Schülke, S.; Vogel, L.; Junker, A.C.; Hanschmann, K.M.; Flaczyk, A.; Vieths, S.; Scheurer, S. A fusion protein consisting of the vaccine adjuvant monophosphoryl lipid A and the allergen ovalbumin boosts allergen-specific Th1, Th2, and Th17 responses in vitro. J. Immunol. Res. 2016, 2016, 4156456. [Google Scholar] [CrossRef] [Green Version]
- Micoli, F.; Costantino, P.; Adamo, R. Potential targets for next generation antimicrobial glycoconjugate vaccines. FEMS Microbiol. Rev. 2018, 42, 388–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nevagi, R.J.; Skwarczynski, M.; Toth, I. Polymers for subunit vaccine delivery. Eur. Polym. 2019, 114, 397–410. [Google Scholar] [CrossRef]
- Khademi, F.; Taheri, R.A.; Avarvand, A.Y.; Vaez, H.; Momtazi-Borojeni, A.A.; Soleimanpour, S. Are chitosan natural polymers suitable as adjuvant/delivery system for anti-tuberculosis vaccines? Microb. Pathog. 2018, 121, 218–223. [Google Scholar] [CrossRef]
- Vedove, E.D.; Costabile, G.; Merkel, O.M. Mannose and Mannose-6-Phosphate Receptor–Targeted Drug Delivery Systems and Their Application in Cancer Therapy. Adv. Healthc. Mater. 2018, 7, 1701398. [Google Scholar] [CrossRef]
- Popescu, R.; Ghica, M.V.; Dinu-Pîrvu, C.E.; Anuța, V.; Lupuliasa, D.; Popa, L. New Opportunity to Formulate Intranasal Vaccines and Drug Delivery Systems Based on Chitosan. Int. J. Mol. Sci. 2020, 21, 5016. [Google Scholar] [CrossRef]
- Sun, B.; Yu, S.; Zhao, D.; Guo, S.; Wang, X.; Zhao, K. Polysaccharides as vaccine adjuvants. Vaccine 2018, 36, 5226–5234. [Google Scholar] [CrossRef] [PubMed]
- Aljohani, S.; Hussein, W.M.; Toth, I.; Simerska, P. Carbohydrates in Vaccine Development. Curr. Drug Deliv. 2019, 16, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Fruk, L.; Franck, C.O.; Fanslau, L.; Popov, A.B.; Tyagi, P. Biopolymer-based Carriers for DNA Vaccine Design. Angew. Chem. 2020, in press. [Google Scholar] [CrossRef]
- Ghaffarifar, F. Plasmid DNA vaccines: Where are we now. Drugs Today 2018, 54, 315–333. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Regulation of adaptive immunity by the innate immune system. Science 2010, 327, 291–295. [Google Scholar] [CrossRef] [Green Version]
- Roche, P.A.; Furuta, K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat. Rev. Immunol. 2015, 15, 203–216. [Google Scholar] [CrossRef]
- De Temmerman, M.L.; Rejman, J.; Demeester, J.; Irvine, D.J.; Gander, B.; De Smedt, S.C. Particulate vaccines: On the quest for optimal delivery and immune response. Drug Discov. Today 2011, 16, 569–582. [Google Scholar] [CrossRef]
- Garrett-Sinha, L.A. B cell immunity. In Management of Infections in the Immunocompromised Host; Springer: Cham, Switzerland, 2018; pp. 43–54. ISBN 978-3-319-77672-9. [Google Scholar] [CrossRef]
- Gao, H.; Gonçalves, C.; Gallego, T.; François-Heude, M.; Malard, V.; Mateo, V.; Lemoine, F.; Cendret, V.; Pilard, F.D.; Moreau, V.; et al. Comparative binding and uptake of liposomes decorated with mannose oligosaccharides by cells expressing the mannose receptor or DC-SIGN. Carbohydr. Res. 2020, 487, 107877. [Google Scholar] [CrossRef]
- Bueter, C.L.; Specht, C.A.; Levitz, S.M. Innate sensing of chitin and chitosan. PLoS Pathog. 2013, 9, e1003080. [Google Scholar] [CrossRef] [Green Version]
- Kanjan, P.; Sahasrabudhe, N.M.; de Haan, B.J.; de Vos, P. Immune effects of β-glucan are determined by combined effects on Dectin-1, TLR2, 4 and 5. J. Funct. Foods. 2017, 37, 433–440. [Google Scholar] [CrossRef]
- Kamphuis, T.; Meijerhof, T.; Stegmann, T.; Lederhofer, J.; Wilschut, J.; de Haan, A. Immunogenicity and protective capacity of a virosomal respiratory syncytial virus vaccine adjuvanted with monophosphoryl lipid A in mice. PLoS ONE 2012, 7, e36812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marciani, D.J. Is fucose the answer to the immunomodulatory paradox of Quillaja saponins? Int. J. Immunopharmacol. 2015, 29, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Zaric, M.; Lyubomska, O.; Poux, C.; Hanna, M.L.; McCrudden, M.T.; Malissen, B.; Ingram, R.J.; Power, U.F.; Scott, C.J.; Donnelly, R.F.; et al. Dissolving microneedle delivery of nanoparticle-encapsulated antigen elicits efficient cross-priming and Th1 immune responses by murine langerhans cells. J. Investig. Dermatol. 2015, 135, 425–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, W.; Bhide, Y.; Marsman, S.; Holtrop, M.; Meijerhof, T.; de Vries-Idema, J.; de Haan, A.; Huckriede, A. Monophosphoryl Lipid A-Adjuvanted Virosomes with Ni-Chelating Lipids for Attachment of Conserved Viral Proteins as Cross-Protective Influenza Vaccine. Biotechnol. J. 2018, 13, 1700645. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Noh, Y.W.; Lim, Y.T. Polymer nanoparticles for cross-presentation of exogenous antigens and enhanced cytotoxic T-lymphocyte immune response. Int. J. Nanomed. 2016, 11, 3753. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.; Maharjan, S.; Cho, K.H.; Cui, L.; Park, I.K.; Choi, Y.J.; Cho, C.S. Chitosan-based particulate systems for the delivery of mucosal vaccines against infectious diseases. Int. J. Biol. Macromol. 2018, 110, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.N. Mucosal vaccine delivery and M cell targeting. In Targeted Drug Delivery: Concepts and Design; Devarajan, V.P., Jain, S., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 313–337. ISBN 978-3-319-11354-8. [Google Scholar] [CrossRef]
- Chen, K.; Cerutti, A. Vaccination strategies to promote mucosal antibody responses. Immunity 2010, 33, 479–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehner, T.; Anton, P.A. Mucosal immunity and vaccination against HIV. AIDS 2002, 16, S125–S132. [Google Scholar] [CrossRef]
- Marasini, N.; Skwarczynski, M.; Toth, I. Intranasal delivery of nanoparticle-based vaccines. Ther. Deliv. 2017, 8, 151–167. [Google Scholar] [CrossRef]
- Marinaro, M.; Kiyono, H.; VanCott, J.L.; Okahashi, N.; van Ginkel, F.W.; Pascual, D.W.; Ban, E.; Jackson, R.J.; Staats, H.F.; McGhee, J.R. Vaccines for selective induction of Th1-and Th2-cell responses and their roles in mucosal immunity. In Essentials of Mucosal Immunology; Kagnoff, M.F., Kiyono, H., Eds.; Academic Press: San Diego, CA, USA, 1996; pp. 461–475. ISBN 978-0-12-394330-9. [Google Scholar] [CrossRef]
- Kumar, R.B. Needle free injection systems. J. Pharm. Innov. 2012, 1, 57–72. [Google Scholar]
- Skwarczynski, M.; Toth, I. Non-invasive mucosal vaccine delivery: Advantages, challenges and the future. Expert Opin. Drug Deliv. 2020, 17, 435–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawson, L.B.; Norton, E.B.; Clements, J.D. Defending the mucosa: Adjuvant and carrier formulations for mucosal immunity. Curr. Opin. Immunol. 2011, 23, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, A.J.; McCoy, K.D.; Johansen, F.E.; Brandtzaeg, P. The immune geography of IgA induction and function. Mucosal Immunol. 2008, 1, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, M.K.; Kang, S.K.; Choi, J.H.; Park, I.K.; Na, H.S.; Lee, H.C.; Kim, E.B.; Lee, N.K.; Nah, J.W.; Choi, Y.J.; et al. Targeted delivery of chitosan nanoparticles to Peyer’s patch using M cell-homing peptide selected by phage display technique. Biomaterials 2010, 31, 7738–7747. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Zhou, T.J.; Fan, Y.T.; He, Y.J.; Pang, T.; Pang, T.; Cho, K.H.; Lu, J.J.; Jiang, H.L.; Cho, C.S. Efficient Mucosal Immunization by Mucoadhesive and pH-Sensitive Polymeric Vaccine Delivery System. Macromol. Res. 2019, 27, 215–226. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, W.; Chen, Y.; Xu, Y.; Wang, B.; Zong, L. Eudragit® L100-coated mannosylated chitosan nanoparticles for oral protein vaccine delivery. Int. J. Biol. Macromol. 2018, 113, 534–542. [Google Scholar] [CrossRef]
- McGeary, R.P.; Olive, C.; Toth, I. Lipid and carbohydrate-based adjuvant/carriers in immunology. J. Pept. Sci. 2003, 9, 405–418. [Google Scholar] [CrossRef]
- Sarkar, I.; Garg, R.; van Drunen Littel-van den Hurk, S. Selection of adjuvants for vaccines targeting specific pathogens. Expert Rev. Vaccines 2019, 18, 505–521. [Google Scholar] [CrossRef]
- Bergmann-Leitner, E.; Leitner, W. Adjuvants in the driver’s seat: How magnitude, type, fine specificity and longevity of immune responses are driven by distinct classes of immune potentiators. Vaccines 2014, 2, 252–296. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Mendieta, S.; Guillen, D.; Hernandez-Pando, R.; Sanchez, S.; Rodriguez-Sanoja, R. Potential of glucans as vaccine adjuvants: A review of the α-glucans case. Carbohydr. Polym. 2017, 165, 103–114. [Google Scholar] [CrossRef]
- Bose, R.J.; Kim, M.; Chang, J.H.; Paulmurugan, R.; Moon, J.J.; Koh, W.G.; Lee, S.H.; Park, H. Biodegradable polymers for modern vaccine development. Ind. Eng. Chem. Res. 2019, 77, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, S.; Skwarczynski, M.; Toth, I. Lipids as activators of innate immunity in peptide vaccine delivery. Curr. Med. Chem. 2020, 27, 2887–2901. [Google Scholar] [CrossRef] [PubMed]
- Lindblad, E.B. Aluminium adjuvants—In retrospect and prospect. Vaccine 2004, 22, 3658–3668. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.G.; Orr, M.T.; Fox, C.B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013, 19, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Mosaiab, T.; Farr, D.C.; Kiefel, M.J.; Houston, T.A. Carbohydrate-based nanocarriers and their application to target macrophages and deliver antimicrobial agents. Adv. Drug Deliv. Rev. 2019, 151, 94–129. [Google Scholar] [CrossRef]
- Nagae, M.; Yoshiki, Y. Structural Aspects of Carbohydrate Recognition Mechanisms of C-Type Lectins. In Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–30. [Google Scholar] [CrossRef]
- Lepenies, B.; Yin, J.; Seeberger, P.H. Applications of synthetic carbohydrates to chemical biology. Curr. Opin. Chem. Biol. 2010, 14, 404–411. [Google Scholar] [CrossRef]
- Van Dissel, J.T.; Joosten, S.A.; Hoff, S.T.; Soonawala, D.; Prins, C.; Hokey, D.A.; O’Dee, D.M.; Graves, A.; Thierry-Carstensen, B.; Andreasen, L.V.; et al. A novel liposomal adjuvant system, CAF01, promotes long-lived Mycobacterium tuberculosis-specific T-cell responses in human. Vaccine 2014, 32, 7098–7107. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Liu, H.; Zhang, X.; Qian, F. Intranasal and oral vaccination with protein-based antigens: Advantages, challenges and formulation strategies. Protein Cell 2015, 6, 480–503. [Google Scholar] [CrossRef] [Green Version]
- Petrovsky, N.; Cooper, P.D. Carbohydrate-based immune adjuvants. Expert Rev. Vaccines 2011, 10, 523–537. [Google Scholar] [CrossRef]
- Jin, J.W.; Peng, W.L.; Tang, S.Q.; Rong, M.Z.; Zhang, M.Q. Antigen uptake and immunoadjuvant activity of pathogen-mimetic hollow silica particles conjugated with β-glucan. J. Mater. Chem. B. 2018, 6, 6288–6301. [Google Scholar] [CrossRef]
- Porporatto, C.; Bianco, I.D.; Correa, S.G. Local and systemic activity of the polysaccharide chitosan at lymphoid tissues after oral administration. J. Leukoc. Biol. 2005, 78, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Geijtenbeek, T.B.H.; Van Kooyk, Y. Pathogens target DC-SIGN to influence their fate DC-SIGN functions as a pathogen receptor with broad specificity. APMIS 2003, 111, 698–714. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, S.; Matsumoto, M.; Takeuchi, O.; Matsuzawa, T.; Ishikawa, E.; Sakuma, M.; Tateno, H.; Uno, J.; Hirabayashi, J.; Mikami, Y.; et al. C-type lectin Mincle is an activating receptor for pathogenic fungus, Malassezia. Proc. Natl. Acad. Sci. USA 2009, 106, 1897–1902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Netea, M.G.; Gow, N.A.R.; Munro, C.A.; Bates, S.; Collins, C.; Ferwerd, G.; Hobson, R.P.; Bertram, G.; Hughes, H.B.; Jansen, T.; et al. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J. Clin. Investig. 2006, 116, 1642–1650. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shi, G.; Zhang, J.; Song, H.; Niu, J.; Shi, S.; Huang, P.; Wang, Y.; Wang, W.; Li, C.; et al. Targeted antigen delivery to dendritic cell via functionalized alginate nanoparticles for cancer immunotherapy. J. Control. Release 2017, 256, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Hu, C.; Fan, F.; Qin, Y.; Huang, C.; Zhang, Z.; Lu, L.; Wang, H.; Sun, H.; Leng, X.; et al. Co-delivery of antigen and dual agonists by programmed mannose-targeted cationic lipid-hybrid polymersomes for enhanced vaccination. Biomaterials 2019, 206, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, B.; Stephenson, R.; Toth, I. Targeting the mannose receptor with mannosylated subunit vaccines. Curr. Med. Chem. 2014, 21, 3405–3418. [Google Scholar] [CrossRef]
- He, L.Z.; Crocker, A.; Lee, J.; Mendoza-Ramirez, J.; Wang, X.T.; Vitale, L.A.; O’Neill, T.; Petromilli, C.; Zhang, H.F.; Lopez, J.; et al. Antigenic targeting of the human mannose receptor induces tumor immunity. J. Immunol. 2007, 178, 6259–6267. [Google Scholar] [CrossRef] [Green Version]
- Dabaghian, M.; Latifi, A.M.; Tebianian, M.; NajmiNejad, H.; Ebrahimi, S.M. Nasal vaccination with r4M2e. HSP70c antigen encapsulated into N-trimethyl chitosan (TMC) nanoparticulate systems: Preparation and immunogenicity in a mouse model. Vaccine 2018, 36, 2886–2895. [Google Scholar] [CrossRef]
- Glaffig, M.; Stergiou, N.; Hartmann, S.; Schmitt, E.; Kunz, H. A synthetic MUC1 anticancer vaccine containing mannose ligands for targeting macrophages and dendritic cells. ChemMedChem 2018, 13, 25–29. [Google Scholar] [CrossRef]
- Karanikas, V.; Thynne, G.; Mitchell, P.; Ong, C.S.; Gunawardana, D.; Blum, R.; Pearson, J.; Lodding, J.; Pietersz, G.; Broadbent, R.; et al. Mannan mucin-1 peptide immunization: Influence of cyclophosphamide and the route of injection. J. Immunother. 2001, 24, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulos, V.; Pietersz, G.A.; Loveland, B.E.; Sandrin, M.S.; McKenzie, I.F. Oxidative/reductive conjugation of mannan to antigen selects for T1 or T2 immune responses. Proc. Natl. Acad. Sci. USA 1995, 92, 10128–10132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zehner, M.; Burgdorf, S. Regulation of antigen transport into the cytosol for cross presentation by ubiquitination of the mannose receptor. Mol. Immunol. 2013, 55, 146–148. [Google Scholar] [CrossRef]
- Wilson, D.S.; Hirosue, S.; Raczy, M.M.; Bonilla-Ramirez, L.; Jeanbart, L.; Wang, R.; Quaglia-Thermes, X. Antigens reversibly conjugated to a polymeric glyco-adjuvant induce protective humoral and cellular immunity. Nat. Mater. 2019, 18, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.E.; Williamson, D.; Titball, R. Vaccine delivery using nanoparticles. Front. Cell. Infect. Microbiol. 2013, 3, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, L.; Fan, Y.T.; Zhou, T.J.; Gong, J.H.; Cui, L.H.; Cho, K.H.; Choi, Y.J.; Jiang, H.L.; Cho, C.S. Chemical modification of chitosan for efficient vaccine delivery. Molecules 2018, 23, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, R.; Xu, J.; Xu, L.; Sun, X.; Chen, Q.; Zhao, Y.; Peng, R.; Liu, Z. Cancer cell membrane-coated adjuvant nanoparticles with mannose modification for effective anticancer vaccination. ACS Nano 2018, 12, 5121–5129. [Google Scholar] [CrossRef]
- Simerska, P.; Moyle, P.M.; Toth, I. Modern lipid-, carbohydrate-, and peptide-based delivery systems for peptide, vaccine, and gene products. Med. Res. Rev. 2011, 31, 520–547. [Google Scholar] [CrossRef]
- Hong, S.J.; Ahn, M.H.; Lee, Y.W.; Pal, S.; Sangshetti, J.; Arote, R.B. Biodegradable Polymeric Nanocarrier-Based Immunotherapy in Hepatitis Vaccination. In Cutting-Edge Enabling Technologies for Regenerative Medicine; Springer: Berlin/Heidelberg, Germany, 2018; pp. 303–320. ISBN 978-981-13-0949-6. [Google Scholar] [CrossRef]
- Malik, A.; Gupta, M.; Gupta, V.; Gogoi, H.; Bhatnagar, R. Novel application of trimethyl chitosan as an adjuvant in vaccine delivery. Int. J. Nanomed. 2018, 13, 7959. [Google Scholar] [CrossRef] [Green Version]
- Jabbal-Gill, I.; Watts, P.; Smith, A. Chitosan-based delivery systems for mucosal vaccines. Expert Opin. Drug Deliv. 2012, 9, 1051–1067. [Google Scholar] [CrossRef]
- Shi, G.N.; Zhang, C.N.; Xu, R.; Niu, J.F.; Song, H.J.; Zhang, X.Y.; Wang, W.W.; Wang, Y.M.; Li, C.; Wei, X.Q.; et al. Enhanced antitumor immunity by targeting dendritic cells with tumor cell lysate-loaded chitosan nanoparticles vaccine. Biomaterials 2017, 113, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, S.; Eichenberger, R.M.; Nevagi, R.J.; Ghaffar, K.A.; Marasini, N.; Dai, Y.; Loukas, A.; Toth, I.; Skwarczynski, M. Lipopeptide-based oral vaccine against hookworm infection. J. Infect. Dis. 2020, 221, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Giddam, A.K.; Reiman, J.M.; Zaman, M.; Skwarczynski, M.; Toth, I.; Good, M.F. A semi-synthetic whole parasite vaccine designed to protect against blood stage malaria. Acta Biomater. 2016, 44, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, P.; Zhuang, Y.; Li, P.; Jiang, B.; Pan, H.; Liu, L.; Cai, L.; Ma, Y. Lymphatic-targeted cationic liposomes: A robust vaccine adjuvant for promoting long-term immunological memory. Vaccine 2014, 32, 5475–5483. [Google Scholar] [CrossRef] [PubMed]
- Irache, J.M.; Salman, H.H.; Gamazo, C.; Espuelas, S. Mannose-targeted systems for the delivery of therapeutics. Expert Opin. Drug Deliv. 2008, 5, 703–724. [Google Scholar] [CrossRef] [PubMed]
- Burgdorf, S.; Schölz, C.; Kautz, A.; Tampé, R.; Kurts, C. Spatial and mechanistic separation of cross-presentation and endogenous antigen presentation. Nat. Immunol. 2008, 9, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.E.; Drickamer, K. Structural requirements for high affinity binding of complex ligands by the macrophage mannose receptor. J. Biol. Chem. 1993, 268, 399–404. [Google Scholar]
- McGreal, E.P.; Rosas, M.; Brown, G.D.; Zamze, S.; Wong, S.Y.; Gordon, S.; Martinez-Pomares, L.; Taylor, P.R. The carbohydrate recognition domain of Dectin-2 is a C-type lectin with specificity for high mannose. Glycobiology 2006, 16, 422–430. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.L.; Zhao, X.Q.; Jiang, C.; You, Y.; Chen, X.P.; Jiang, Y.Y.; Jia, X.M.; Lin, X. C-Type Lectin Receptors Dectin-3 and Dectin-2 Form a Heterodimeric Pattern-Recognition Receptor for Host Defense against Fungal Infection. Immunity 2013, 39, 324–334. [Google Scholar] [CrossRef] [Green Version]
- Kottom, T.J.; Hebrink, D.M.; Monteiro, J.T.; Lepenies, B.; Carmona, E.M.; Wuethrich, M.; Dos Santo Dias, L.; Limper, A.H. Myeloid C-type lectin receptors that recognize fungal mannans interact with Pneumocystis organisms and major surface glycoprotein. J. Med. Microbiol. 2019, 68, 1649–1654. [Google Scholar] [CrossRef]
- Cambi, A.; Gijzen, K.; De Vries, I.J.M.; Torensma, R.; Joosten, B.; Adema, G.J.; Netea, M.G.; Kullberg, B.J.; Romani, L.; Figdor, C.G. The C-type lectin DC-SIGN (CD209) is an antigen-uptake receptor for Candida albicans on dendritic cells. Eur. J. Immunol. 2003, 33, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Kohatsu, L.; Hsu, D.K.; Jegalian, A.G.; Liu, F.T.; Baum, L.G. Galectin-3 Induces Death of Candida Species Expressing Specific β-1,2-Linked Mannans. J. Immunol. 2006, 177, 4718–4726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, K.; Yang, X.L.; Yudate, T.; Chung, J.S.; Wu, J.; Luby-Phelps, K.; Kimberly, R.P.; Underhill, D.; Cruz, P.D.; Ariizumi, K. Dectin-2 Is a Pattern Recognition Receptor for Fungi That Couples with the Fc Receptor γ Chain to Induce Innate Immune Responses. J. Biol. Chem. 2006, 281, 38854–38866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jouault, T.; Ibata-Ombetta, S.; Takeuchi, O.; Trinel, P.A.; Sacchetti, P.; Lefebvre, P.; Akira, S.; Poulain, D. Candida albicans phospholipomannan is sensed through toll-like receptors. J. Infect. Dis. 2003, 188, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Bartheldyová, E.; Knotigová, P.T.; Zachová, K.; Mašek, J.; Kulich, P.; Effenberg, R.; Zyka, D.; Hubatka, F.; Kotouček, J.; Čelechovská, H.; et al. N-Oxy lipid-based click chemistry for orthogonal coupling of mannan onto nanoliposomes prepared by microfluidic mixing: Synthesis of lipids, characterisation of mannan-coated nanoliposomes and in vitro stimulation of dendritic cells. Carbohydr. Polym. 2019, 207, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Vendele, I.; Willment, J.A.; Silva, L.M.; Palma, A.S.; Chai, W.; Liu, Y.; Feizi, T.; Spyrou, M.; Stappers, M.H.; Brown, G.D.; et al. Mannan detecting C-type lectin receptor probes recognise immune epitopes with diverse chemical, spatial and phylogenetic heterogeneity in fungal cell walls. PLoS Pathog. 2020, 16, e1007927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- François-Heude, M.; Méndez-Ardoy, A.; Cendret, V.; Lafite, P.; Daniellou, R.; Mellet, C.O.; Fernández, J.M.G.; Moreau, V.; Djedaïni-Pilard, F. Synthesis of High-Mannose Oligosaccharide Analogues through Click Chemistry: True Functional Mimics of Their Natural Counterparts against Lectins? Chem. Eur. J. 2015, 21, 1978–1991. [Google Scholar] [CrossRef]
- Han, J.; Zhao, D.; Li, D.; Wang, X.; Jin, Z.; Zhao, K. Polymer-based nanomaterials and applications for vaccines and drugs. Polymers 2018, 10, 31. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Qi, C.; Guo, Y.; Zhou, W.; Zhang, Y. Toll-like receptor 4-related immunostimulatory polysaccharides: Primary structure, activity relationships, and possible interaction models. Carbohydr. Polym. 2016, 149, 186–206. [Google Scholar] [CrossRef]
- Yoo, M.K.; Park, I.Y.; Kim, I.Y.; Park, I.K.; Kwon, J.S.; Jeong, H.J.; Jeong, Y.Y.; Cho, C.S. Superparamagnetic iron oxide nanoparticles coated with mannan for macrophage targeting. J. Nanosci. Nanotechnol. 2008, 8, 5196–5202. [Google Scholar] [CrossRef]
- Wu, Y.; Yan, C.; He, J.A.; Xiong, W.; Wu, S.; Liu, S.; Cai, Z. Reversible Mannosylation as a Covalent Binding Adjuvant Enhances Immune Responses for Porcine Circovirus Type 2 Vaccine. ACS Omega 2018, 3, 17341–17347. [Google Scholar] [CrossRef]
- Stambas, J.; Pietersz, G.; McKenzie, I.; Cheers, C. Oxidised mannan as a novel adjuvant inducing mucosal IgA production. Vaccine 2002, 20, 1068–1078. [Google Scholar] [CrossRef]
- Cordeiro, A.S.; Alonso, M.J. Recent advances in vaccine delivery. Pharm. Pat. Anal. 2016, 5, 49–73. [Google Scholar] [CrossRef] [PubMed]
- Soto, E.; Ostroff, G.R. Oral Macrophage Mediated Gene Delivery System. NSTI Nanotech 2007, 2, 378–381. [Google Scholar]
- Goodridge, H.S.; Reyes, C.N.; Becker, C.A.; Katsumoto, T.R.; Ma, J.; Wolf, A.J.; Bose, N.; Chan, A.S.; Magee, A.S.; Danielson, M.E.; et al. Activation of the innate immune receptor Dectin-1 upon formation of a ‘phagocytic synapse’. Nature 2011, 472, 471–475. [Google Scholar] [CrossRef]
- Dedloff, M.R.; Effler, C.S.; Holban, A.M.; Gestal, M.C. Use of Biopolymers in Mucosally-Administered Vaccinations for Respiratory Disease. Materials 2019, 12, 2445. [Google Scholar] [CrossRef] [Green Version]
- Netea, M.G.; Van De Veerdonk, F.; Verschueren, I.; Van Der Meer, J.W.; Kullberg, B.J. Role of TLR1 and TLR6 in the host defense against disseminated candidiasis. FEMS Microbiol. Immunol. 2008, 52, 118–123. [Google Scholar] [CrossRef]
- Kagimura, F.Y.; da Cunha, M.A.A.; Barbosa, A.M.; Dekker, R.F.H.; Malfatti, C.R.M. Biological activities of derivatized d-glucans: A review. Int. J. Biol. Macromol. 2015, 72, 588–598. [Google Scholar] [CrossRef]
- De Smet, R.; Demoor, T.; Verschuere, S.; Dullaers, M.; Ostroff, G.R.; Leclercq, G.; Allais, L.; Pilette, C.; Dierendonck, M.; De Geest, B.G.; et al. β-Glucan microparticles are good candidates for mucosal antigen delivery in oral vaccination. J. Control. Release 2013, 172, 671–678. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, J.Y.; Ryu, H.S.; Park, H.G.; Kim, Y.O.; Kang, J.S.; Kim, H.M.; Hong, J.T.; Kim, Y.; Han, S.B. Induction of dendritic cell maturation by β-glucan isolated from Sparassis crispa. Int. Immunopharmacol. 2010, 10, 1284–1294. [Google Scholar] [CrossRef]
- Di Carlo, F.J.; Fiore, J.V. On the composition of zymosan. Science 1958, 127, 756–757. [Google Scholar] [CrossRef] [PubMed]
- Slack, E.C.; Robinson, M.J.; Hernanz-Falcón, P.; Brown, G.D.; Williams, D.L.; Schweighoffer, E.; Tybulewicz, V.L.; Reis e Sousa, C. Syk-dependent ERK activation regulates IL-2 and IL-10 production by DC stimulated with zymosan. Eur. J. Immunol. 2007, 37, 1600–1612. [Google Scholar] [CrossRef] [PubMed]
- Rogers, N.C.; Slack, E.C.; Edwards, A.D.; Nolte, M.A.; Schulz, O.; Schweighoffer, E.; Williams, D.L.; Gordon, S.; Tybulewicz, V.L.; Brown, G.D.; et al. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity 2005, 22, 507–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ainai, A.; Ichinohe, T.; Tamura, S.I.; Kurata, T.; Sata, T.; Tashiro, M.; Hasegawa, H. Zymosan enhances the mucosal adjuvant activity of poly (I: C) in a nasal influenza vaccine. J. Med. Virol. 2010, 82, 476–484. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.; Boudrant, J.; Meyer, D.; Manno, N.; DeMarchis, M.; Paoletti, M.G. Current views on fungal chitin/chitosan, human chitinases, food preservation, glucans, pectins and inulin: A tribute to Henri Braconnot, precursor of the carbohydrate polymers science, on the chitin bicentennial. Carbohydr. Polym. 2012, 87, 995–1012. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.N. Trimethyl chitosan and its applications in drug delivery. J. Mater. Sci. Mater. Med. 2009, 20, 1057. [Google Scholar] [CrossRef]
- Marasini, N.; Giddam, A.K.; Khalil, Z.G.; Hussein, W.M.; Capon, R.J.; Batzloff, M.R.; Good, M.F.; Toth, I.; Skwarczynski, M. Double adjuvanting strategy for peptide-based vaccines: Trimethyl chitosan nanoparticles for lipopeptide delivery. Nanomedicine 2016, 11, 3223–3235. [Google Scholar] [CrossRef]
- Slütter, B.; Plapied, L.; Fievez, V.; Sande, M.A.; des Rieux, A.; Schneider, Y.J.; Van Riet, E.; Jiskoot, W.; Préat, V. Mechanistic study of the adjuvant effect of biodegradable nanoparticles in mucosal vaccination. J. Control. Release 2009, 138, 113–121. [Google Scholar] [CrossRef]
- Kulkarni, A.D.; Patel, H.M.; Surana, S.J.; Vanjari, Y.H.; Belgamwar, V.S.; Pardeshi, C.V. N, N, N-Trimethyl chitosan: An advanced polymer with myriad of opportunities in nanomedicine. Carbohydr. Polym. 2017, 157, 875–902. [Google Scholar] [CrossRef]
- Vila, A.; Sánchez, A.; Janes, K.; Behrens, I.; Kissel, T.; Jato, J.L.V.; Alonso, M.J. Low molecular weight chitosan nanoparticles as new carriers for nasal vaccine delivery in mice. Eur. J. Pharm. Biopharm. 2004, 57, 123–131. [Google Scholar] [CrossRef]
- Zhao, L.; Skwarczynski, M.; Toth, I. Polyelectrolyte-based platforms for the delivery of peptides and proteins. ACS Biomater. Sci. Eng. 2019, 5, 4937–4950. [Google Scholar] [CrossRef]
- Nevagi, R.J.; Khalil, Z.G.; Hussein, W.M.; Powell, J.; Batzloff, M.R.; Capon, R.J.; Good, M.F.; Skwarczynski, M.; Toth, I. Polyglutamic acid-trimethyl chitosan-based intranasal peptide nano-vaccine induces potent immune responses against group A streptococcus. Acta Biomater. 2018, 80, 278–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nevagi, R.J.; Dai, W.; Khalil, Z.G.; Hussein, W.M.; Capon, R.J.; Skwarczynski, M.; Toth, I. Structure-activity relationship of group A streptococcus lipopeptide vaccine candidates in trimethyl chitosan-based self-adjuvanting delivery system. Eur. J. Med. Chem. 2019, 179, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Nevagi, R.J.; Dai, W.; Khalil, Z.G.; Hussein, W.M.; Capon, R.J.; Skwarczynski, M.; Toth, I. Self-assembly of trimethyl chitosan and poly (anionic amino acid)-peptide antigen conjugate to produce a potent self-adjuvanting nanovaccine delivery system. Bioorg. Med. Chem. 2019, 27, 3082–3088. [Google Scholar] [CrossRef]
- Bal, S.M.; Slütter, B.; Verheul, R.; Bouwstra, J.A.; Jiskoot, W. Adjuvanted, antigen loaded N-trimethyl chitosan nanoparticles for nasal and intradermal vaccination: Adjuvant-and site-dependent immunogenicity in mice. Eur. J. Pharm. Sci. 2012, 45, 475–481. [Google Scholar] [CrossRef]
- Zhang, G.; Jia, P.; Liu, H.; Hu, T.; Du, Y. Conjugation of chitosan oligosaccharides enhances immune response to porcine circovirus vaccine by activating macrophages. Immunobiology 2018, 223, 663–670. [Google Scholar] [CrossRef]
- Park, J.; Babensee, J.E. Differential functional effects of biomaterials on dendritic cell maturation. Acta Biomater. 2012, 8, 3606–3617. [Google Scholar] [CrossRef] [Green Version]
- Xue, M.; Hu, S.; Lu, Y.; Zhang, Y.; Jiang, X.; An, S.; Guo, Y.; Zhou, X.; Hou, H.; Jiang, C. Development of chitosan nanoparticles as drug delivery system for a prototype capsid inhibitor. Int. J. Pharm. 2015, 495, 771–782. [Google Scholar] [CrossRef]
- Xia, Y.; Fan, Q.; Hao, D.; Wu, J.; Ma, G.; Su, Z. Chitosan-based mucosal adjuvants: Sunrise on the ocean. Vaccine 2015, 33, 5997–6010. [Google Scholar] [CrossRef]
- Pei, M.; Liang, J.; Zhang, C.; Wang, X.; Zhang, C.; Ma, G.; Sun, H. Chitosan/calcium phosphates nanosheet as a vaccine carrier for effective cross-presentation of exogenous antigens. Carbohydr. Polym. 2019, 224, 115–172. [Google Scholar] [CrossRef]
- Fan, Y.; Sahdev, P.; Ochyl, L.J.; Akerberg, J.J.; Moon, J.J. Cationic liposome–hyaluronic acid hybrid nanoparticles for intranasal vaccination with subunit antigens. J. Control. Release 2015, 208, 121–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bussio, J.I.; Molina-Perea, C.; González-Aramundiz, J.V. Hyaluronic Acid Nanocapsules as a Platform for Needle-Free Vaccination. Pharmaceutics 2019, 11, 246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic acid in the third millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef] [Green Version]
- Necas, J.B.L.B.P.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef] [Green Version]
- Termeer, C.; Benedix, F.; Sleeman, J.; Fieber, C.; Voith, U.; Ahrens, T.; Miyake, K.; Freudenberg, M.; Galanos, C.; Simon, J.C. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J. Exp. Med. 2002, 195, 99–111. [Google Scholar] [CrossRef] [Green Version]
- Gariboldi, S.; Palazzo, M.; Zanobbio, L.; Selleri, S.; Sommariva, M.; Sfondrini, L.; Cavicchini, S.; Balsari, A.; Rumio, C. Low molecular weight hyaluronic acid increases the self-defense of skin epithelium by induction of β-defensin 2 via TLR2 and TLR4. J. Immunol. 2008, 181, 2103–2110. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Cao, F.; Liu, X.; Wang, H.; Zhang, C.; Sun, H.; Wang, C.; Leng, X.; Song, C.; Kong, D.; et al. Hyaluronic acid-modified cationic lipid–PLGA hybrid nanoparticles as a nanovaccine induce robust humoral and cellular immune responses. ACS Appl. Mater. Interfaces 2016, 8, 11969–11979. [Google Scholar] [CrossRef] [PubMed]
- Verheul, R.J.; Slütter, B.; Bal, S.M.; Bouwstra, J.A.; Jiskoot, W.; Hennink, W.E. Covalently stabilized trimethyl chitosan-hyaluronic acid nanoparticles for nasal and intradermal vaccination. J. Control. Release 2011, 156, 46–52. [Google Scholar] [CrossRef]
- Ran, R.; Liu, Y.; Gao, H.; Kuang, Q.; Zhang, Q.; Tang, J.; Huang, K.; Chen, X.; Zhang, Z.; He, Q. Enhanced gene delivery efficiency of cationic liposomes coated with PEGylated hyaluronic acid for anti P-glycoprotein siRNA: A potential candidate for overcoming multi-drug resistance. Int. J. Pharm. 2014, 477, 590–600. [Google Scholar] [CrossRef]
- Campoccia, D.; Doherty, P.; Radice, M.; Brun, P.; Abatangelo, G.; Williams, D.F. Semisynthetic resorbable materials from hyaluronan esterification. Biomaterials 1998, 19, 2101–2127. [Google Scholar] [CrossRef]
- Partidos, C.D.; Pizza, M.; Rappuoli, R.; Steward, M.W. The adjuvant effect of a non-toxic mutant of heat-labile enterotoxin of Escherichia coli for the induction of measles virus-specific CTL responses after intranasal co-immunization with a synthetic peptide. Immunology 1996, 89, 483–487. [Google Scholar] [CrossRef]
- Singh, M.; Briones, M.; O’Hagan, D.T. A novel bioadhesive intranasal delivery system for inactivated influenza vaccines. J. Control. Release 2001, 70, 267–276. [Google Scholar] [CrossRef]
- Kim, S.; Patel, D.S.; Park, S.; Slusky, J.; Klauda, J.B.; Widmalm, G.; Im, W. Bilayer properties of lipid A from various Gram-negative bacteria. Biophys. J. 2016, 111, 1750–1760. [Google Scholar] [CrossRef]
- Mattsby-Baltzer, I.; Gemski, P.; Alving, C.R. Heterogeneity of lipid A: Comparison of lipid A types from different gram-negative bacteria. J. Bacteriol. 1984, 159, 900–904. [Google Scholar] [CrossRef] [Green Version]
- Persing, D.H.; Coler, R.N.; Lacy, M.J.; Johnson, D.A.; Baldridge, J.R.; Hershberg, R.M.; Reed, S.G. Taking toll: Lipid A mimetics as adjuvants and immunomodulators. Trends Microbiol. 2002, 10, s32–s37. [Google Scholar] [CrossRef]
- Casella, C.R.; Mitchell, T.C. Putting endotoxin to work for us: Monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell. Mol. Life Sci. 2008, 65, 3231. [Google Scholar] [CrossRef] [Green Version]
- Alloatti, A.; Kotsias, F.; Pauwels, A.M.; Carpier, J.M.; Jouve, M.; Timmerman, E.; Pace, L.; Vargas, P.; Maurin, M.; Gehrmann, U.; et al. Toll-like receptor 4 engagement on dendritic cells restrains phago-lysosome fusion and promotes cross-presentation of antigens. Immunity 2015, 43, 1087–1100. [Google Scholar] [CrossRef] [Green Version]
- Choi, A.; Bouzya, B.; Franco, K.D.C.; Stadlbauer, D.; Rajabhathor, A.; Rouxel, R.N.; Mainil, R.; Van der Wielen, M.; Palese, P.; García-Sastre, A.; et al. Chimeric hemagglutinin-based influenza virus vaccines induce protective stalk-specific humoral immunity and cellular responses in mice. Immunohorizons 2019, 3, 133–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, H.P.; Murugappan, S.; Ter Veer, W.; Meijerhof, T.; de Haan, A.; Frijlink, H.W.; Wilschut, J.; Hinrichs, W.L.; Huckriede, A. Evaluation of monophosphoryl lipid A as adjuvant for pulmonary delivered influenza vaccine. J. Control. Release 2014, 174, 51–62. [Google Scholar] [CrossRef]
- Chong, C.S.; Cao, M.; Wong, W.W.; Fischer, K.P.; Addison, W.R.; Kwon, G.S.; Tyrrell, D.L.; Samuel, J. Enhancement of T helper type 1 immune responses against hepatitis B virus core antigen by PLGA nanoparticle vaccine delivery. J. Control. Release 2005, 102, 85–99. [Google Scholar] [CrossRef]
- Hu, X.; Liu, R.; Zhu, N. Enhancement of humoral and cellular immune responses by monophosphoryl lipid A (MPLA) as an adjuvant to the rabies vaccine in BALB/c mice. Immunobiology 2013, 218, 1524–1528. [Google Scholar] [CrossRef] [PubMed]
- Golkar, M.; Shokrgozar, M.A.; Rafati, S.; Musset, K.; Assmar, M.; Sadaie, R.; Cesbron-Delauw, M.F.; Mercier, C. Evaluation of protective effect of recombinant dense granule antigens GRA2 and GRA6 formulated in monophosphoryl lipid A (MPL) adjuvant against Toxoplasma chronic infection in mice. Vaccine 2007, 25, 4301–4311. [Google Scholar] [CrossRef] [PubMed]
- Baldridge, J.R.; Yorgensen, Y.; Ward, J.R.; Ulrich, J.T. Monophosphoryl lipid A enhances mucosal and systemic immunity to vaccine antigens following intranasal administration. Vaccine 2000, 18, 2416–2425. [Google Scholar] [CrossRef]
- Patel, P.; Salapatek, A.M.F. Pollinex® Quattro: A novel and well–tolerated, ultra-short–course allergy vaccine. Expert Rev. Vaccines 2006, 5, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Kundi, M. New hepatitis B vaccine formulated with an improved adjuvant system. Expert Rev. Vaccines 2007, 6, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Seth, L.; Ferlez, K.M.B.; Kaba, S.A.; Musser, D.M.; Emadi, S.; Matyas, G.R.; Beck, Z.; Alving, C.R.; Burkhard, P.; Lanar, D.E. Development of a self-assembling protein nanoparticle vaccine targeting Plasmodium falciparum Circumsporozoite Protein delivered in three Army Liposome Formulation adjuvants. Vaccine 2017, 35, 5448–5454. [Google Scholar] [CrossRef]
- Zollinger, W.D.; Babcock, J.G.; Moran, E.E.; Brandt, B.L.; Matyas, G.R.; Wassef, N.M.; Alving, C.R. Phase I study of a Neisseria meningitidis liposomal vaccine containing purified outer membrane proteins and detoxified lipooligosaccharide. Vaccine 2012, 30, 712–721. [Google Scholar] [CrossRef]
- Stanberry, L.R.; Spruance, S.L.; Cunningham, A.L.; Bernstein, D.I.; Mindel, A.; Sacks, S.; Tyring, S.; Aoki, F.Y.; Slaoui, M.; Denis, M.; et al. Glycoprotein-D–adjuvant vaccine to prevent genital herpes. N. Engl. J. Med. 2002, 347, 1652–1661. [Google Scholar] [CrossRef]
- Lee, Y.; Ko, E.J.; Kim, K.H.; Lee, Y.T.; Hwang, H.S.; Kwon, Y.M.; Graham, B.S.; Kang, S.M. A unique combination adjuvant modulates immune responses preventing vaccine-enhanced pulmonary histopathology after a single dose vaccination with fusion protein and challenge with respiratory syncytial virus. Virology 2019, 534, 1–13. [Google Scholar] [CrossRef]
- Pirahmadi, S.; Zakeri, S.; Mehrizi, A.A.; Djadid, N.D.; Raz, A.A.; Sani, J.J. Combining MPL, CpG ODN, and QS-21 adjuvants induce strong and persistent functional antibodies and T cell responses against cell-traversal protein for ookinetes and sporozoites (CelTOS) of Plasmodium falciparum in BALB/c mice. Infect Immun. 2019, 87, e00911-18. [Google Scholar] [CrossRef] [Green Version]
- Didierlaurent, A.M.; Laupèze, B.; Di Pasquale, A.; Hergli, N.; Collignon, C.; Garçon, N. Adjuvant system AS01: Helping to overcome the challenges of modern vaccines. Expert Rev. Vaccines 2017, 16, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Gutjahr, A.; Tiraby, G.; Perouzel, E.; Verrier, B.; Paul, S. Triggering intracellular receptors for vaccine adjuvantation. Trends Immunol. 2016, 37, 573–587. [Google Scholar] [CrossRef]
- Mata, E.; Salvador, A.; Igartua, M.; Hernández, R.M.; Pedraz, J.L. Malaria vaccine adjuvants: Latest update and challenges in preclinical and clinical research. BioMed Res. Int. 2013, 282913. [Google Scholar] [CrossRef]
- Romanowski, B.; de Borba, P.C.; Naud, P.S.; Roteli-Martins, C.M.; De Carvalho, N.S.; Teixeira, J.C.; Aoki, F.; Ramjattan, B.; Shier, R.M.; Somani, R.; et al. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: Analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet 2009, 374, 1975–1985. [Google Scholar] [CrossRef]
- Alving, C.R.; Peachman, K.K.; Matyas, G.R.; Rao, M.; Beck, Z. Army Liposome Formulation (ALF) family of vaccine adjuvants. Expert Rev. Vaccines 2020, 19, 279–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamani, P.; Navashenaq, J.G.; Nikpoor, A.R.; Hatamipour, M.; Oskuee, R.K.; Badiee, A.; Jaafari, M.R. MPL nano-liposomal vaccine containing P5 HER2/neu-derived peptide pulsed PADRE as an effective vaccine in a mice TUBO model of breast cancer. J. Control. Release 2019, 303, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Shariat, S.; Badiee, A.; Jalali, S.A.; Mansourian, M.; Yazdani, M.; Mortazavi, S.A.; Jaafari, M.R. P5 HER2/neu-derived peptide conjugated to liposomes containing MPL adjuvant as an effective prophylactic vaccine formulation for breast cancer. Cancer Lett. 2014, 355, 54–60. [Google Scholar] [CrossRef]
- Alving, C.R.; Detrick, B.; Richards, R.L.; Lewis, M.G.; Shafferman, A.; Eddy, G.A. Novel adjuvant strategies for experimental malaria and AIDS vaccines. Ann. N. Y. Acad. Sci. 1993, 690, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, A.; Schumack, N.M.; Gariepy, C.L.; Eggleston, H.; Nunez, G.; Espinoza, N.; Nieto, M.; Castillo, R.; Rojas, J.; McCoy, A.J.; et al. Enhanced Immunogenicity and Protective Efficacy of a Campylobacter jejuni Conjugate Vaccine Coadministered with Liposomes Containing Monophosphoryl Lipid A and QS-21. mSphere 2019, 4, e00101-119. [Google Scholar] [CrossRef] [Green Version]
- Torres, O.B.; Matyas, G.R.; Rao, M.; Peachman, K.K.; Jalah, R.; Beck, Z.; Michael, N.L.; Rice, K.C.; Jacobson, A.E.; Alving, C.R. Heroin-HIV-1 (H2) vaccine: Induction of dual immunologic effects with a heroin hapten-conjugate and an HIV-1 envelope V2 peptide with liposomal lipid A as an adjuvant. NPJ Vaccines 2017, 2, 1–8. [Google Scholar] [CrossRef]
- Boks, M.A.; Ambrosini, M.; Bruijns, S.C.; Kalay, H.; Van Bloois, L.; Storm, G.; Garcia-Vallejo, J.J.; Van Kooyk, Y. MPLA incorporation into DC-targeting glycoliposomes favours anti-tumour T cell responses. J. Control. Release 2015, 216, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Alving, C.R.; Rao, M.; Steers, N.J.; Matyas, G.R.; Mayorov, A.V. Liposomes containing lipid A: An effective, safe, generic adjuvant system for synthetic vaccines. Expert Rev. Vaccines 2012, 11, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Fries, L.F.; Gordon, D.M.; Richards, R.L.; Egan, J.E.; Hollingdale, M.R.; Gross, M.; Silverman, C.; Alving, C.R. Liposomal malaria vaccine in humans: A safe and potent adjuvant strategy. Proc. Natl. Acad. Sci. USA 1992, 89, 358–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braganza, C.D.; Teunissen, T.; Timmer, M.S.; Stocker, B.L. Identification and biological activity of synthetic macrophage inducible C-type lectin ligands. Front. Immunol. 2018, 8, 1940. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.J.; Miller, S.; Buhl, C.; Child, R.; Whitacre, M.; Schoener, R.; Ettenger, G.; Burkhart, D.; Ryter, K.; Evans, J.T. Species-specific structural requirements of alpha-branched trehalose diester Mincle agonists. Front. Immunol. 2019, 10, 338. [Google Scholar] [CrossRef]
- Gram, G.J.; Karlsson, I.; Agger, E.M.; Andersen, P.; Fomsgaard, A. A novel liposome-based adjuvant CAF01 for induction of CD8+ cytotoxic T-lymphocytes (CTL) to HIV-1 minimal CTL peptides in HLA-A* 0201 transgenic mice. PLoS ONE 2009, 4, e6950. [Google Scholar] [CrossRef]
- Lindenstrøm, T.; Agger, E.M.; Korsholm, K.S.; Darrah, P.A.; Aagaard, C.; Seder, R.A.; Rosenkrands, I.; Andersen, P. Tuberculosis subunit vaccination provides long-term protective immunity characterized by multifunctional CD4 memory T cells. J. Immunol. 2009, 182, 8047–8055. [Google Scholar] [CrossRef]
- Christensen, D.; Christensen, J.P.; Korsholm, K.S.; Isling, L.K.; Erneholm, K.; Thomsen, A.R.; Andersen, P. Seasonal influenza split vaccines confer partial cross-protection against heterologous influenza virus in ferrets when combined with the CAF01 adjuvant. Front. Immunol. 2018, 8, 1928. [Google Scholar] [CrossRef] [Green Version]
- Christensen, D.; Mortensen, R.; Rosenkrands, I.; Dietrich, J.; Andersen, P. Vaccine-induced Th17 cells are established as resident memory cells in the lung and promote local IgA responses. Mucosal Immunol. 2017, 10, 260–270. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, G.K.; Andersen, P.; Christensen, D. Immunocorrelates of CAF family adjuvants. Semin. Immunol. 2018, 39, 4–13. [Google Scholar] [CrossRef]
- Wern, J.E.; Sorensen, M.R.; Olsen, A.W.; Andersen, P.; Follmann, F. Simultaneous subcutaneous and intranasal administration of a CAF01-adjuvanted chlamydia vaccine elicits elevated IgA and protective Th1/Th17 responses in the genital tract. Front. Immunol. 2017, 8, 569. [Google Scholar] [CrossRef] [Green Version]
- Olsen, A.W.; Lorenzen, E.K.; Rosenkrands, I.; Follmann, F.; Andersen, P. Protective effect of vaccine promoted neutralizing antibodies against the intracellular pathogen Chlamydia trachomatis. Front. Immunol. 2017, 8, 1652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakur, A.; Rodríguez-Rodríguez, C.; Saatchi, K.; Rose, F.; Esposito, T.; Nosrati, Z.; Andersen, P.; Christensen, D.; Häfeli, U.O.; Foged, C. Dual-isotope SPECT/CT imaging of the tuberculosis subunit vaccine H56/CAF01: Induction of strong systemic and mucosal IgA and T-cell responses in mice upon subcutaneous prime and intrapulmonary boost immunization. Front. Immunol. 2018, 9, 2825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordly, P.; Rose, F.; Christensen, D.; Nielsen, H.M.; Andersen, P.; Agger, E.M.; Foged, C. Immunity by formulation design: Induction of high CD8+ T-cell responses by poly (I: C) incorporated into the CAF01 adjuvant via a double emulsion method. J. Control. Release 2011, 150, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Korsholm, K.S.; Karlsson, I.; Tang, S.T.; Brandt, L.; Agger, E.M.; Aagaard, C.; Andersen, P.; Fomsgaard, A. Broadening of the T-cell repertoire to HIV-1 Gag p24 by vaccination of HLA-A2/DR transgenic mice with overlapping peptides in the CAF05 adjuvant. PLoS ONE 2013, 8, e63575. [Google Scholar] [CrossRef] [Green Version]
- Nordly, P.; Agger, E.M.; Andersen, P.; Nielsen, H.M.; Foged, C. Incorporation of the TLR4 agonist monophosphoryl lipid A into the bilayer of DDA/TDB liposomes: Physico-Chemical characterization and induction of CD8+ T-cell responses in vivo. Pharm. Res. 2011, 28, 553–562. [Google Scholar] [CrossRef]
- Vangala, A.; Kirby, D.; Rosenkrands, I.; Agger, E.M.; Andersen, P.; Perrie, Y. A comparative study of cationic liposome and niosome-based adjuvant systems for protein subunit vaccines: Characterisation, environmental scanning electron microscopy and immunisation studies in mice. J. Pharm. Pharmacol. 2006, 58, 787–799. [Google Scholar] [CrossRef]
- Davidsen, J.; Rosenkrands, I.; Christensen, D.; Vangala, A.; Kirby, D.; Perrie, Y.; Agger, E.M.; Andersen, P. Characterization of cationic liposomes based on dimethyldioctadecylammonium and synthetic cord factor from M. tuberculosis (trehalose 6, 6′-dibehenate)—A novel adjuvant inducing both strong CMI and antibody responses. Biochim. Biophys. Acta Biomembr. 2005, 1718, 22–31. [Google Scholar] [CrossRef] [Green Version]
- Kamstrup, S.; San Martin, R.; Doberti, A.; Grande, H.; Dalsgaard, K. Preparation and characterisation of quillaja saponin with less heterogeneity than Quil-A. Vaccine 2000, 18, 2244–2249. [Google Scholar] [CrossRef]
- Fernández-Tejada, A.; Tan, D.S.; Gin, D.Y. Development of improved vaccine adjuvants based on the saponin natural product QS-21 through chemical synthesis. Acc. Chem. Res. 2016, 49, 1741–1756. [Google Scholar] [CrossRef]
- Pink, J.R.; Kieny, M.P. 4th Meeting on Novel Adjuvants Currently in close to Human Clinical Testing, World Health Organization. Vaccine 2004, 22, 2097–2102. [Google Scholar] [CrossRef]
- Oda, K.; Matsuda, H.; Murakami, T.; Katayama, S.; Ohgitani, T.; Yoshikawa, M. Adjuvant and haemolytic activities of 47 saponins derived from medicinal and food plants. Biol. Chem. 2000, 381, 67–74. [Google Scholar] [CrossRef]
- Sun, H.; Chen, L.; Wang, J.; Wang, K.; Zhou, J. Structure–function relationship of the saponins from the roots of Platycodon grandiflorum for hemolytic and adjuvant activity. Int. Immunopharmacol. 2011, 11, 2047–2056. [Google Scholar] [CrossRef]
- Den Brok, M.H.; Büll, C.; Wassink, M.; De Graaf, A.M.; Wagenaars, J.A.; Minderman, M.; Thakur, M.; Amigorena, S.; Rijke, E.O.; Schrier, C.C.; et al. Saponin-based adjuvants induce cross-presentation in dendritic cells by intracellular lipid body formation. Nat. Commun. 2016, 7, 13324. [Google Scholar] [CrossRef]
- Welsby, I.; Detienne, S.; N’Kuli, F.; Thomas, S.; Wouters, S.; Bechtold, V.; De Wit, D.; Gineste, R.; Reinheckel, T.; Elouahabi, A.; et al. Lysosome-dependent activation of human dendritic cells by the vaccine adjuvant QS-21. Front. Immunol. 2017, 7, 663. [Google Scholar] [CrossRef] [Green Version]
- Marciani, D.J. Vaccine Adjuvants: Role and Mechanisms of Action in Vaccine Immunogenicity. Drug Discov. Today 2003, 8, 934–943. [Google Scholar] [CrossRef]
- Moghimipour, E.; Handali, S. Saponin: Properties, methods of evaluation and applications. Annu. Res. Rev. Biol. 2015, 5, 207–220. [Google Scholar] [CrossRef] [Green Version]
- Kashala, O.; Amador, R.; Valero, M.V.; Moreno, A.; Barbosa, A.; Nickel, B.; Daubenberger, C.A.; Guzman, F.; Pluschke, G.; Patarroyo, M.E. Safety, tolerability and immunogenicity of new formulations of the Plasmodium falciparum malaria peptide vaccine SPf66 combined with the immunological adjuvant QS-21. Vaccine 2002, 20, 2263–2277. [Google Scholar] [CrossRef]
- Ragupathi, G.; Gardner, J.R.; Livingston, P.O.; Gin, D.Y. Natural and synthetic saponin adjuvant QS-21 for vaccines against cancer. Expert Rev. Vaccines 2011, 10, 463–470. [Google Scholar] [CrossRef]
- Ghirardello, M.; Ruiz-de-Angulo, A.; Sacristan, N.; Barriales, D.; Jiménez-Barbero, J.; Poveda, A.; Corzana, F.; Anguita, J.; Fernández-Tejada, A. Exploiting structure–activity relationships of QS-21 in the design and synthesis of streamlined saponin vaccine adjuvants. Chem. Commun. 2020, 56, 719–722. [Google Scholar] [CrossRef] [Green Version]
- Heidari, A.R.; Boroumand-Noughabi, S.; Nosratabadi, R.; Arab, F.L.; Tabasi, N.; Rastin, M.; Mahmoudi, M. Acylated and deacylated quillaja saponin-21 adjuvants have opposite roles when utilized for immunization of C57BL/6 mice model with MOG35-55 peptide. Mult. Scler. Relat. Disord. 2019, 29, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.P.; Li, Y.D.; Luo, L.L.; Liu, Y.Q.; Li, Y.; Guo, C.; Li, Z.D.; Xie, X.R.; Song, H.X.; Yang, L.P.; et al. Astragalus saponins and liposome constitute an efficacious adjuvant formulation for cancer vaccines. Cancer Biother. Radiopharm. 2018, 33, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Morein, B.; Sundquist, B.; Hoglund, S.; Dalsgaard, K.; Osterhaus, A. ISCOM, a novel structure for antigenic presentation of membrane proteins from enveloped viruses. Nature 1984, 308, 457–460. [Google Scholar] [CrossRef]
- Krug, L.M.; Ragupathi, G.; Hood, C.; George, C.; Hong, F.; Shen, R.; Abrey, L.; Jennings, H.J.; Kris, M.G.; Livingston, P.O. Immunization with N-propionyl polysialic acid-KLH conjugate in patients with small cell lung cancer is safe and induces IgM antibodies reactive with SCLC cells and bactericidal against group B meningococci. Cancer Immunol. Immunother. 2012, 61, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, J.S.; Co, M.; Green, S.; Longtine, K.; Longtine, J.; O’Neill, M.A.; Adams, J.P.; Rothman, A.L.; Yu, Q.; Johnson-Leva, R.; et al. The safety and tolerability of an HIV-1 DNA prime-protein boost vaccine (DP6–001) in healthy adult volunteers. Vaccine 2008, 26, 4420–4424. [Google Scholar] [CrossRef] [Green Version]
- Vandepapelière, P.; Horsmans, Y.; Moris, P.; Van Mechelen, M.; Janssens, M.; Koutsoukos, M.; Van Belle, P.; Clement, F.; Hanon, E.; Wettendorff, M.; et al. Vaccine adjuvant systems containing monophosphoryl lipid A and QS21 induce strong and persistent humoral and T cell responses against hepatitis B surface antigen in healthy adult volunteers. Vaccine 2008, 26, 1375–1386. [Google Scholar] [CrossRef] [PubMed]
- Rts, S.C.T.P. Efficacy and safety of RTS, S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: Final results of a phase 3, individually randomised, controlled trial. Lancet 2015, 386, 31–45. [Google Scholar] [CrossRef] [Green Version]
- Kawano, T.; Cui, J.; Koezuka, Y.; Toura, I.; Kaneko, Y.; Motoki, K.; Ueno, H.; Nakagawa, R.; Sato, H.; Kondo, E.; et al. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science 1997, 278, 1626–1629. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Shimizu, K.; Smith, C.; Bonifaz, L.; Steinman, R.M. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to co-administered protein. J. Exp. Med. 2003, 198, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Reilly, E.C.; Thompson, E.A.; Aspeslagh, S.; Wands, J.R.; Elewaut, D.; Brossay, L. Activated i NKT Cells Promote Memory CD8+ T Cell Differentiation during Viral Infection. PLoS ONE 2012, 7, e37991. [Google Scholar] [CrossRef] [Green Version]
- Ko, S.Y.; Ko, H.J.; Chang, W.S.; Park, S.H.; Kweon, M.N.; Kang, C.Y. Agalactosylceramide can act as a nasal vaccine adjuvant inducing protective immune responses against viral infections and tumor. J. Immunol. 2005, 175, 3309–3317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sainz, V.; Moura, L.I.; Peres, C.; Matos, A.I.; Viana, A.S.; Wagner, A.M.; Ramirez, J.E.V.; Barata, T.S.; Gaspar, M.; Brocchini, S.; et al. α-Galactosylceramide and peptide-based nano-vaccine synergistically induced a strong tumor suppressive effect in melanoma. Acta Biomater. 2018, 76, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Moschos, S.A.; Bramwell, V.W.; Somavarapu, S.; Alpar, H.O. Adjuvant synergy: The effects of nasal coadministration of adjuvants. Immunol. Cell Biol. 2004, 82, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Maisonneuve, C.; Bertholet, S.; Philpott, D.J.; De Gregorio, E. Unleashing the potential of NOD- and Toll-like agonists as vaccine adjuvants. Proc. Natl. Acad. Sci. USA 2014, 111, 12294–12299. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.J.; Chen, J.; Hou, J.B.; Zheng, Y.; Yu, Y.N.; He, H.; Zhang, Y.P.; Feng, X.L.; Zheng, Q.S. The Immunological Functions of Muramyl Dipeptide Compound Adjuvant on Humoral, Cellular-Mediated and Mucosal Immune Responses to PEDV Inactivated Vaccine in Mice. Protein Pept. Lett. 2018, 25, 908–913. [Google Scholar] [CrossRef]
- Shafique, M.; Meijerhof, T.; Wilschut, J.; de Haan, A. Evaluation of an intranasal virosomal vaccine against respiratory syncytial virus in mice: Effect of TLR2 and NOD2 ligands on induction of systemic and mucosal immune responses. PLoS ONE 2013, 8, e61287. [Google Scholar] [CrossRef] [Green Version]
- Zom, G.G.; Willems, M.M.; Meeuwenoord, N.; Reintjens, N.R.; Tondini, E.; Khan, S.; Overkleeft, H.S.; van der Marel, G.A.; Codee, J.D.; Ossendorp, F.; et al. Dual Synthetic Peptide Conjugate Vaccine Simultaneously Triggers TLR2 and NOD2 and Activates Human Dendritic Cells. Bioconjug. Chem. 2019, 30, 1150–1161. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashiri, S.; Koirala, P.; Toth, I.; Skwarczynski, M. Carbohydrate Immune Adjuvants in Subunit Vaccines. Pharmaceutics 2020, 12, 965. https://doi.org/10.3390/pharmaceutics12100965
Bashiri S, Koirala P, Toth I, Skwarczynski M. Carbohydrate Immune Adjuvants in Subunit Vaccines. Pharmaceutics. 2020; 12(10):965. https://doi.org/10.3390/pharmaceutics12100965
Chicago/Turabian StyleBashiri, Sahra, Prashamsa Koirala, Istvan Toth, and Mariusz Skwarczynski. 2020. "Carbohydrate Immune Adjuvants in Subunit Vaccines" Pharmaceutics 12, no. 10: 965. https://doi.org/10.3390/pharmaceutics12100965
APA StyleBashiri, S., Koirala, P., Toth, I., & Skwarczynski, M. (2020). Carbohydrate Immune Adjuvants in Subunit Vaccines. Pharmaceutics, 12(10), 965. https://doi.org/10.3390/pharmaceutics12100965