Biomimetic Artificial Membrane Permeability Assay over Franz Cell Apparatus Using BCS Model Drugs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Impregnation of Membrane Support
2.2.2. Electronic Paramagnetic Resonance (EPR)
2.2.3. Permeation Studies
2.2.4. Permeability Calculations
3. Results
3.1. EPR Analysis and Membrane Stability
3.2. Membrane Validation and Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Petereit, A.C.; Swinney, K.; Mensch, J.; Mackie, C.; Stokbroekx, S.; Brewster, M.; Dressman, J.B. Prediction of blood-brain barrier penetration of poorly soluble drug candidates using surface activity profiling. Eur. J. Pharm. Biopharm. 2010, 75, 405–410. [Google Scholar] [CrossRef]
- Waring, M.J.; Arrowsmith, J.; Leach, A.R.; Leeson, P.D.; Mandrell, S.; Owen, R.M.; Pairaudeau, G.; Pennie, W.D.; Pickett, S.D.; Wang, J.; et al. An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nat. Rev. Drug Discov. 2015, 14, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A Theoretical Basis for a Biopharmaceutic Drug Classification: The Correlation of in vitro Drug Product Dissolution and in vivo Bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.-Y.Y.; Benet, L.Z.Z. Predicting Drug Disposition via Application of BCS: Transport/Absorption/Elimination Interplay and Development of a Biopharmaceutics Drug Disposition Classification System; Kluwer Academic Publishers-Plenum Publishers: Amsterdam, The Netherlands, 2005; Volume 22, pp. 11–23. [Google Scholar]
- Corti, G.; Maestrelli, F.; Cirri, M.; Furlanetto, S.; Mura, P. Development and evaluation of an in vitro method for prediction of human drug absorption I. Assessment of artificial membrane composition. Eur. J. Pharm. Sci. 2006, 27, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Ruell, J.A.; Tsinman, K.L.; Avdeef, A. PAMPA—A drug absorption in vitro model. 5. Unstirred water layer in iso-pH mapping assays and pKa(flux)—Optimized design (pOD-PAMPA). Eur. J. Pharm. Sci. 2003, 20, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Kortejärvi, H.; Urtti, A.; Yliperttula, M. Pharmacokinetic simulation of biowaiver criteria: The effects of gastric emptying, dissolution, absorption and elimination rates. Eur. J. Pharm. Sci. 2007, 30, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Matsson, P.; Doak, B.C.; Over, B.; Kihlberg, J. Cell permeability beyond the rule of 5. Adv. Drug Deliv. Rev. 2016, 101, 42–61. [Google Scholar] [CrossRef]
- Benet, L.Z.; Hosey, C.M.; Ursu, O.; Oprea, T.I. BDDCS, the Rule of 5 and drugability. Adv. Drug Deliv. Rev. 2016, 101, 89–98. [Google Scholar] [CrossRef]
- Bergström, C.A.S.; Holm, R.; Jørgensen, S.A.; Andersson, S.B.E.; Artursson, P.; Beato, S.; Borde, A.; Box, K.; Brewster, M.; Dressman, J.; et al. Early pharmaceutical profiling to predict oral drug absorption: Current status and unmet needs. Eur. J. Pharm. Sci. 2014, 57, 173–199. [Google Scholar] [CrossRef]
- Lennernäs, H. Regional intestinal drug permeation: Biopharmaceutics and drug development. Eur. J. Pharm. Sci. 2014, 57, 333–341. [Google Scholar] [CrossRef]
- Sjögren, E.; Eriksson, J.; Vedin, C.; Breitholtz, K.; Hilgendorf, C. Excised segments of rat small intestine in Ussing chamber studies: A comparison of native and stripped tissue viability and permeability to drugs. Int. J. Pharm. 2016, 505, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.; Toguchi, H.; Nishibayashi, T.; Higaki, K.; Sugita, A.; Koganei, K.; Kamada, N.; Kitazume, M.T.; Hisamatsu, T.; Sato, T.; et al. Establishment of Novel Prediction System of Intestinal Absorption in Humans Using Human Intestinal Tissues. J. Pharm. Sci. 2013, 102, 2564–2571. [Google Scholar] [CrossRef] [PubMed]
- Hilgendorf, C.; Spahn-Langguth, H.; Regårdh, C.G.; Lipka, E.; Amidon, G.L.; Langguth, P. Caco-2 versus Caco-2/HT29-MTX co-cultured cell lines: Permeabilities via diffusion, inside- and outside-directed carrier-mediated transport. J. Pharm. Sci. 2000, 89, 63–75. [Google Scholar] [CrossRef]
- Artursson, P.; Karlsson, J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem. Biophys. Res. Commun. 1991, 175, 885. [Google Scholar] [CrossRef]
- Corti, G.; Maestrelli, F.; Cirri, M.; Zerrouk, N.; Mura, P. Development and evaluation of an in vitro method for prediction of human drug absorption II. Demonstration of the method suitability. Eur. J. Pharm. Sci. 2006, 27, 354–362. [Google Scholar] [CrossRef]
- Faller, B. Artificial membrane assays to assess permeability. Curr. Drug Metab. 2008, 9, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Mirmehrabi, M.; Rohani, S.; Perry, L. Thermodynamic modeling of activity coefficient and prediction of solubility: Part 2. Semipredictive or semiempirical models. J. Pharm. Sci. 2006, 95, 798–809. [Google Scholar] [CrossRef]
- Lozoya-Agullo, I.; González-Álvarez, I.; González-Álvarez, M.; Merino-Sanjuán, M.; Bermejo, M. In Situ Perfusion Model in Rat Colon for Drug Absorption Studies: Comparison with Small Intestine and Caco-2 Cell Model. J. Pharm. Sci. 2015, 104, 3136–3145. [Google Scholar] [CrossRef]
- Yamashita, S.; Furubayashi, T.; Kataoka, M.; Sakane, T.; Sezaki, H.; Tokuda, H. Optimized conditions for prediction of intestinal drug permeability using Caco-2 cells. Eur. J. Pharm. Sci. 2000, 10, 195–204. [Google Scholar] [CrossRef]
- Oltra-Noguera, D.; Mangas-Sanjuan, V.; Centelles-Sangüesa, A.; Gonzalez-Garcia, I.; Sanchez-Castaño, G.; Gonzalez-Alvarez, M.; Casabo, V.-G.; Merino, V.; Gonzalez-Alvarez, I.; Bermejo, M. Variability of permeability estimation from different protocols of subculture and transport experiments in cell monolayers. J. Pharmacol. Toxicol. Methods 2015, 71, 21–32. [Google Scholar] [CrossRef]
- Di Cagno, M.; Bibi, H.A.; Bauer-Brandl, A. New biomimetic barrier PermeapadTM for efficient investigation of passive permeability of drugs. Eur. J. Pharm. Sci. 2015, 73, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Berben, P.; Bauer-Brandl, A.; Brandl, M.; Faller, B.; Flaten, G.E.; Jacobsen, A.-C.; Brouwers, J.; Augustijns, P. Drug permeability profiling using cell-free permeation tools: Overview and applications. Eur. J. Pharm. Sci. 2018, 119, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Billat, P.-A.; Roger, E.; Faure, S.; Lagarce, F. Models for drug absorption from the small intestine: Where are we and where are we going? Drug Discov. Today 2017, 22, 761–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flaten, G.E.; Bunjes, H.; Luthman, K.; Brandl, M. Drug permeability across a phospholipid vesicle-based barrier 2. Characterization of barrier structure, storage stability and stability towards pH changes. Eur. J. Pharm. Sci. 2006, 28, 336–343. [Google Scholar] [CrossRef] [Green Version]
- OECD. Joint Meeting of the Chemicals Committee and the Working Party on Chemicals, Pesticides and Biotechnology. Ser. Chem. Accid. 2017, 33, 1–117. [Google Scholar]
- Selzer, D.; Abdel-Mottaleb, M.M.A.; Hahn, T.; Schaefer, U.F.; Neumann, D. Finite and infinite dosing: Difficulties in measurements, evaluations and predictions. Adv. Drug Deliv. Rev. 2013, 65, 278–294. [Google Scholar] [CrossRef]
- Kasim, N.A.; Whitehouse, M.; Ramachandran, C.; Bermejo, M.; Lennernäs, H.; Hussain, A.S.; Junginger, H.E.; Stavchansky, S.A.; Midha, K.K.; Shah, V.P.; et al. Molecular Properties of WHO Essential Drugs and Provisional Biopharmaceutical Classification. Mol. Pharm. 2004, 1, 85–96. [Google Scholar] [CrossRef]
- Tavelin, S.; Gråsjö, J.; Taipalensuu, J.; Ocklind, G.; Artursson, P. Applications of epithelial cell culture in studies of drug transport. Methods Mol. Biol. 2002, 188, 233–272. [Google Scholar] [CrossRef]
- Mangas-Sanjuan, V.; González-Álvarez, I.; González-Álvarez, M.; Casabó, V.G.; Bermejo, M. Modified nonsink equation for permeability estimation in cell monolayers: Comparison with standard methods. Mol. Pharm. 2014, 11, 1403–1414. [Google Scholar] [CrossRef]
- Kim, J.-S.; Mitchell, S.; Kijek, P.; Tsume, Y.; Hilfinger, J.; Amidon, G.L. The suitability of an in situ perfusion model for permeability determinations: Utility for BCS class I biowaiver requests. Mol. Pharm. 2006, 3, 686–694. [Google Scholar] [CrossRef]
- Collado, M.I.; Goñi, F.M.; Alonso, A.; Marsh, D. Domain Formation in Sphingomyelin/Cholesterol Mixed Membranes Studied by Spin-Label Electron Spin Resonance Spectroscopy. Biochemistry 2005, 44, 4911–4918. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, M.; Avdeef, A.; Ruiz, A.; Nalda, R.; Ruell, J.A.; Tsinman, O.; González, I.; Fernández, C.; Sánchez, G.; Garrigues, T.M.; et al. PAMPA—A drug absorption in vitro model 7. Comparing rat in situ, Caco-2, and PAMPA permeability of fluoroquinolones. Eur. J. Pharm. Sci. 2004, 21, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Flaten, G.E.; Luthman, K.; Vasskog, T.; Brandl, M. Drug permeability across a phospholipid vesicle-based barrier 4. The effect of tensides, co-solvents and pH changes on barrier integrity and on drug permeability. Eur. J. Pharm. Sci. 2008, 34, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Naderkhani, E.; Isaksson, J.; Ryzhakov, A.; Flaten, G.E. Development of a biomimetic phospholipid vesicle-based permeation assay for the estimation of intestinal drug permeability. J. Pharm. Sci. 2014, 103, 1882–1890. [Google Scholar] [CrossRef] [Green Version]
- Kansy, M.; Senner, F.; Gubernator, K. Physicochemical high throughput screening: Parallel artificial membrane permeation assay in the description of passive absorption processes. J. Med. Chem. 1998, 41, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration Guidance for Industry: Waiver of In vivo Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System. Available online: https://www.fda.gov/media/70963/download (accessed on 20 December 2017).
- Lindenberg, M.; Kopp, S.; Dressman, J.B. Classification of orally administered drugs on the World Health Organization Model list of Essential Medicines according to the biopharmaceutics classification system. Eur. J. Pharm. Biopharm. 2004, 58, 265–278. [Google Scholar] [CrossRef]
- Zhu, C.; Jiang, L.; Chen, T.M.; Hwang, K.K. A comparative study of artificial membrane permeability assay for high throughput profiling of drug absorption potential. Eur. J. Med. Chem. 2002, 37, 399–407. [Google Scholar] [CrossRef]
- Sinkó, B.; Garrigues, T.M.; Balogh, G.T.; Nagy, Z.K.; Tsinman, O.; Avdeef, A.; Takács-Novák, K. Skin-PAMPA: A new method for fast prediction of skin penetration. Eur. J. Pharm. Sci. 2012, 45, 698–707. [Google Scholar] [CrossRef]
- Mensch, J.; Melis, A.; Mackie, C.; Verreck, G.; Brewster, M.E.; Augustijns, P. Evaluation of various PAMPA models to identify the most discriminating method for the prediction of BBB permeability. Eur. J. Pharm. Biopharm. 2010, 74, 495–502. [Google Scholar] [CrossRef]
- Huque, F.T.T.; Box, K.; Platts, J.A.; Comer, J. Permeability through DOPC/dodecane membranes: measurement and LFER modelling. Eur. J. Pharm. Sci. 2004, 23, 223–232. [Google Scholar] [CrossRef]



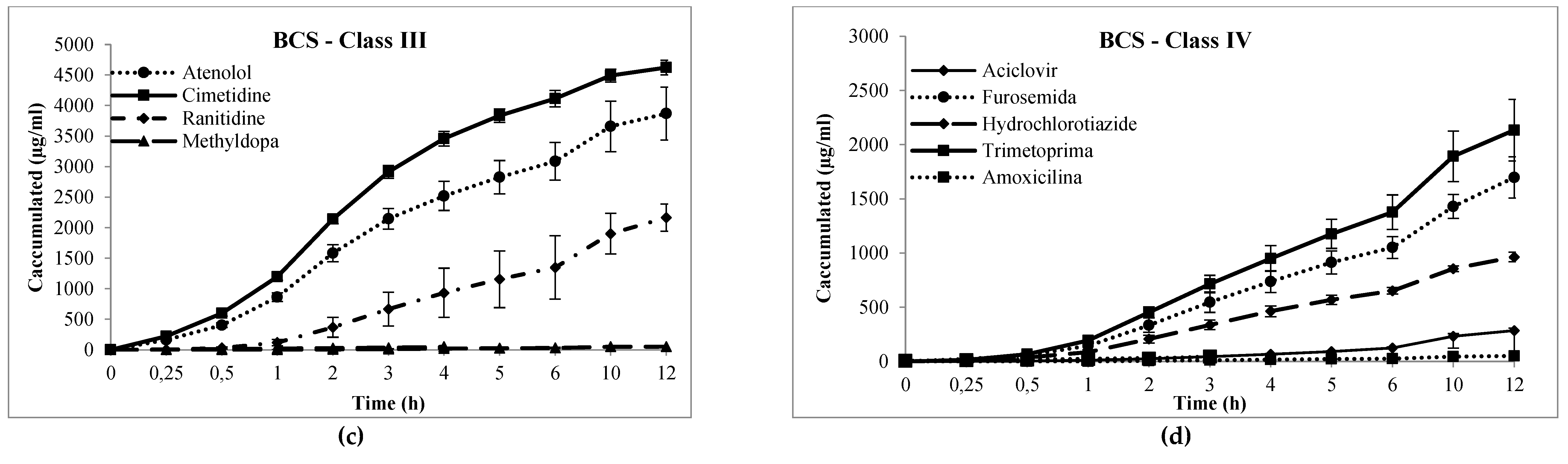
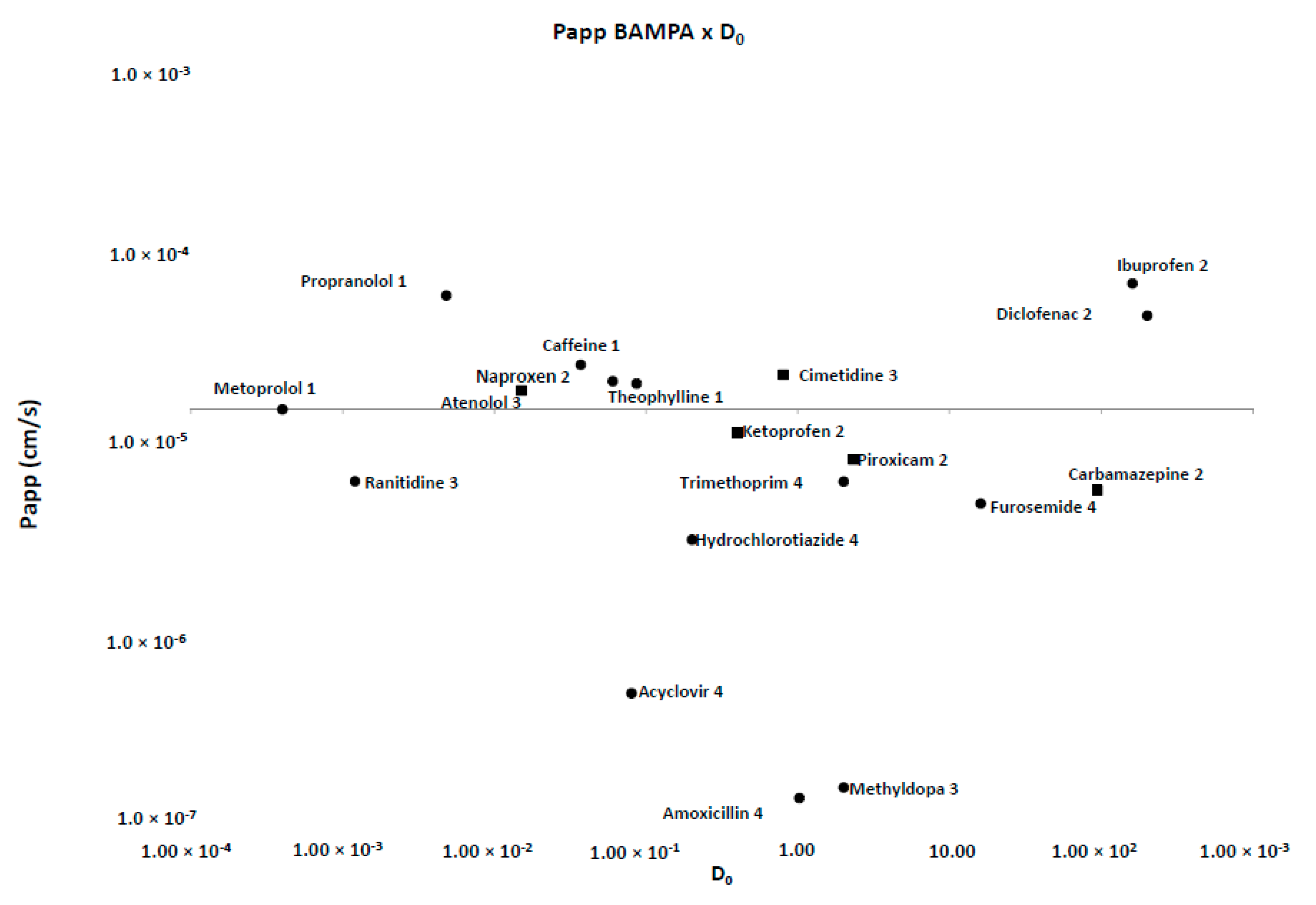

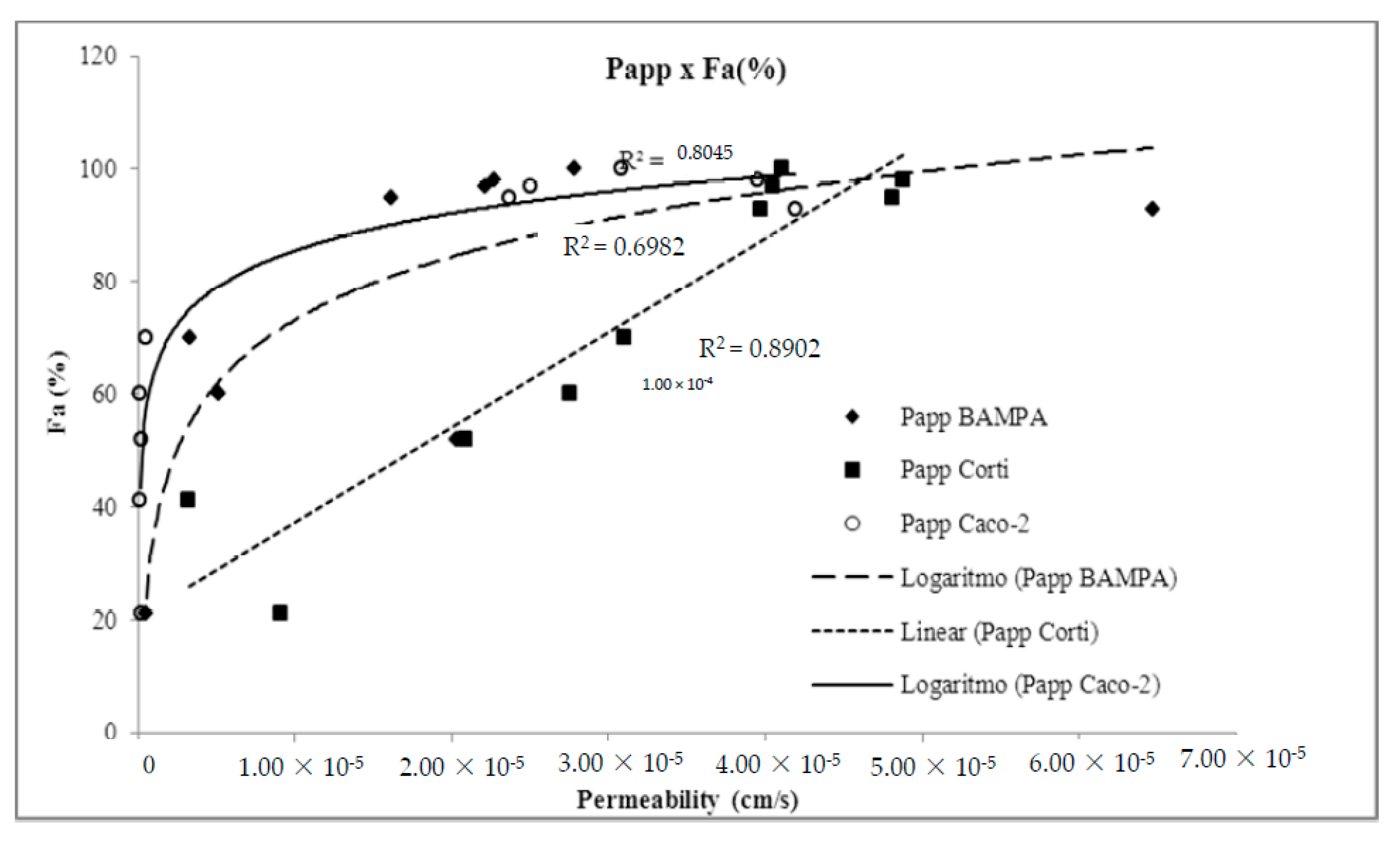
| Drug | Chromatographic Conditions (Stationary and Mobile Phase; λ; Flow Rate; Injection Volume) | LOQ (μg/mL) |
|---|---|---|
| Acyclovir | C-18 (5 µm; 250 × 4.2 mm), acetic acid: water (1:1000); 254 nm; 3.0 mL/min; 20 µL | 46.3 |
| Amoxicillin | C18 (5 µm; 250 × 4.0 mm); acetonitrile e phosphate buffer pH 5.0 (4:96); 230 nm, 1.5 mL/min, 10 μL | 1.00 |
| Atenolol | C-18 (5 µm; 300 × 3.9 mm); Dissolve 1.1 g of sodium heptane sulfonate and 0.71 g of sodium phosphate dibasic anhydrous in 700 mL of water. Add 2 mL of dibutylamine. Adjust pH 3.0. Add methanol (300 mL); 226 nm; 0.6 mL/min; 10 µL | 3.4 |
| Caffeine | C-18 (5 µm; 150 × 4.6 mm); Solution of 1.64 g anhydrous sodium acetate in 2000 mL of water. Take 1910 mL of this solution add acetonitrile (50 mL), tetrahydrofuran (40 mL). Adjust pH 4.5 with glacial acetic acid; 275 nm; 1.0 mL/min; 10 µL | 19.0 |
| Carbamazepine | CN ((250 mm × 4,.6 mm); Water, methanol, and tetrahydrofuran (85:12:3), 0.22 mL formic acid and 0.5 mL triethylamine; 230 nm, 1.5 mL/min, 20 μL | 0.03 |
| Cimetidine | C-18 (5 µm; 300 × 3.9 mm); 20% methanol in 0.3% phosphoric acid solution; 220 nm; 2.0 mL/min; 50 µL | 1.0 |
| Diclofenac sodium | C-8 (5 µm; 250 × 4.6 mm); phosphate buffer pH 2.5 and methanol (30:70); 254 nm; 1.0 mL/min; 10 µL | 0.20 |
| Furosemide | C-18 (5 µm; 250 × 4.6 mm); Water, tetrahydrofuran, and glacial acetic acid (70:30:1); 254 nm; 1.0 mL/min; 20 µL | 16.6 |
| Hydrochlor-thiazide | C-18 (5 µm; 150 × 4.6 mm); Solution A: acetonitrile and methanol (3:1). Solution B: 0.5% formic acid. Gradient: 0–3 min. Sol A: Sol B (3:97), 5–14 min. Sol A: Sol. B (3 to 36:97 to 64), 14–18 min. The Sol. A: Sol B (36 to 3:64 to 97), 18–20 min. Sol A: Sol B (3:97); 275 nm; 1.0 mL/min; 10 µL | 7.8 |
| Ibuprofen | C-18 (5 µm; 250 × 4.6 mm); 4% chloroacetic acid pH 3.0 and acetonitrile (40:60); 254 nm; 2.0 mL/min; 10 µL | 13.9 |
| Ketoprophen | C18 (3 µm; 150 × 4.6 mm); water, acetonitrile, and phosphate buffer pH3.5 (55:43:22); 233 nm, 1.0 mL/min, 20 μL | 1.56 |
| Metoprolol | C-18 (5 µm; 300 × 3.9 mm); 961 mg of pentane sulfonate, 82 mg of anhydrous sodium acetate, 550 mL of methanol, 470 mL of water and 0.57 mL of acetic acid; 254 nm; 1.0 mL/min; 30 µL | 13.8 |
| Methyldopa | C18 (5 µm; 300 × 3.9 mm); Monobasic phosphate buffer pH 3.5; 280 nm, 1.0 mL/min, 50 μL | 0.12 |
| Naproxen | C-18 (5 µm; 150 × 4.6 mm); Acetonitrile, water, and glacial acetic acid (50:49:1); 254 nm; 1.2 mL/min; 20 µL | 3.6 |
| Piroxicam | C-18 (5 µm; 250 × 4.6 mm); Buffer solution containing 7.72 g of anhydrous citric acid in 400 mL of water and 5.35 g dibasic sodium phosphate in 100 mL of water, mix the two solutions and adjust volume to 1000 mL with water.Mix buffer and methanol (55:45); 254 nm; 1.2 mL/min; 20 µL | 4.0 |
| Propranolol | C-8 (5 µm; 250 × 4.6 mm); Dissolve 0.5 g of sodium dodecyl sulfate in 18 mL of 0.15 M phosphoric acid. Add 90 mL of acetonitrile, 90 mL of methanol, dilute with water to complete 250 mL; 290 nm; 1.5 mL/min; 20 µL | 8.2 |
| Ranitidine | C-18 (3.5 μm; 10 × 4.6 mm); buffer phosphate pH 7.1: acetonitrile (80:20); 230 nm, 1.5 mL/min, 35°C, 10 μL | 7.4 |
| Trimethoprim | C-18 (5 μm; 250 × 4.2 mm); 1% glacial acetic acid: acetonitrile (21:4); 254 nm, 2 mL/min, 10 μL | 0.15 |
| Theophylline | C-18 (5 μm; 300 × 4.0 mm); 7% acetonitrile in sodium acetate buffer; 280 nm, 1 mL/min, 10 μL | 0.22 |
| Verapamil | C-18 (5 µm; 150 × 4.6 mm); 0.015 N sodium acetate in 3.3% glacial acetic acid. add acetonitrile and 2-amino-heptane (70:30:0.5); 278 nm; 0.9 mL/min; 10 µL | 0.50 |
| Drug | 5 BCS | 1 Fa(%) | Papp × 10−6 cm/s | 5 LogP | 5 Log D pH 7.4 | 5 pKa | 4,5 Intrinsic Solubility (mg/mL) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Franz–PAMPA | Non-Sink Arthurson | 1 Caco-2 | 2 Corti | 3 PAMPA pH 7,4 | 3 Permeapad™ | |||||||
| Metoprolol | I | 95 | 15.8 (HP) | 59.0 | 23.7 (HP) | 48.1 (HP) | 3.5 | 1.0 | 1.9 | −0.2 | 9.6 | 1000.0 |
| Caffeine | 100 | 36.2 (HP) | 53.3 | 30.8 (HP) | 41.1 | 10.8 | 20.4 | −0.1 | 0.02 | 0.6 | 21.17 | |
| Propranolol | 93 | 33.1 (HP) | 88.4 | 41.9 (HP) | 39.7 | 23.5 | nC | 2.65 | 1.3 | 9.5 | 33.0 | |
| Theophylline | 97 | 22.1 (HP) | --- | 25.0 (HP) | 40.5 | -- | 7.2 | −0.25 | −0.05 | 0.6 & 8.55 | 8.33 | |
| Carbamazepine | II | 100 | 5.97 | --- | - | - | 11.3 | nC | 2.5 | 1.8 | 1.0 & 13.9 | 0.12 |
| Diclofenac | 100 | 68.1 (HP) | 104.9 | - | - | 12.5 | nC | 4.4 | 1.2 | 3.99 | 0.001 | |
| Ibuprofen | 93 | 57.5 (HP) | 36.0 | 52.5 (HP) | - | 6.8 | 16.6 | 3.1 | 0.7 | 4.9 | 0.01 | |
| Ketoprofen (AT) | 100 | 12.1 | --- | 20.1 | 42.7 | 16.7 | nC | 3.3 | −1.51 | 4.5 | 0.051 | |
| Naproxen | 98 | 2.89 (HP) | 1.7 | 39.5 (HP) | 48.8 (HP) | 10.6 | nC | 3.2 | 0.2 | 4.2 | 33.0 | |
| Piroxicam | 100 | 11.0 | 9.6 | 35.6 (HP) | - | 8.2 | nC | 2.0 | −0.07 | 2.33 & 5.1 | 0.11 | |
| Verapamil (AT) | 98 | 5.39 | 5.0 | 15.8 | 41.6 | 7.4 | 9.3 | 3.8 | 2.7 | 8.9 | 0.44 | |
| Atenolol | III | 52 | 25.8 (HP) | 22.0 | 0.2 | 20.9 | 0.0 | 4.3 | 0.2 | 9.6 | 26.5 | |
| Cimetidine | 93 | 35.6 (HP) | 31.0 | 0.7 | - | 0.0 | nC | 0.4 | 0.4 | 6.8 | 6.0 | |
| Methyldopa | 41 | --- | --- | 0.2 | 3.2 | -- | nC | 0.4 | 1.7 & 9.9 | 10.0 | ||
| Ranitidine (AT) | 55 | 6.81 | 5.3 | 0.5 | 21.5 | 0.5 | nC | 0.3 | −0.3 | 2.1 & 8.1 | 100.0 | |
| Acyclovir | IV | 21 | 0.40 | 0.4 | 0.3 | 9.1 | 0.0 | 7.9 | −1.7 | −1.7 | 2.3 & 9.3 | 10.0 |
| Amoxicillin | 93 | 0.85 | 0.07 | 0.8 | - | 1.5 | nC | 0.9 | --- | 3.2 & 11.7 | 4.0 | |
| Furosemide | 60 | 4.57 | 4.5 | 0.1 | 27.5 | 0.6 | nC | 2.3 | −0.7 | 3.5 & 10.6 | 0.01 | |
| Hydrochlorothiazide | 70 | 2.74 | 2.7 | 0.5 | 31.0 | 0.1 | nC | −0.1 | −0.1 | 7.9 | 1.0 | |
| Trimethoprim (AT) | 97 | 6.61 | 7.7 | 83.0 (HP) | 45.5 | 5.0 | nC | 0.9 | 0.7 | 7.1 | 0.4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza Teixeira, L.; Vila Chagas, T.; Alonso, A.; Gonzalez-Alvarez, I.; Bermejo, M.; Polli, J.; Rezende, K.R. Biomimetic Artificial Membrane Permeability Assay over Franz Cell Apparatus Using BCS Model Drugs. Pharmaceutics 2020, 12, 988. https://doi.org/10.3390/pharmaceutics12100988
de Souza Teixeira L, Vila Chagas T, Alonso A, Gonzalez-Alvarez I, Bermejo M, Polli J, Rezende KR. Biomimetic Artificial Membrane Permeability Assay over Franz Cell Apparatus Using BCS Model Drugs. Pharmaceutics. 2020; 12(10):988. https://doi.org/10.3390/pharmaceutics12100988
Chicago/Turabian Stylede Souza Teixeira, Leonardo, Tatiana Vila Chagas, Antonio Alonso, Isabel Gonzalez-Alvarez, Marival Bermejo, James Polli, and Kênnia Rocha Rezende. 2020. "Biomimetic Artificial Membrane Permeability Assay over Franz Cell Apparatus Using BCS Model Drugs" Pharmaceutics 12, no. 10: 988. https://doi.org/10.3390/pharmaceutics12100988
APA Stylede Souza Teixeira, L., Vila Chagas, T., Alonso, A., Gonzalez-Alvarez, I., Bermejo, M., Polli, J., & Rezende, K. R. (2020). Biomimetic Artificial Membrane Permeability Assay over Franz Cell Apparatus Using BCS Model Drugs. Pharmaceutics, 12(10), 988. https://doi.org/10.3390/pharmaceutics12100988








