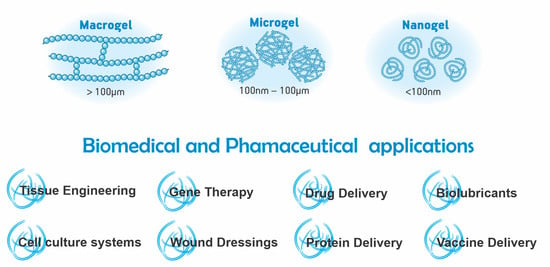
An Updated Review of Macro, Micro, and Nanostructured Hydrogels for Biomedical and Pharmaceutical Applications
Abstract
:1. Introduction
2. Macrogels
2.1. Definition
2.2. Methods of Synthesis
2.3. Biomedical Applications
2.4. Pharmaceutical Applications
3. Microgels
3.1. Definition
3.2. Methods of Synthesis
3.3. Biomedical Applications
3.4. Pharmaceutical Applications
4. Nanogels
4.1. Definition
4.2. Methods of Synthesis
4.3. Biomedical Applications
4.4. Pharmaceutical Applications
5. Grafted Hydrogel Chains
5.1. Definition
5.2. Methods of Synthesis
- -
- Grafting by radical polymerizations: This method is among the most common as it is relatively easy to apply to a great variety of surfaces and it works on virtually any monomer containing vinyl groups. In these procedures, typically, functional groups active on radical polymerization (vinyl groups, azo groups, peroxides, i.e.,) are immobilized through chemical means on the substrate that will be grafted. Subsequently, a radical polymerization reaction is performed in the presence of a crosslinker (or even the absence of a crosslinker depending on the nature of the monomer). As mentioned before, these reactions are the most versatile in terms of applicability to different substrates. Nevertheless, these kinds of reactions provide little to no synthetic control over the finer structure of the hydrogel since it is impossible to control polymeric chain length and polymer dispersity; additionally, it is not possible to achieve the formation of specific polymeric architectures (block copolymers, star copolymers, dendrimers, etc.) with these procedures. This in turn affects how useful the final hydrogel product is. Another disadvantage of this method is the presence of residual toxic initiators and crosslinkers on the final product, which limit the application of these kinds of grated materials in biomedicine [117,118].
- -
- Grafting by controlled radical polymerizations: Controlled radical polymerizations include procedures such as atom-transfer radical polymerization (ATRP), reverse addition-fragmentation chain-transfer (RAFT) polymerization, ring-opening metathesis polymerization (ROMP), and nitroxide-mediated radical polymerization (NMP). These procedures are also widely used for the formation of grafts of hydrogels because of their strong versatility in producing exotic architectures that may be very useful when crafting complicated aerogels. These procedures use special initiators coupled with catalysts that allow for polymerization with very controlled molecular weight distributions, control over the molecular weights of the polymers, and overall control on all the structural parameters of the polymer chains. Although these procedures are very useful, their use is limited by the elevated cost of the initiator-catalysts systems and the synthetic challenge of producing monomers (and substrates to graft) that are compatible with some of these procedures. Additionally, the issue with toxic residues on the products still limits the applications of these polymers in some areas such as the biomedical fields [119,120].
- -
- Grafting polymerizations induced by radiation: This final classification for the grafting of hydrogels is very important since it tends to overcome the problem of residual toxic polymerization initiators, crosslinkers, and spacers. In these procedures, reactive monomers are activated by high energy radiation, ranging from UV to gamma radiation. The latter even being able to form cross-linkages without the use of chemical additives as it has been mentioned for plain hydrogels. These materials have then had many applications, including several the biomedical field [69].
5.3. Biomedical Applications
5.4. Pharmaceutical Applications
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Peppas, N.A.; Hoffman, A.S. Hydrogels. In Biomaterials Science—An Introduction to Materials in Medicine, 3rd ed.; Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J.E., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 166–179. [Google Scholar] [CrossRef]
- Feksa, L.R.; Troian, E.A.; Muller, C.D.; Viegas, F.; Machado, A.B.; Rech, V.C. Hydrogels for biomedical applications. In Nanostructures for the Engineering of Cells, Tissues and Organs—From Design to Applications; Grumezescu, A.M., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 403–438. [Google Scholar] [CrossRef]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidi, F.; Zhao, W.; Xiao, H.; Jin, Y.; Saeb, M.R.; Zhao, C. Radical polymerization as a versatile tool for surface grafting of thin hydrogel films. Polym. Chem. 2020, 11, 4355–4381. [Google Scholar] [CrossRef]
- Karg, M.; Pich, A.; Hellweg, T.; Hoare, T.; Lyon, L.A.; Crassous, J.J.; Suzuki, D.; Gumerov, R.A.; Schneider, S.; Potemkin, I.I.; et al. Nanogels and Microgels: From Model Colloids to Applications, Recent Developments, and Future Trends. Langmuir: ACS J. Surf. Colloids 2019, 35, 6231–6255. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.; Gaba, P. Hydrogel: An Updated Primer. J. Crit. Rev. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Cha, G.D.; Lee, W.H.; Lim, C.; Choi, M.K.; Kim, D.H. Materials engineering, processing, and device application of hydrogel nanocomposites. Nanoscale 2020, 12, 10456–10473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Sun, P.; Li, P.; Xue, A.; Zhang, X.; Zhang, H.; Jin, X. A magnetic chitosan hydrogel for sustained and prolonged delivery of Bacillus Calmette-Guerin in the treatment of bladder cancer. Biomaterials 2013, 34, 10258–10266. [Google Scholar] [CrossRef] [PubMed]
- Jordan-Lloyd, D. Colloid Chemistry; Chemical Catalogue Company: New York, NY, USA, 1926. [Google Scholar]
- Rosiak, J.M. Hydrogel dressings. Radiation Effects on Polymers. In ACS Symposium Series 475; Shalaby, R.L.C.a.S.W., Ed.; ACS: Washington, DC, USA, 1991; p. 271. [Google Scholar]
- Singh, H.; Vasudevan, P.; Ray, A.R. Polymeric hydrogels: Preparation and biomedical applications. J. Sci. Ind. Res. 1980, 39, 162. [Google Scholar]
- Kaetsu, I. Immobilization of biofunctional substances. Radiat. Phys. Chem. 1981, 18, 343–356. [Google Scholar] [CrossRef]
- Peppas, N.A. Hydrogels in Medicine and Pharmacy; CRC Press: Boca Raton, FL, USA, 1986. [Google Scholar]
- De Rossi, D.; Kajiware, K.; Osada, Y.; Yamauchi, A. Polymer Gels. Fundamentals and Biomedical Applications; Plenum Press: New York, NY, USA, 1991. [Google Scholar]
- Rosiak, J.M.; Ulanski, P.; Rzeznicki, A. Hydrogels for biomedical purposes. Nucl. Instrum. Methods Phys. Res. Sect. B 1995, 105, 18–23. [Google Scholar] [CrossRef]
- Rosiak, J.M.; Ulanski, P.; Pajewski, L.A.; Yoshii, F.; Makuuchi, K. Radiation formation of hydrogels for biomedical purposes. Some remarks and comments. Radiat. Phys. Chem. 1995, 46, 161–168. [Google Scholar] [CrossRef]
- Rosiak, J.M.; Ulanski, P. Synthesis of hydrogels by irradiation of polymers in aqueous solution. Radiat. Phys. Chem. 1999, 55, 139–151. [Google Scholar] [CrossRef]
- Rosiak, J.M.; Yoshii, F. Hydrogels and their medical applications. Nucl. Instrum. Methods Phys. Res. Sect. B 1999, 151, 56–64. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medice: From Molecular Principles to Bionamotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Crescenzi, V.; Cornelio, L.; Di Meo, C.; Nardeccia, S.; Lamanna, R. Novel hydrogels via click chemistry: Synthesis and potential biomedical applications. Biomacromolecules 2007, 8, 1844–1850. [Google Scholar] [CrossRef] [PubMed]
- Klouda, L.; Mikos, A.G. Thermoresponsive hydrogels in biomedical applications. Eur. J. Pharm. Biopharm. 2008, 68, 34–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagur-Grodzinski, J. Polymeric gels and hydrogels for biomedical and pharmaceutical applications. Polym. Adv. Technol. 2010, 21, 27–47. [Google Scholar] [CrossRef]
- Rimmer, S. Biomedical Hydrogels: Biochemistry, Manufacture and Medical Applications; Woodhead Publishing Limited: Cambridge, UK, 2011. [Google Scholar]
- Seliktar, D. Designing cell-compatible hydrogels for biomedical applications. Science 2012, 336, 1124–1128. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Annabi, N.; Tamayol, A.; Uquillas, J.A.; Akbari, M.; Bertassoni, L.E.; Cha, C.; Camci-Unal, G.; Dokmeci, M.R.; Peppas, N.A.; Khademhosseini, A. 25th Anniversary Article: Rational Design and Applications of Hydrogels in Regenerative Medicine. Adv. Mater. 2014, 26, 26–85. [Google Scholar] [CrossRef]
- Dong, R.; Pang, Y.; Su, Y.; Zhu, X. Supramolecular hydrogels: Synthesis, properties and their biomedical applications. Biomater. Sci. 2015, 3, 937–954. [Google Scholar] [CrossRef]
- Calo, E.; Khutorianskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, A.S. Environmentally sensitive polymers and hydrogels—“Smart” biomaterials. MRS Bull. 1991, 16, 42–46. [Google Scholar] [CrossRef]
- Kaetsu, I.; Uchida, K.; Morita, Y.; Okubo, M. Synthesis of electro-responsive hydrogels by radiation polymerization of sodium acrylate. Int. J. Radiat. Appl. Instrum. Part C Radiat. Phys. Chem. 1992, 40, 157–160. [Google Scholar] [CrossRef]
- Hirasa, O. Research trends of stimuli-responsive polymer hydrogels in Japan. J. Intell. Mater. Syst. Struct. 1993, 4, 538–542. [Google Scholar] [CrossRef]
- Yoshida, R.; Ichijo, H.; Hakuta, T.; Yamaguchi, T. Self-oscillating swelling and deswelling of polymer gels. Macromol. Rapid Commun. 1995, 16, 305–310. [Google Scholar] [CrossRef]
- Kadłubowski, S.; Henke, A.; Ulański, P.; Rosiak, J.M. Hydrogels of polyvinylpyrrolidone (PVP) and poly(acrylic acid) (PAA) synthesized by radiation-induced crosslinking of homopolymers. Radiat. Phys. Chem. 2010, 79, 261–266. [Google Scholar] [CrossRef]
- Sharpe, L.A.; Daily, A.; Horava, S.; Peppas, N.A. Therapeutic applications of hydrogels in oral drug delivery. Expert Opin. Drug Deliv. 2014, 11, 901–915. [Google Scholar] [CrossRef] [Green Version]
- Shiga, T.; Hirose, Y.; Okada, A.; Kurauchi, T.; Kamigaito, O. Design of biomimetic machinery systems with polymer gel. In Proceedings of the 1st Japan International SAMPE Symposium, Tokyo, Japan, 28 November–1 December 1989; p. 659. [Google Scholar]
- Brasch, U.; Burchard, W. Preparation and solution properties of microhydrogels from poly(vinyl alcohol). Macromol. Chem. Phys. 1996, 197, 223–235. [Google Scholar] [CrossRef]
- Badiger, M.V.; McNeill, M.E.; Graham, N.B. Porogens in the preparation of microporous hydrogels based on poly(ethylene oxides). Biomaterials 1993, 14, 1059–1063. [Google Scholar] [CrossRef]
- Dusek, K.; Sedlacek, B. Structure and properties of hydrophilic polymers and their gels. XI. Microsyneresis in swollen poly(ethylene glycol methacrylate) gels induced by changes in temperature. Collect. Czechoslov. Chem. Commun. 1969, 34, 136–157. [Google Scholar] [CrossRef]
- Camacho-Cruz, L.A.; Velazco-Medel, M.A.; Bucio, E. Aqueous polymerizations. In Green Sustainable Process for Chemical and Environmental Engineering and Science Organic Synthesis in Water and Supercritical Water; Inamuddin, Boddula, R., Asiri, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 275–318. [Google Scholar] [CrossRef]
- Rezai, E.; Lahrman, F.H.; Iwasaki, T. Porous, Absorbent Macrostructures of Bonded Absorbent Particles Surface Crosslinked with Cationic Amino-Epichlorohydrin Adducts. U.S. Patent No. 5,324,561, 23 June 1994. [Google Scholar]
- Rosiak, J.M. Radiation formation of hydrogels. J. Control. Release 1994, 31, 9–19. [Google Scholar] [CrossRef]
- Charlesby, A. Atomic Radiation and Polymers; Pergamon Press: Oxford, UK, 1960. [Google Scholar]
- Chapiro, A. Radiation Chemistry of Polymeric Systems; Interscience: New York, NY, USA, 1962. [Google Scholar]
- Hoffman, A.S. A review of the use of radiation plus chemical and biochemical processing treatments to prepare novel biomaterials. Radiat. Phys. Chem. 1981, 18, 323–342. [Google Scholar] [CrossRef]
- Wichterle, O.; Lim, D. Hydrophilic gels for biological use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Yoshi, F.; Zhanshan, Y.; Isobe, K.; Shinozaki, K.; Makuuchi, K. Electron beam crosslinked PEO and PEO/PVA hydrogels for wound dressing. Radiat. Phys. Chem. 1999, 55, 133–138. [Google Scholar] [CrossRef]
- Oliveira, J.P.R.; Melendez-Ortiz, H.I.; Bucio, E.; Alves, P.T.; Lima, M.I.S.; Goulart, L.R.; Mathor, M.B.; Varca, G.H.C.; Lugao, A.B. Current Methods Applied to Biomaterials—Characterization Approaches, Safety Assessment and Biological International Standards. Curr. Top. Med. Chem. 2018, 18, 256–274. [Google Scholar] [CrossRef]
- Byrnea, M.E.; Parka, K.; Peppasa, N.A. Molecular imprinting within hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 149–161. [Google Scholar] [CrossRef]
- Mahinroosta, M.; Jomeh Farsangi, Z.; Allahverdi, A.; Shakoori, Z. Hydrogels as intelligent materials: A brief review of synthesis, properties and applications. Mater. Today Chem. 2018, 8, 42–55. [Google Scholar] [CrossRef]
- Tomme, S.R.V.; Stom, G.; Hennink, W.E. In situ gelling hydrogels for pharmaceutical and biomedical applications. Int. J. Pharm. 2008, 355, 1–18. [Google Scholar] [CrossRef]
- Mandru, M.; Bercea, M.; Gradinaru, L.M.; Ciobanu, C.; Drobota, M.; Vlad, S.; Albulescu, R. Polyurethane/poly(vinyl alcohol) hydrogels: Preparation, characterization and drug delivery. Eur. Polym. J. 2019, 118, 137–145. [Google Scholar] [CrossRef]
- Sirousazar, M.; Taleblou, N.; Roufegari-Nejad, E. Hydrogel and nanocomposite hydrogel drug-delivery systems for treatment of cancers. In Materials for Biomedical Engineering—Nanomaterials-Based Drug Delivery; Holban, A.-M., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 293–329. [Google Scholar] [CrossRef]
- Kim, H.S.; Yang, J.; Kim, K.; Shin, U.S. Biodegradable and injectable hydrogels as an immunosuppressive drug delivery system. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 472–481. [Google Scholar] [CrossRef]
- Funke, W.; Okay, O.; Joos-Müller, B. Microgels-Intramolecularly Crossünked Macromolecules with a Globular Structure. Adv. Polym. Sci. 1998, 136, 139–234. [Google Scholar] [CrossRef]
- IUPAC. Compendium of Chemical Terminology (the “Gold Book”); Blackwell, A.D.M.a.A.W., Ed.; Scientific Publications: Oxford, UK, 1997. [Google Scholar] [CrossRef]
- Oh, J.K.; Lee, D.I.; Park, J.M. Biopolymer-based microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2009, 34, 1261–1282. [Google Scholar] [CrossRef]
- Plamper, F.A.; Richtering, W. Functional Microgels and Microgel Systems. Acc. Chem. Res. 2017, 50, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A. Hidrogels and drug delivery. Curr. Opin. Colloid Interface Sci. 1997, 2, 531–537. [Google Scholar] [CrossRef]
- Oh, J.K.; Drumright, R.; Siegwart, D.J.; Matyjaszewski, K. The development of microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2008, 33, 448–477. [Google Scholar] [CrossRef]
- Das, M.; Sanson, N.; Fava, D.; Kumacheva, E. Microgels Loaded with Gold Nanorods: Photothermally Triggered Volume Transitions under Physiological Conditions. Langmuir: ACS J. Surf. Colloids 2007, 23, 196–201. [Google Scholar] [CrossRef]
- Seyfoori, A.; Koshkaki, M.R.; Majidzadeh-A, K. Nanohybrid Stimuli-Responsive Microgels: A New Approach in Cancer Therapy. In Nanoarchitectonics for Smart Delivery and Drug Targeting; Holban, A.M., Ed.; William Andrew: Norwich, NY, USA, 2016; pp. 715–742. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef]
- Pelton, R.; Hoare, T. Microgels and their synthesis: An introduction. In Microgel Suspensions—Fundamentals and Applications; Fernandez-Nieves, A., Wyss, H., Mattsson, J., Weitz, D.A., Eds.; Wiley-VCH: Weinheim, Germany, 2011; pp. 3–32. [Google Scholar]
- Farjami, T.; Madadlou, A. Fabrication methods of biopolymeric microgels and microgel-based hydrogels. Food Hydrocoll. 2017, 62, 262–272. [Google Scholar] [CrossRef]
- McClements, D.J. Designing biopolymer microgels to encapsulate, protect and deliver bioactive components: Physicochemical aspects. Adv. Colloid Interface Sci. 2017, 240, 31–59. [Google Scholar] [CrossRef] [Green Version]
- Sung, M.-R.; Xiao, H.; Decker, E.A.; McClements, D.J. Fabrication, characterization and properties of filled hydrogel particles formed by the emulsion-template method. J. Food Eng. 2015, 155, 16–21. [Google Scholar] [CrossRef]
- Sing, C.E. Development of the modern theory of polymeric complex coacervation. Adv. Colloid Interface Sci. 2017, 239, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Makuuchi, K. Critical review of radiation processing of hydrogel and polysaccharide. Radiat. Phys. Chem. 2010, 79, 267–271. [Google Scholar] [CrossRef]
- Pino-Ramos, V.H.; Ramos-Ballesteros, A.; Lopez-Saucedo, F.; Lopez-Barriguete, J.E.; Varca, G.H.C.; Bucio, E. Radiation Grafting for the Functionalization and Development of Smart Polymeric Materials. Top. Curr. Chem. 2016, 374, 63. [Google Scholar] [CrossRef] [PubMed]
- Khutoryanskiy, V.V.; Mun, G.A.; Nurkeeva, Z.S.; Dubolazov, A.V. pH and salt effects on interpolymer complexation via hydrogen bonding in aqueous solutions. Polym. Int. 2004, 53, 1382–1387. [Google Scholar] [CrossRef]
- Contreras-García, A.; Bucio, E. Biomedical Devices Based on Smart Polymers. In Responsive Materials and Methods; Tiwari, A., Kobayashi, H., Eds.; Scrivener Publishing LLC: Beverly, MA, USA, 2014; pp. 105–122. [Google Scholar]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Ranquin, A.; Versées, W.; Meier, W.; Steyaert, J.; Gelder, P.V. Therapeutic Nanoreactors: Combining Chemistry and Biology in a Novel Triblock Copolymer Drug Delivery System. Nano Lett. 2005, 5, 2220–2224. [Google Scholar] [CrossRef] [PubMed]
- Eswaramma, S.; Reddy, N.S.; Rao, K.S.V.K. Carbohydrate polymer based pH-sensitive IPN microgels: Synthesis, characterization and drug release characteristics. Mater. Chem. Phys. 2017, 195, 176–186. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, S.; Yildirimer, L.; Zhao, H.; Ding, R.; Wang, H.; Cui, W.; Weitz, D. Injectable Stem Cell-Laden Photocrosslinkable Microspheres Fabricated Using Microfluidics for Rapid Generation of Osteogenic Tissue Constructs. Adv. Funct. Mater. 2016, 26, 2809–2819. [Google Scholar] [CrossRef]
- Hou, Y.; Xie, W.; Achazi, K.; Cuellar-Camacho, J.L.; Melzig, M.F.; Chen, W.; Haag, R. Injectable degradable PVA microgels prepared by microfluidic technology for controlled osteogenic differentiation of mesenchymal stem cells. Acta Biomater. 2018, 77, 28–37. [Google Scholar] [CrossRef]
- Busatto, C.A.; Labie, H.; Lapeyre, V.; Auzely-Velty, R.; Perro, A.; Casis, N.; Luna, J.; Estenoz, D.A.; Ravaine, V. Oil-in-microgel strategy for enzymatic-triggered release of hydrophobic drugs. J. Colloid Interface Sci. 2017, 493, 356–364. [Google Scholar] [CrossRef]
- Wilke, P.; Coger, V.; Nachev, M.; Schachschal, S.; Million, N.; Barcikowski, S.; Sures, B.; Reimers, K.; Vogt, P.M.; Pich, A. Biocompatible microgel-modified electrospun fibers for zinc ion release. Polymer 2015, 61, 163–173. [Google Scholar] [CrossRef]
- Lopez, V.C.; Hadgraft, J.; Snowden, M.J. The use of colloidal microgels as a (trans)dermal drug delivery system. Int. J. Pharm. 2005, 292, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Bottcher, C.; Haag, R. Enzymatically crosslinked dendritic polyglycerol nanogels for encapsulation of catalytically active proteins. Soft Matter 2015, 11, 972–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jooybar, E.; Abdekhodaie, M.J.; Mousavi, A.; Zoetebier, B.; Dijkstra, P.J. Enzymatically crosslinked hyaluronic acid microgels as a vehicle for sustained delivery of cationic proteins. Eur. Polym. J. 2019, 115, 234–243. [Google Scholar] [CrossRef]
- Bysell, H.; Mansson, R.; Hansson, P.; Malmsten, M. Microgels and microcapsules in peptide and protein drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 1172–1185. [Google Scholar] [CrossRef] [PubMed]
- Foster, G.A.; Headen, D.M.; Gonzalez-Garcia, C.; Salmeron-Sanchez, M.; Shirwan, H.; Garcia, A.J. Protease-degradable microgels for protein delivery for vascularization. Biomaterials 2017, 113, 170–175. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Guo, L.; Dong, S.; Cui, J.; Hao, J. Microgels in biomaterials and nanomedicines. Adv. Colloid Interface Sci. 2019, 266, 1–20. [Google Scholar] [CrossRef]
- Matsumoto, A.; Ishii, T.; Nishida, J.; Matsumoto, H.; Kataoka, K.; Miyahara, Y. A synthetic approach toward a self-regulated insulin delivery system. Angew. Chem. 2012, 51, 2124–2128. [Google Scholar] [CrossRef]
- Torres, O.; Andablo-Reyes, E.; Murray, B.S.; Sarkar, A. Emulsion Microgel Particles as High-Performance Bio-Lubricants. ACS Appl. Mater. Interfaces 2018, 10, 26893–26905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahdieh, Z.; Holian, A. Electrospun fibers loaded with ball-milled poly(n-isopropylacrylamide) microgel particles for smart delivery applications. J. Appl. Polym. Sci. 2020, 137, 49786. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Design and engineering of nanogels for cancer treatment. Drug Discov. Today 2011, 16, 457–463. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Nostrum, C.F.V.; Mastrobattista, E.; Vermonden, T.; Hernnink, W.E. Nanogels for intracelular delivery of biotherapeutics. J. Control. Release 2017, 259, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Sutekin, S.D.; Guven, O. Application of radiation for the synthesis of poly(n-vinyl pyrrolidone) nanogels with controlled sizes from aqueous solutions. Appl. Radiat. Isot. 2019, 145, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhai, Y.; Wang, J.; Zhai, G. New progress and prospects: The application of nanogel in drug delivery. Mater. Sci. Eng. 2016, 60, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Neamtu, I.; Rusu, A.G.; Diaconu, A.; Nita, L.E.; Chiriac, A.P. Basic concepts and recent advances in nanogels as carriers for medical applications. Drug Deliv. 2017, 24, 539–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadlubowski, S. Radiation-induced synthesis of nanogels based on poly(N-vinyl-2-pyrrolidone)—A review. Radiat. Phys. Chem. 2014, 102, 29–39. [Google Scholar] [CrossRef]
- Kendre, P.N.; Satav, T.S. Current trends and concepts in the design and development of nanogel carrier systems. Polym. Bull. 2019, 76, 1595–1617. [Google Scholar] [CrossRef]
- Ghaffarlou, M.; Sütekin, S.D.; Güven, O. Preparation of nanogels by radiation-induced cross-linking of interpolymer complexes of poly (acrylic acid) with poly (vinyl pyrrolidone) in aqueous medium. Radiat. Phys. Chem. 2018, 142, 130–136. [Google Scholar] [CrossRef]
- Molina, M.; Giulbudagian, M.; Calderón, M. Positively Charged Thermoresponsive Nanogels for Anticancer Drug Delivery. Macromol. Chem. Phys. 2014, 215, 2414–2419. [Google Scholar] [CrossRef]
- Giulbudagian, M.; Asadian-Birjand, M.; Steinhilber, D.; Achazi, K.; Molina, M.; Calderón, M. Fabrication of thermoresponsive nanogels by thermo-nanoprecipitation and in situ encapsulation of bioactives. Polym. Chem. 2014, 5, 6909–6913. [Google Scholar] [CrossRef] [Green Version]
- Pikabea, A.; Villar-Álvarez, E.; Forcada, J.; Taboada, P. pH-controlled doxorubicin delivery from PDEAEMA-based nanogels. J. Mol. Liq. 2018, 266, 321–329. [Google Scholar] [CrossRef]
- Rezaei, S.J.T.; Norouzi, K.; Hesami, A.; Malekzadeh, A.M.; Ramazani, A.; Amani, V.; Ahmadi, R. Au(III) complexes loaded pH-responsive magnetic nanogels for cancer therapy. Appl. Organomet. Chem. 2018, 32, e4303. [Google Scholar] [CrossRef]
- Kang, H.; Trondoli, A.C.; Zhu, G.; Chen, Y.; Chang, Y.; Liu, H.; Huang, Y.; Zhang, X.; Tan, W. Near-infrared light-responsive core-shell nanogels for targeted drug delivery. ACS Nano 2011, 5, 5094–5099. [Google Scholar] [CrossRef] [Green Version]
- Vicario-de-la-Torre, M.; Forcada, J. The Potential of Stimuli-Responsive Nanogels in Drug and Active Molecule Delivery for Targeted Therapy. Gels 2017, 3, 16. [Google Scholar] [CrossRef] [Green Version]
- Matusiak, M.; Kadlubowski, S.; Ulanski, P. Radiation-induced synthesis of poly(acrylic acid) nanogels. Radiat. Phys. Chem. 2018, 142, 125–129. [Google Scholar] [CrossRef]
- Chacko, R.T.; Ventura, J.; Zhuang, J.; Thayumanavan, S. Polymer nanogels: A versatile nanoscopic drug delivery platform. Adv. Drug Deliv. Rev. 2012, 64, 836–851. [Google Scholar] [CrossRef] [Green Version]
- Jawaid, M.; Mohammad, F. Nanocellulose and Nanohydrogel Matrices: Biotechnological and Biomedical Applications; Wiley-VCH: Weinheim, Germany, 2017; 384p. [Google Scholar]
- Khoee, S.; Asadi, H. Nanogels: Chemical Approaches to Preparation. In Encyclopedia of Biomedical Polymers and Polymeric Biomaterials; Tayler and Francis: New York, NY, USA, 2016; pp. 5266–5293. [Google Scholar] [CrossRef]
- Wu, W.; Zhou, S. Hybrid micro-/nanogels for optical sensing and intracellular imaging. Nano Rev. 2010, 1, 5730. [Google Scholar] [CrossRef]
- Lanzalacoa, S.; Sirés, I.; Sabatinoa, M.A.; Dispenza, C.; Onofrio Scialdone, O.; Galia, A. Synthesis of polymer nanogels by electro-Fenton process: Investigation of the effect of main operation parameters. Electrochim. Acta 2017, 246, 812–822. [Google Scholar] [CrossRef] [Green Version]
- An, J.; Weaver, A.; Kim, B.; Barkatt, A.; Poster, D.; Vreeland, W.N.; Silverman, J.; Al-Sheikhly, M. Radiation-induced synthesis of poly(vinylpyrrolidone) nanogel. Polymer 2011, 52, 5746–5755. [Google Scholar] [CrossRef]
- Vashist, A.; Kaushik, A.; Vashist, A.; Bala, J.; Nikkhah-Moshaie, R.; Sagar, V.; Nair, M. Nanogels as potential drug nanocarriers for CNS drug delivery. Drug Discov. Today 2018, 23, 1436–1443. [Google Scholar] [CrossRef]
- Picone, P.; Sabatino, M.A.; Ditta, L.A.; Amatoc, A.; San Biagio, P.L.; Mulèc, F.; Giacomazzad, D.; Dispenza, C.; Di Carlo, M. Nose-to-brain delivery of insulin enhanced by a nanogel carrier. J. Control. Release 2018, 270, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Jammalamadaka, U.; Tappa, K. Nanogels for Pharmaceutical and Biomedical Applications and Their Fabrication Using 3D Printing. Technol. Mater. 2018, 11, 302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as Pharmaceutical Carriers: Finite Networks of Infinite Capabilities. Angew. Chem. Int. Ed. 2009, 48, 5418–5429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, M.; Wang, Y.; Zhao, Y.; Xie, R.; Yodsanit, N.; Johnston, K.; Gong, S. Double-Network Nanogel as a Nonviral Vector for DNA Delivery. ACS Appl. Mater. Interfaces 2019, 11, 42865–42872. [Google Scholar] [CrossRef]
- Saloni, J.; Kumar, A.R.; Saumya, S.; Lal, S.S.; Mukesh, S. An Overview of Nanogel—Novel Drug Delivery System. Asian J. Pharm. Res. Dev. 2019, 7, 47–55. [Google Scholar] [CrossRef]
- Yadav, H.K.S.; Al Halabi, N.A.; Alsalloum, G.A. Nanogels as Novel Drug Delivery Systems—A Review. J. Pharm. Pharm. Res. 2017, 1, 1–8. [Google Scholar]
- Sharma, A.; Garg, T.; Aman, A.; Panchal, K.; Sharma, R.; Kumar, S.; Markandeywar, T. Nanogel—An advanced drug delivery tool: Current and future. Artif. Cells Nanomed. Biotechnol. 2016, 44, 165–177. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- White, J.L.; Sasaki, A. Free Radical Graft Polymerization. Polym. Plast. Technol. Eng. 2003, 42, 711–735. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Spanswick, J. Controlled/living radical polymerization. Mater. Today 2005, 8, 26–33. [Google Scholar] [CrossRef]
- Grubbs, R.B.; Grubbs, R.H. 50th Anniversary Perspective: Living Polymerization—Emphasizing the Molecule in Macromolecules. Macromolecules 2017, 50, 6979–6997. [Google Scholar] [CrossRef]
- Ratner, B.D.; Weathersby, P.K.; Hoffman, A.S.; Kelly, M.A.; Scharpen, L.H. Radiation-grafted hydrogels for biomaterial applications as studied by the ESCA technique. J. Appl. Polym. Sci. 1978, 22, 643–664. [Google Scholar] [CrossRef]
- Huh, K.M.; Baek, N.; Park, K. Enhanced Swelling Rate of Poly(ethylene glycol)-Grafted Superporous Hydrogels. J. Bioact. Compat. Polym. 2005, 20, 231–243. [Google Scholar] [CrossRef]
- Ma, S.; Yan, C.; Cai, M.; Yang, J.; Wang, X.; Zhou, F.; Liu, W. Continuous Surface Polymerization via Fe(II)-Mediated Redox Reaction for Thick Hydrogel Coatings on Versatile Substrates. Adv. Mater. 2018, 30, 1803371. [Google Scholar] [CrossRef]
- Yang, K.; Han, Q.; Chen, B.; Zheng, Y.; Zhang, K.; Li, Q.; Wang, J. Antimicrobial hydrogels: Promising materials for medical application. Int. J. Nanomed. 2018, 13, 2217–2263. [Google Scholar] [CrossRef] [Green Version]
- Veiga, A.S.; Schneider, J.P. Antimicrobial hydrogels for the treatment of infection. Biopolymers 2013, 100, 637–644. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Li, P.; Qi, X.; Sharif, A.R.M.; Poon, Y.F.; Cao, Y.; Chang, M.W.; Leong, S.S.J.; Chan-Park, M.B. A photopolymerized antimicrobial hydrogel coating derived from epsilon-poly-l-lysine. Biomaterials 2011, 32, 2704–2712. [Google Scholar] [CrossRef]
- Moreau, D.; Chauvet, C.; Etienne, F.; Rannou, F.P.; Corté, L. Hydrogel films and coatings by swelling-induced gelation. Proc. Natl. Acad. Sci. USA 2016, 113, 13295–13300. [Google Scholar] [CrossRef] [Green Version]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Hu, H.; Yang, Z.; He, L.; Kong, Y.; Fei, B.; Xin, J.H. Smart hydrogel-functionalized textile system with moisture management property for skin application. Smart Mater. Struct. 2014, 23, 125027. [Google Scholar] [CrossRef]
- Kim, C.-L.; Kim, D.-E. Durability and Self-healing Effects of Hydrogel Coatings with respect to Contact Condition. Sci. Rep. 2017, 7, 6896. [Google Scholar] [CrossRef] [PubMed]
- Werzer, O.; Tumphart, S.; Keimel, R.; Christian, P.; Coclite, A.M. Drug release from thin films encapsulated by a temperature-responsive hydrogel. Soft Matter 2019, 15, 1853–1859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muir, B.V.O.; Myung, D.; Knoll, W.; Frank, C.W. Grafting of Cross-Linked Hydrogel Networks to Titanium Surfaces. ACS Appl. Mater. Interfaces 2014, 6, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Song, H.; Loh, J.L.C.; She, J.; Deng, L.; Liu, B. Grafting antibiofilm polymer hydrogel film onto catheter by SARA SI-ATRP. J. Biomater. Sci. Polym. Ed. 2018, 29, 2106–2123. [Google Scholar] [CrossRef] [PubMed]
- Velazco-Medel, M.A.; Camacho-Cruz, L.A.; Bucio, E. Modification of PDMS with acrylic acid and acrylic acid/ethylene glycol dimethacrylate by simultaneous polymerization assisted by gamma radiation. Radiat. Phys. Chem. 2020, 171, 108754. [Google Scholar] [CrossRef]
- Mahanta, A.K.; Maiti, P. Injectable Hydrogel through Hydrophobic Grafting on Chitosan for Controlled Drug Delivery. ACS Appl. Bio Mater. 2019, 2, 5415–5426. [Google Scholar] [CrossRef]
- Jommanee, N.; Chanthad, C.; Manokruang, K. Preparation of injectable hydrogels from temperature and pH responsive grafted chitosan with tuned gelation temperature suitable for tumor acidic environment. Carbohydr. Polym. 2018, 198, 486–494. [Google Scholar] [CrossRef]
- Iatridi, Z.; Saravanou, S.-F.; Tsitsilianis, C. Injectable self-assembling hydrogel from alginate grafted by P(N-isopropylacrylamide-co-N-tert-butylacrylamide) random copolymers. Carbohydr. Polym. 2019, 219, 344–352. [Google Scholar] [CrossRef]







| Method | Brief Description | |
|---|---|---|
| Homogeneous Nucleation | Emulsion Polymerization | Water-soluble monomers and crosslink agents are mixed in an aqueous medium. In this case, initially, a homogeneous solution is obtained. To avoid the formation of a macrogel, it is of great importance that the polymer formed is not soluble under the conditions for polymerization [63]. |
| Emulsification: W/O Heterogeneous Emulsion | Emulsion Polymerization | Water-soluble monomers and bioactive components are dispersed in an oil medium with surfactants and high shear forces, forming a colloidal system. To promote particle gelation inside the water droplets, several methods may be used such as chemical crosslink agents or stimulating it by temperature according to the polymer critical temperature [65,66]. |
| Inverse Microemulsion Polymerization | Monomer aqueous droplets are dispersed in an oil phase by a homogenizer or mechanical stirrer. Inside these aqueous droplets, drugs and other substances of interest may be incorporated. Crosslinking agents are used for this process [59]. | |
| Membrane Emulsification | In this process, an emulsion is passed through the pores of a membrane made of glass or ceramic into a nanofluid phase to form a microgel [61]. | |
| Heterogeneous controlled/living radical polymerization | This process may be performed by several methods such as stable radical polymerization, reversible addition-fragmentation chain transfer, and transfer radical polymerization [61]. | |
| Polymer Complexation | Microgels are obtained by mixing polymeric solutions of opposite charges, forming polyelectrolyte complexes [67]. | |
| Radiation | To produce microgels by radiation, the polymer solution is placed in molds, which may be the final packages, then they are exposed to γ-rays that will crosslink and sterilize the microgels. In this case, crosslinking occurs from the water radiolysis that generates hydroxyl radicals that react with polymer chains allowing them to combine. Radiation may also be used to make polymer surface modifications and obtain physical-chemical properties of interest [68,69]. | |
| Physical-Based Methods for Microgel Fabrication | Photolithographic Techniques | In this method, a monomer solution containing a photoinitiator, and the crosslinking agent is exposed to ultraviolet or laser light which causes the curing reaction. Masks and stamps are used to control the size and shape of the microgel [59]. |
| Micromolding Method | This method is like photoligraphy, but in this case, the polymer solution is placed in a mold, and gelation happens by temperature change or by adding a gelling agent [64]. | |
| Microfluidic and Droplet Formation | It combines the synthesis of polymer particles and microencapsulation. In this case, polymer solutions are injected in an oil phase and then crosslinked. The difference here is the use of specially designed devices that allow specific morphologies and structures for the particles formed [65]. | |
| Traditional Method | Simultaneous polymerization and crosslinking | Emulsion polymerization [92,103] |
| Precipitation polymerization [92] | ||
| Inverse emulsion polymerization [92,103] | ||
| Dispersion polymerization [92] | ||
| Controlled radical polymerization [105] | ||
| Atom transfer radical polymerization (ATRP) [92,103] | ||
| Reversible addition-fragmentation chain transfer (RAFT) polymerization [92] | ||
| Degenerative chain transfer polymerization represented by iodine-mediated polymerization [106] | ||
| Uncontrolled radical polymerization [92] | ||
| Crosslinking of polymer precursors | Disulfide-based crosslinking [92,103,105] | |
| Amine-based crosslinking [92,103,105] | ||
| Imine crosslinking [92,105] | ||
| Click chemistry-based crosslinking [92,103] | ||
| Photoinduced crosslinking [92,103,105] | ||
| Physical crosslinkingc [92,103,105] | ||
| Controlled aggregation by physical self-assembly of hydrophilic polymers [90] | ||
| Template-assisted fabrication of nanogel particles | Photolithography [90,92,94] | |
| Micromolding techniques [90,92,94] | ||
| Novel Methods | Novel pullulan chemistry modification [94] | |
| Novel photochemical approach [94] | ||
| Novel radical polymerization with inverse mini-emulsion technology [94] | ||
| Addition-fragmentation transfer process [94] | ||
| Chemical modification [94] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, C.S.A.d.; Balogh, T.S.; Varca, J.P.R.O.; Varca, G.H.C.; Lugão, A.B.; A. Camacho-Cruz, L.; Bucio, E.; Kadlubowski, S.S. An Updated Review of Macro, Micro, and Nanostructured Hydrogels for Biomedical and Pharmaceutical Applications. Pharmaceutics 2020, 12, 970. https://doi.org/10.3390/pharmaceutics12100970
Lima CSAd, Balogh TS, Varca JPRO, Varca GHC, Lugão AB, A. Camacho-Cruz L, Bucio E, Kadlubowski SS. An Updated Review of Macro, Micro, and Nanostructured Hydrogels for Biomedical and Pharmaceutical Applications. Pharmaceutics. 2020; 12(10):970. https://doi.org/10.3390/pharmaceutics12100970
Chicago/Turabian StyleLima, Caroline S. A. de, Tatiana S. Balogh, Justine P. R. O. Varca, Gustavo H. C. Varca, Ademar B. Lugão, Luis A. Camacho-Cruz, Emilio Bucio, and Slawomir S. Kadlubowski. 2020. "An Updated Review of Macro, Micro, and Nanostructured Hydrogels for Biomedical and Pharmaceutical Applications" Pharmaceutics 12, no. 10: 970. https://doi.org/10.3390/pharmaceutics12100970
APA StyleLima, C. S. A. d., Balogh, T. S., Varca, J. P. R. O., Varca, G. H. C., Lugão, A. B., A. Camacho-Cruz, L., Bucio, E., & Kadlubowski, S. S. (2020). An Updated Review of Macro, Micro, and Nanostructured Hydrogels for Biomedical and Pharmaceutical Applications. Pharmaceutics, 12(10), 970. https://doi.org/10.3390/pharmaceutics12100970