Extraction, Characterization and Incorporation of Hypericum scruglii Extract in Ad Hoc Formulated Phospholipid Vesicles Designed for the Treatment of Skin Diseases Connected with Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Phytocomplexes
2.3. Determination of the Total Phenolic Content
2.4. Estimation of the Antiradical Properties of the Extracts
2.5. Polyphenol Characterization and Quantification by High Performance Liquid Chromatography (HPLC)
2.6. Vesicle Preparation
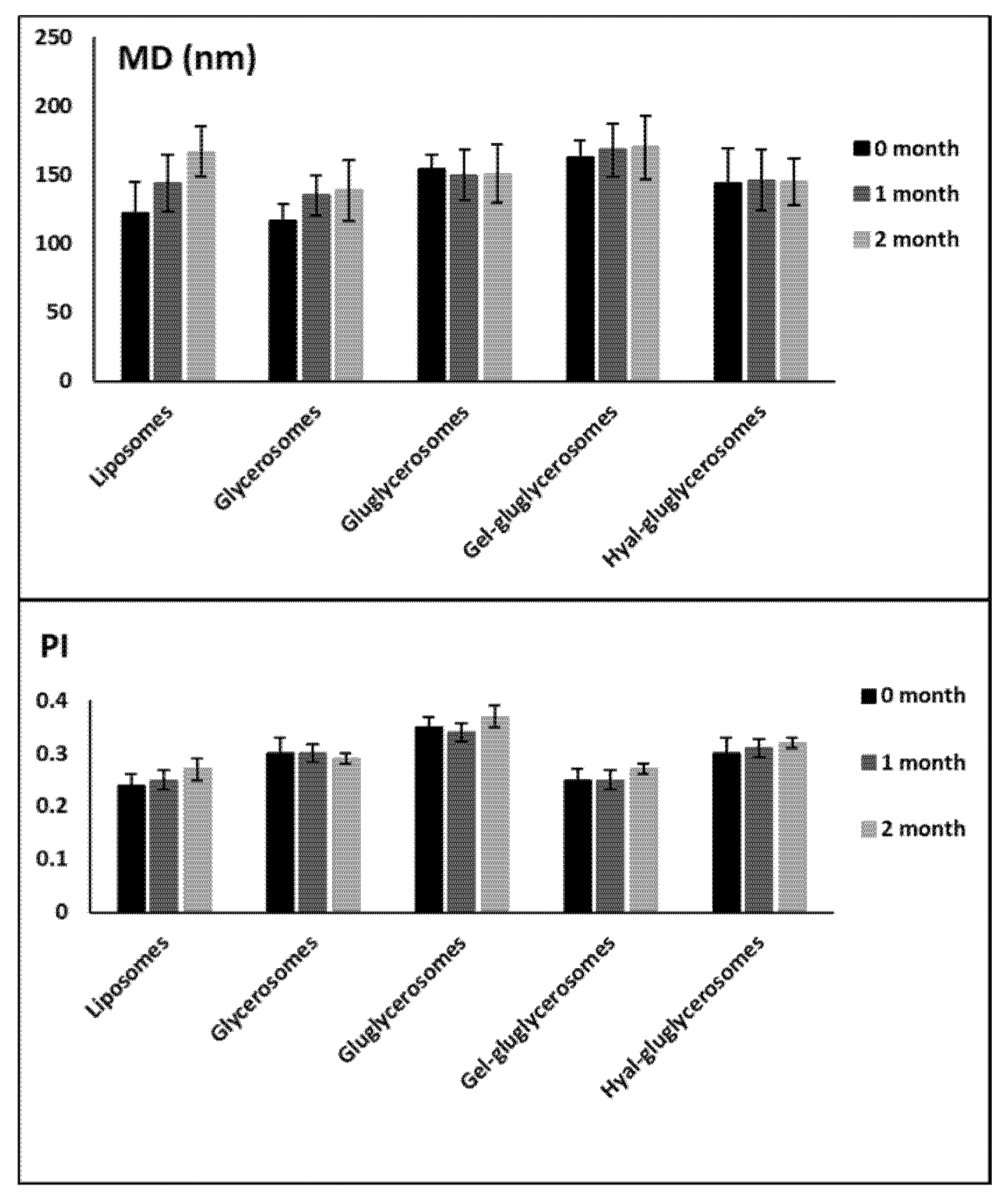
2.7. Evaluation of Physico-chemical Properties and Stability on Storage of Vesicles
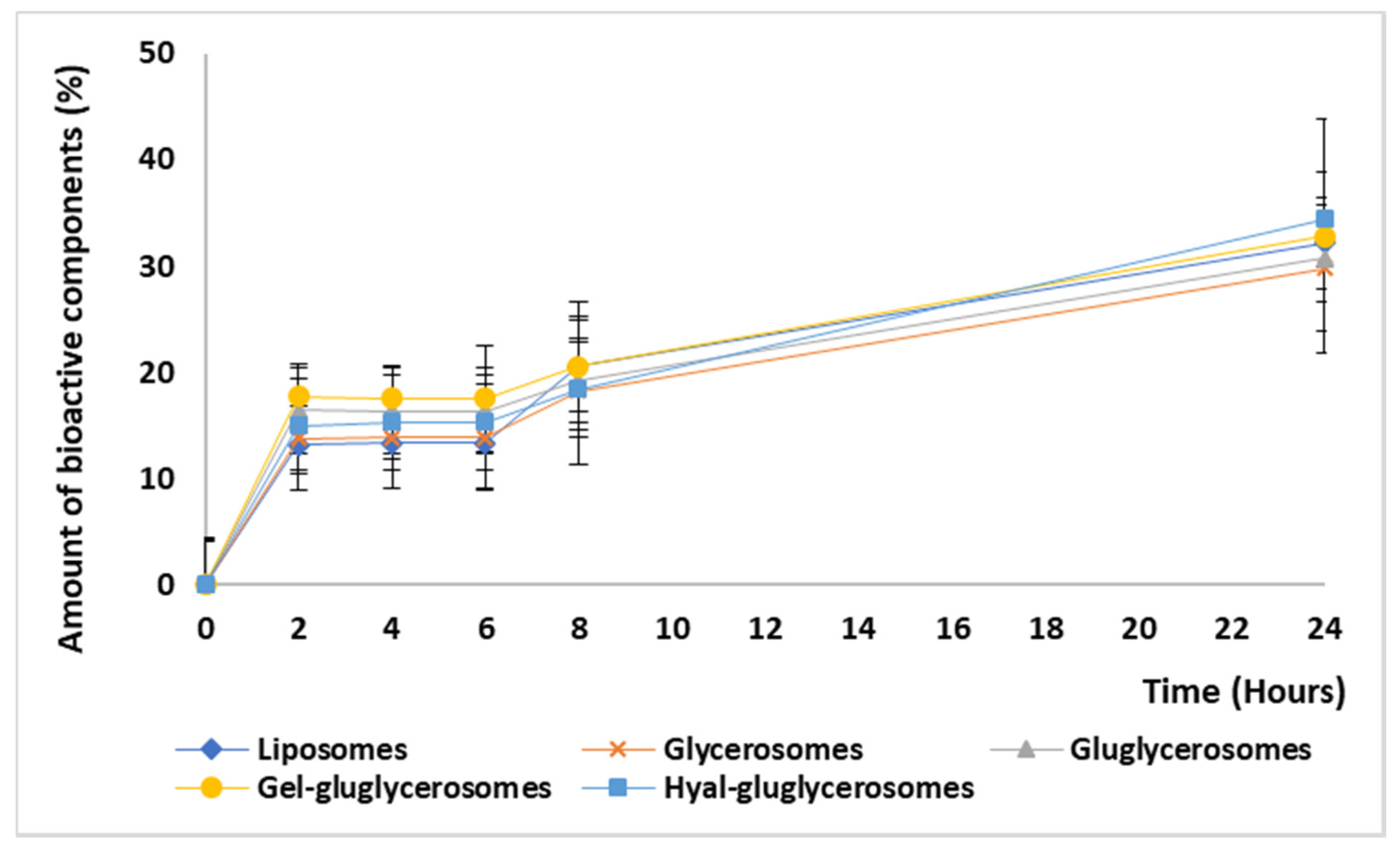
2.8. Release Studies
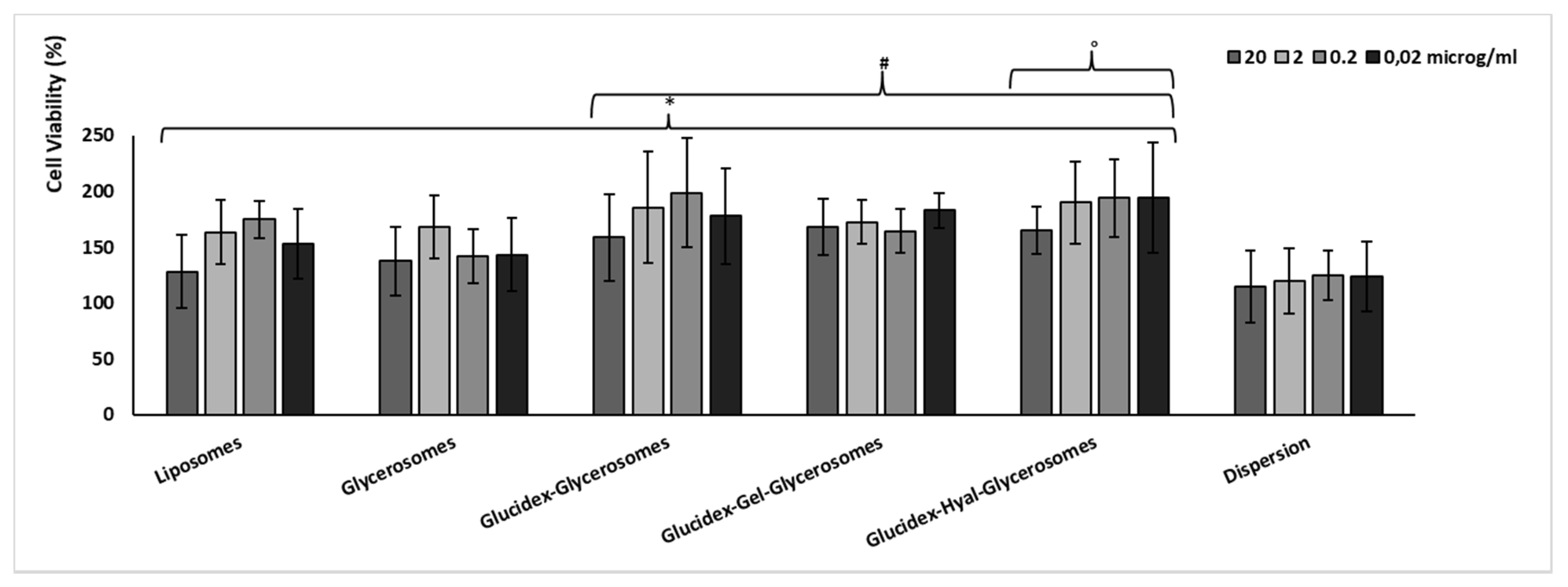
2.9. Cell Viability and Protection against Oxidative Stress
2.10. Ability of H. Scruglii Extract Loaded into Vesicles to Promote Cell Proliferation and Migration: Scratch Assay
2.11. Uptake of Fluorescent Vesicles by Keratinocytes
2.12. Statistical Analysis of Data
3. Results
3.1. Extract Characterization
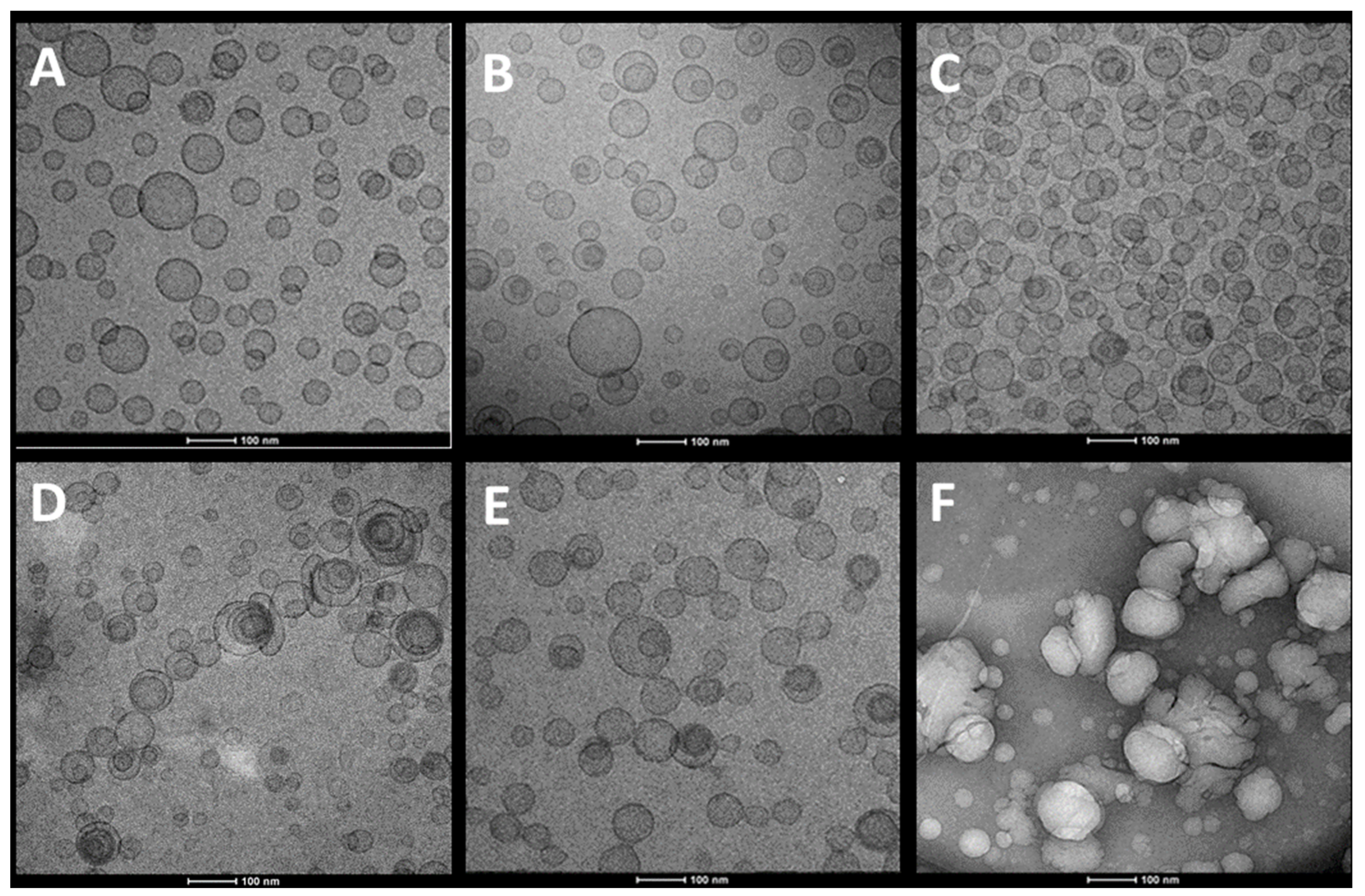
3.2. Vesicle Characterization
3.3. Release Studies
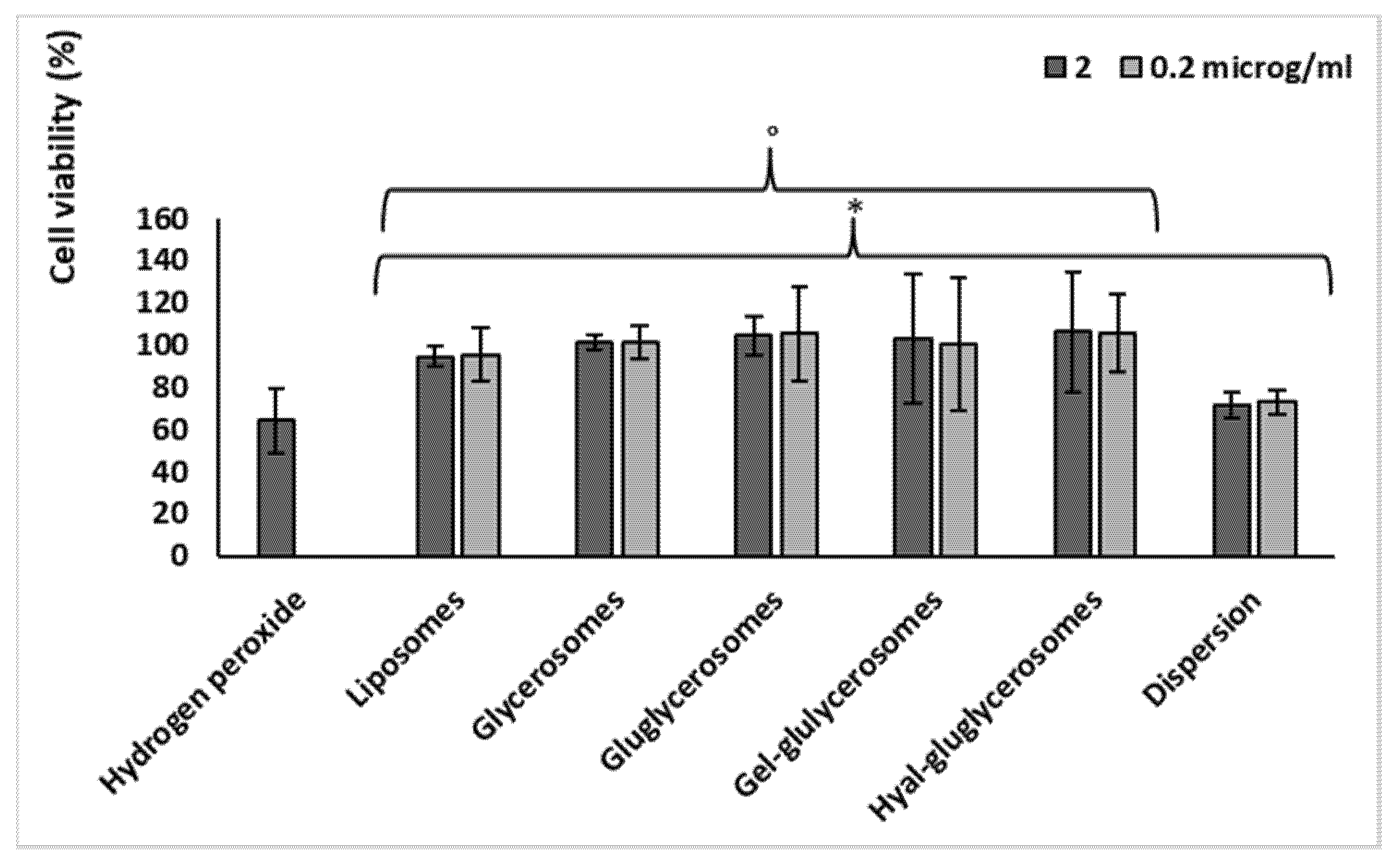
3.4. Biocompatibility and Protective Effect against Oxidative Stress of H. scruglii Extract-Loaded Vesicles
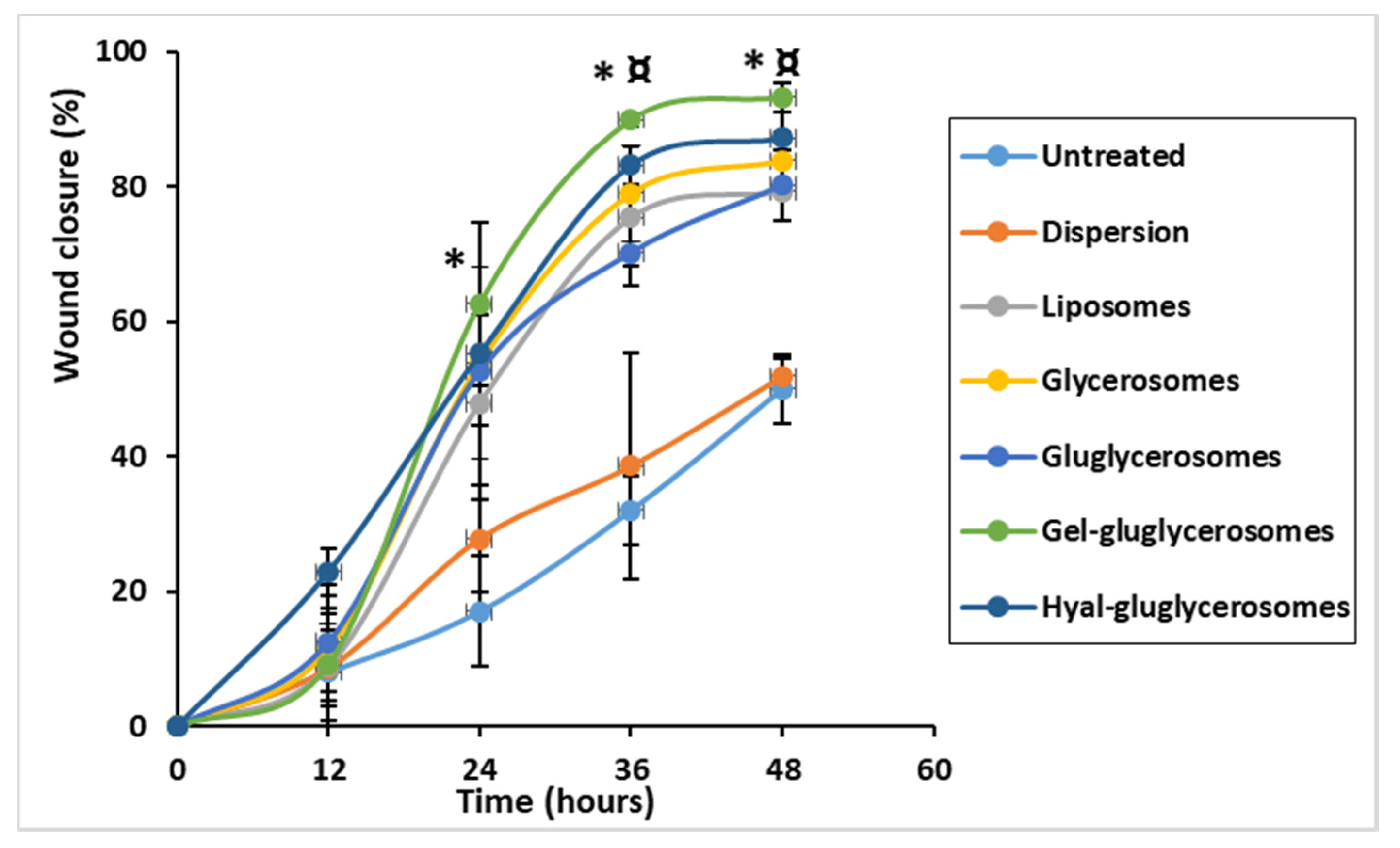
3.5. Wound Healing Activity
3.6. Uptake of Fluorescently Labelled Vesicles by Keratinocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gefen, A. Innovations and Emerging Technologies in Wound Care; Academic Press: London, UK, 2019. [Google Scholar]
- Fore, J. A review of skin and the effects of aging on skin structure and function. Ostomy/Wound Manag. 2006, 52, 24–35. [Google Scholar]
- Birch-Machin, M.A.; Russell, E.V.; Latimer, J.A. Mitochondrial DNA damage as a biomarker for ultraviolet radiation exposure and oxidative stress. Br. J. Dermatol. 2013, 169, 9–14. [Google Scholar] [CrossRef]
- Packer, L.; Valacchi, G. Antioxidants and the Response of Skin to Oxidative Stress: Vitamin E as a Key Indicator. Ski. Pharmacol. Physiol. 2002, 15, 282–290. [Google Scholar] [CrossRef]
- Birch-machin, M.A.; Swalwell, H. How mitochondria record the effects of UV exposure and oxidative stress using human skin as a model tissue. Mutagenesis 2010, 25, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Church, D.; Elsayed, S.; Reid, O.; Winston, B.; Lindsay, R. Burn Wound Infections. Clin. Microbiol. Rev. 2006, 19, 403–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godbout, J.P.; Glaser, R. Stress-Induced Immune Dysregulation: Implications for Wound Healing, Infectious Disease and Cancer. J. Neuroimmune Pharmacol. 2006, 1, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Briganti, S.; Picardo, M. Antioxidant activity, lipid peroxidation and skin diseases. What’s new. J. Eur. Acad. Dermatol. Venereol. 2003, 17, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, N.; Hamdani, M. Plants used to treat skin diseases. Pharm. Rev. 2014, 8, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch. Biochem. Biophys. 2008, 476, 107–112. [Google Scholar] [CrossRef]
- Chua, L.S.; Lee, S.Y.; Abdullah, N.; Sarmidi, M.R. Review on Labisia pumila (Kacip Fatimah): Bioactive phytochemicals and skin collagen synthesis promoting herb. Fitoterapia 2012, 83, 1322–1335. [Google Scholar] [CrossRef]
- Nicolaou, A. Eicosanoids in skin inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 131–138. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.; Mikhal’chik, E.V.; Suprun, M.V.; Papacharalambous, M.; Truhanov, A.I.; Korkina, L.G. Skin Antiageing and Systemic Redox Effects of Supplementation with Marine Collagen Peptides and Plant-Derived Antioxidants: A Single-Blind Case-Control Clinical Study. Oxid. Med. Cell. Longev. 2016, 2016, 4389410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sala, M.; Diab, R.; Elaissari, A.; Fessi, H. Lipid nanocarriers as skin drug delivery systems: Properties, mechanisms of skin interactions and medical applications. Int. J. Pharm. 2018, 535, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tsai, P.; Ramezanli, T.; Michniak-Kohn, B.B. Polymeric nanoparticles-based topical delivery systems for the treatment of dermatological diseases. WIREs Nanomed. Nanobiotechnol. 2013, 5, 205–218. [Google Scholar] [CrossRef] [Green Version]
- Cristiano, M.C.; Froiio, F.; Spaccapelo, R.; Mancuso, A.; Nisticò, S.P.; Udongo, B.P.; Fresta, M.; Paolino, D. Sulforaphane-loaded ultradeformable vesicles as a potential natural nanomedicine for the treatment of skin cancer diseases. Pharmaceutics 2020, 12, 6. [Google Scholar] [CrossRef] [Green Version]
- Coradini, K.; Lima, F.O.; Oliveira, C.M.; Chaves, P.S.; Athayde, M.L.; Carvalho, L.M.; Beck, R.C.R. Co-encapsulation of resveratrol and curcumin in lipid-core nanocapsules improves their in vitro antioxidant effects. Eur. J. Pharm. Biopharm. 2014, 88, 178–185. [Google Scholar] [CrossRef]
- Scalia, S.; Franceschinis, E.; Bertelli, D.; Iannuccelli, V. Comparative Evaluation of the Effect of Permeation Enhancers, Lipid Nanoparticles and Colloidal Silica on in vivo Human Skin Penetration of Quercetin. Ski. Pharmacol. Physiol. 2013, 26, 57–67. [Google Scholar] [CrossRef]
- Pivetta, T.P.; Simões, S.; Araújo, M.M.; Carvalho, T.; Arruda, C.; Marcato, P.D. Development of nanoparticles from natural lipids for topical delivery of thymol: Investigation of its anti-inflammatory properties. Colloids Surf. B Biointerfaces 2018, 164, 281–290. [Google Scholar] [CrossRef]
- Bacchetta, G.; Brullo, S.; Salmeri, C. Hypericum scruglii sp. nov.(Guttiferae) from Sardinia. Nord. J. Bot. 2010, 28, 469–474. [Google Scholar] [CrossRef]
- Sanna, C.; Scognamiglio, M.; Fiorentino, A.; Corona, A.; Graziani, V.; Caredda, A.; Cortis, P.; Montisci, M.; Ceresola, R.; Canducci, F.; et al. Prenylated phloroglucinols from Hypericum scruglii, an endemic species of Sardinia (Italy), as new dual HIV-1 inhibitors effective on HIV-1 replication. PLoS ONE 2018, 13, e0195168. [Google Scholar] [CrossRef] [Green Version]
- Porceddu, M.; Sanna, M.; Serra, S.; Manconi, M.; Bacchetta, G. Seed germination requirements of Hypericum scruglii, an endangered medicinal plant species of Sardinia (Italy). Botany 2020, 98, 615–621. [Google Scholar] [CrossRef]
- Mandrone, M.; Scognamiglio, M.; Fiorentino, A.; Sanna, C.; Cornioli, L.; Antognoni, F.; Bonvicini, F.; Poli, F. Phytochemical profile and α-glucosidase inhibitory activity of Sardinian Hypericum scruglii and Hypericum hircinum. Fitoterapia 2017, 120, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, G.; Dordevic, A.; Smelcerovic, A. Do other Hypericum species have medical potential as St. John’s wort (Hypericum perforatum)? Curr. Med. Chem. 2013, 20, 2273–2295. [Google Scholar] [CrossRef] [PubMed]
- Saddiqe, Z.; Naeem, I.; Maimoona, A. A review of the antibacterial activity of Hypericum perforatum L. J. Ethnopharmacol. 2010, 131, 511–521. [Google Scholar] [CrossRef]
- Zhang, X.-W.; Ye, Y.-S.; Xia, F.; Yang, X.-W.; Xu, G. Diverse Polyphenols from Hypericum faberi. Nat. Prod. Bioprospecting 2019, 9, 215–221. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Ji, Y.; Zhang, X.; Kennelly, E.J.; Long, C. Ethnopharmacology of Hypericum species in China: A comprehensive review on ethnobotany, phytochemistry and pharmacology. J. Ethnopharmacol. 2020, 254, 112686. [Google Scholar] [CrossRef] [PubMed]
- Chiocchio, I.; Mandrone, M.; Sanna, C.; Maxia, A.; Tacchini, M.; Poli, F. Screening of a hundred plant extracts as tyrosinase and elastase inhibitors, two enzymatic targets of cosmetic interest. Ind. Crops Prod. 2018, 122, 498–505. [Google Scholar] [CrossRef]
- Libbey, L.M.; Walradt, J.P. 3,5-di-Tert-butyl-4-hydroxytoluene (BHT) as an artifact from diethyl ether. Lipids 1968, 3, 561. [Google Scholar] [CrossRef]
- Kallithraka, S.; Mohdaly, A.A.A.; Makris, D.P.; Kefalas, P. Determination of major anthocyanin pigments in Hellenic native grape varieties (Vitis vinifera sp.): Association with antiradical activity. J. Food Compos. Anal. 2005, 18, 375–386. [Google Scholar] [CrossRef]
- Taamalli, A.; Arráez-Román, D.; Barrajón-Catalán, E.; Ruiz-Torres, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Use of advanced techniques for the extraction of phenolic compounds from Tunisian olive leaves: Phenolic composition and cytotoxicity against human breast cancer cells. Food Chem. Toxicol. 2012, 50, 1817–1825. [Google Scholar] [CrossRef] [Green Version]
- Rajha, H.N.; Abi-Khattar, A.M.; El Kantar, S.; Boussetta, N.; Lebovka, N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Comparison of aqueous extraction efficiency and biological activities of polyphenols from pomegranate peels assisted by infrared, ultrasound, pulsed electric fields and high-voltage electrical discharges. Innov. Food Sci. Emerg. Technol. 2019, 58, 102212. [Google Scholar] [CrossRef]
- Rajha, H.N.; Mhanna, T.; El Kantar, S.; El Khoury, A.; Louka, N.; Maroun, R.G. Innovative process of polyphenol recovery from pomegranate peels by combining green deep eutectic solvents and a new infrared technology. LWT 2019, 111, 138–146. [Google Scholar] [CrossRef]
- Castangia, I.; Manca, M.L.; Matricardi, P.; Sinico, C.; Lampis, S.; Fernàndez-Busquets, X.; Fadda, A.M.; Manconi, M. Effect of diclofenac and glycol intercalation on structural assembly of phospholipid lamellar vesicles. Int. J. Pharm. 2013, 456, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Castangia, I.; Caddeo, C.; Manca, M.L.; Casu, L.; Latorre, A.C.; Díez-Sales, O.; Ruiz-Saurí, A.; Bacchetta, G.; Fadda, A.M.; Manconi, M. Delivery of liquorice extract by liposomes and hyalurosomes to protect the skin against oxidative stress injuries. Carbohydr. Polym. 2015, 134, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.Y.K. A Simplified Method for Quantifying Cell Migration/Wound Healing in 96-Well Plates. J. Biomol. Screen. 2010, 15, 427–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manca, M.L.; Mir-Palomo, S.; Caddeo, C.; Nacher, A.; Díez-Sales, O.; Peris, J.E.; Pedraz, J.L.; Fadda, A.M.; Manconi, M. Sorbitol-penetration enhancer containing vesicles loaded with baicalin for the protection and regeneration of skin injured by oxidative stress and UV radiation. Int. J. Pharm. 2019, 555, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Manca, M.L.; Castangia, I.; Zaru, M.; Nácher, A.; Valenti, D.; Fernàndez-Busquets, X.; Fadda, A.M.; Manconi, M. Development of curcumin loaded sodium hyaluronate immobilized vesicles (hyalurosomes) and their potential on skin inflammation and wound restoring. Biomaterials 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Akgün, D.; Gültekin, M.-O.; Yücetepe, A.; Altin, G.; Gibis, M.; Weiss, J. Food Hydrocolloids Stirred-type yoghurt incorporated with sour cherry extract in chitosan-coated liposomes. Food Hydrocoll. 2020, 101, 105532. [Google Scholar] [CrossRef]
- Catalán-Latorre, A.; Pleguezuelos-Villa, M.; Castangia, I.; Manca, M.L.; Caddeo, C.; Nácher, A.; Díez-Sales, O.; Peris, J.E.; Pons, R.; Escribano-Ferrer, E.; et al. Nutriosomes: Prebiotic delivery systems combining phospholipids, a soluble dextrin and curcumin to counteract intestinal oxidative stress and inflammation. Nanoscale 2018, 10, 1957–1969. [Google Scholar] [CrossRef] [Green Version]
- Manconi, M.; Mura, S.; Manca, M.L.; Fadda, A.M.; Dolz, M.; Hernandez, M.J.; Casanovas, A.; Diez-Sales, O. Chitosomes as drug delivery systems for C-phycocyanin: Preparation and characterization. Int. J. Pharm. 2010, 392, 92–100. [Google Scholar] [CrossRef]
- Atrooz, O.M. The incorporation effects of methanolic extracts of some plant seeds on the stability of phosphatidylcholine liposomes. Pak. J. Biol. Sci. 2007, 10, 1643–1648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Z. Encapsulation of polyphenols: A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Krämer, S.D.; Jakits-Deiser, C.; Wunderli-Allenspach, H. Free fatty acids cause pH-dependent changes in drug-lipid membrane interactions around physiological pH. Pharm. Res. 1997, 14, 827–832. [Google Scholar] [CrossRef]
- Manca, M.L.; Peris, J.E.; Melis, V.; Valenti, D.; Cardia, M.C.; Lattuada, D.; Escribano-Ferrer, E.; Fadda, A.M.; Manconi, M. Nanoincorporation of curcumin in polymer-glycerosomes and evaluation of their in vitro-in vivo suitability as pulmonary delivery systems. RSC Adv. 2015, 5, 105149–105159. [Google Scholar] [CrossRef]
- Murthy, S.N.; Zhao, Y.L.; Sen, A.; Hui, S.W. Cyclodextrin enhanced transdermal delivery of piroxicam and carboxyfluorescein by electroporation. J. Control. Release 2004, 99, 393–402. [Google Scholar] [CrossRef]
- Lewis, S.; Pandey, S.; Udupa, N. Design and evaluation of matrix type and membrane controlled transdermal delivery systems of nicotine suitable for use in smoking cessation. Indian J. Pharm. Sci. 2006, 68, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Allen, T.M.; Moase, E.H. opportunities for targeted liposomal drug delivery. Adv. Drug Deliv. Rev. 1996, 21, 117–133. [Google Scholar] [CrossRef]
- Chithrani, D.B.; Dunne, M.; Stewart, J.; Allen, C.; Jaffray, D.A. Cellular uptake and transport of gold nanoparticles incorporated in a liposomal carrier. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 161–169. [Google Scholar] [CrossRef]
- Pes, I.; Baykal, T.; Alper, M.; Yes, E. Investigations on the in vivo wound healing potential of Hypericum perforatum L. Ipek Pes. 2010, 127, 468–477. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Verpoorte, R.; Suresh, B. Evaluation of in-vivo wound healing activity of Hypericum patulum (Family: Hypericaceae) leaf extract on different wound model in rats. J. Ethnopharmacol. 2000, 70, 315–321. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Suresh, B. The evaluation of wound-healing potential of Hypericum hookerianum leaf and stem extracts. J. Altern. Complement. Med. 2000, 6, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Süntar, I.P.; Akkol, E.K.; Yilmazer, D.; Baykal, T.; Kirmizibekmez, H.; Alper, M.; Yeşilada, E. Investigations on the in vivo wound healing potential of Hypericum perforatum L. J. Ethnopharmacol. 2010, 127, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Orčić, D.Z.; Mimica-duki, N.M.; Franci, M.M.; Petrovi, S.S.; Jovin, E.Đ. Antioxidant activity relationship of phenolic compounds in Hypericum perforatum L. Chem Cent J. 2011, 5, 34. [Google Scholar] [CrossRef] [Green Version]
- Pistelli, L.; Bertoli, A.; Zucconelli, S.; Morelli, I. Antimicrobial activity of crude extracts and pure compounds of Hypericum hircinum. Fitoterapia 2000, 71, 138–140. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [Green Version]
- Cai, M.S.; Xing, J.; Corke, H. Antioxidant Phenolic Constituents in Roots of Rheum officinale and Rubia cordifolia: Structure—Radical Scavenging Activity Relationships. J. Agric. Food Chem. 2004, 52, 7884–7890. [Google Scholar] [CrossRef] [PubMed]
- Wittenauer, J.; Mäckle, S.; Sußmann, D.; Schweiggert-weisz, U.; Carle, R. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia 2015, 101, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Genc, Y.; Dereli, F.T.G.; Saracoglu, I.; Kupeli, E. The inhibitory effects of isolated constituents from Plantago major subsp. major L. on collagenase, elastase and hyaluronidase enzymes: Potential wound healer. Saudi Pharm. J. 2020, 28, 101–106. [Google Scholar] [CrossRef]
- Derycke, A.S.L.; De Witte, P.A.M. Transferrin-mediated targeting of hypericin embedded in sterically stabilized PEG-liposomes. Int. J. Oncol. 2002, 20, 181–187. [Google Scholar] [CrossRef]
- Plenagl, N.; Seitz, B.S.; Duse, L.; Pinnapireddy, S.R.; Jedelska, J.; Brüßler, J.; Bakowsky, U. Hypericin inclusion complexes encapsulated in liposomes for antimicrobial photodynamic therapy. Int. J. Pharm. 2019, 570, 118666. [Google Scholar] [CrossRef]
- Manca, M.L.; Matricardi, P.; Cencetti, C.; Peris, J.E.; Melis, V.; Carbone, C.; Escribano, E.; Zaru, M.; Fadda, A.M.; Manconi, M. Combination of argan oil and phospholipids for the development of an effective liposome-like formulation able to improve skin hydration and allantoin dermal delivery. Int. J. Pharm. 2016, 505, 204–211. [Google Scholar] [CrossRef]
- Yang, S.; Liu, L.; Han, J.; Tang, Y. Encapsulating plant ingredients for dermocosmetic application: An updated review of delivery systems and characterization techniques. Int. J. Cosmet. Sci. 2020, 42, 16–28. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Hu, X.; Chelliah, R.; Oh, D. Biogenic silver nanoparticles-polyvinylpyrrolidone based glycerosomes coating to expand the shelf life of fresh-cut bell pepper (Capsicum annuum L. var. grossum (L.) Sendt). Postharvest Biol. Technol. 2020, 160, 111039. [Google Scholar] [CrossRef]
- Mir-Palomo, S.; Nácher, A.; Díez-Sales, O.; Buso, M.A.O.; Caddeo, C.; Manca, M.L.; Manconi, M.; Fadda, M.A. Inhibition of skin in fl ammation by baicalin ultradeformable vesicles. Int. J. Pharm. 2016, 511, 23–29. [Google Scholar] [CrossRef]
- Moolakkadath, T.; Aqil, M.; Ahad, A.; Imam, S.S.; Praveen, A.; Sultana, Y.; Mujeeb, M. Preparation and optimization of fi setin loaded glycerol based soft nanovesicles by Box-Behnken design. Int. J. Pharm. 2020, 578, 119125. [Google Scholar] [CrossRef] [PubMed]
- Manca, M.L.; Cencetti, C.; Matricardi, P.; Castangia, I.; Zaru, M.; Sales, O.D.; Nacher, A.; Valenti, D.; Maccioni, A.M.; Fadda, A.M.; et al. Glycerosomes: Use of hydrogenated soy phosphatidylcholine mixture and its effect on vesicle features and diclofenac skin penetration. Int. J. Pharm. 2016, 511, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Manca, M.L.; Castangia, I.; Caddeo, C.; Pando, D.; Escribano, E.; Valenti, D.; Lampis, S.; Zaru, M.; Fadda, A.M.; Manconi, M. Improvement of quercetin protective effect against oxidative stress skin damages by incorporation in nanovesicles. Colloids Surf. B Biointerfaces 2014, 123, 566–574. [Google Scholar] [CrossRef]
- Zhang, K.; Li, N. Essential oil-mediated glycerosomes increase transdermal paeoniflorin delivery: Optimization, characterization, and evaluation in vitro and in vivo. Int. J. Nanomed. 2017, 12, 3521–3532. [Google Scholar] [CrossRef] [Green Version]
- Manconi, M.; Manca, M.L.; Valenti, D.; Escribano, E.; Hillaireau, H.; Fadda, A.M.; Fattal, E. Chitosan and hyaluronan coated liposomes for pulmonary administration of curcumin. Int. J. Pharm. 2017, 525, 203–210. [Google Scholar] [CrossRef]
- Manconi, M.; Manca, M.L.; Marongiu, F.; Caddeo, C.; Castangia, I.; Petretto, G.L.; Pintore, G.; Sarais, G.; D’Hallewin, G.; Zaru, M.; et al. Chemical characterization of Citrus limon var. pompia and incorporation in phospholipid vesicles for skin delivery. Int. J. Pharm. 2016, 506, 449–457. [Google Scholar] [CrossRef]
- Dong, C.; Rogers, J.A. Acacia-Gelatin Microencapsulated Liposomes: Preparation, Stability, and Release of Acetylsalicylic Acid. Pharm. Res. 1993, 10, 141–146. [Google Scholar] [CrossRef] [PubMed]
- DiTizio, V.; Karlgard, C.; Lilge, L.; Khoury, A.E.; Mittelman, M.W.; DiCosmo, F. Localized drug delivery using crosslinked gelatin gels containing liposomes: Factors influencing liposome stability and drug release. J. Biomed. Mater. Res. 2000, 51, 96–106. [Google Scholar] [CrossRef]
- Shishova, N.V.; Davydova, G.A.; Kombarova, N.A.; Poltavtsev, A.M.; Zaraisky, E.I.; Poltavtseva, R.A. The use of nanosized liposomes from vegetable phospholipids in combination with albumine and some polysaccharides as cryoprotective agents in the course of cryopreservation. IOP Conf. Ser. Mater. Sci. Eng. 2020, 747, 012074. [Google Scholar] [CrossRef]
- Manconi, M.; Caddeo, C.; Manca, M.L.; Fadda, A.M. Oral delivery of natural compounds by phospholipid vesicles. Nanomedicine 2020, 15, 1795–1803. [Google Scholar] [CrossRef]
- Hu, F.; Yan, Y.; Wang, C.; Liu, Y.; Wang, J.; Zhou, F.; Zeng, Q.; Zhou, X.; Chen, J.; Wang, A.; et al. Article Effect and Mechanism of Ganoderma lucidum Polysaccharides on Human Fibroblasts and Skin Wound Healing in Mice. Chin. J. Integr. Med. 2019, 25, 203–209. [Google Scholar] [CrossRef]
- Kumar, S.; Marrero-Berrios, I.; Kabat, M.; Berthiaume, F. Recent advances in the use of algal polysaccharides for skin wound healing. Curr. Pharm. Des. 2019, 25, 1236–1248. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Dutta, J.; Dutta, P.K.; Koh, J. A systematic study on chitosan-liposome based systems for biomedical applications. Int. J. Biol. Macromol. 2020, 160, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Manca, M.L.; Casula, E.; Marongiu, F.; Bacchetta, G.; Sarais, G.; Zaru, M.; Escribano-Ferrer, E.; Peris, J.E.; Usach, I.; Fais, S.; et al. From waste to health: Sustainable exploitation of grape pomace seed extract to manufacture antioxidant, regenerative and prebiotic nanovesicles within circular economy. Sci. Rep. 2020, 10, 14184. [Google Scholar] [CrossRef] [PubMed]
- Manconi, M.; Marongiu, F.; Manca, M.L.; Caddeo, C.; Sarais, G.; Cencetti, C.; Pucci, L.; Longo, V.; Bacchetta, G.; Fadda, A.M. Nanoincorporation of bioactive compounds from red grape pomaces: In vitro and ex vivo evaluation of antioxidant activity. Int. J. Pharm. 2017, 523, 159–166. [Google Scholar] [CrossRef]



| S75 (mg/mL) | Extract (mg/mL) | Glucidex (mg/mL) | Gelatin (mg/mL) | Hyaluronan (mg/mL) | Glycerol (mL) | Water (mL) | |
|---|---|---|---|---|---|---|---|
| Liposomes | 120 | 10 | 0 | 0 | 0 | - | 1 |
| Glycerosomes | 120 | 10 | 0 | 0 | 0 | 0.25 | 0.75 |
| Gluglycerosomes | 120 | 10 | 25 | 0 | 0 | 0.25 | 0.75 |
| Gel-gluglycerosomes | 120 | 10 | 25 | 1 | 0 | 0.25 | 0.75 |
| Hyal-gluglycerosomes | 120 | 10 | 25 | 0 | 1 | 0.25 | 0.75 |
| DM (nm) | PI | ZP (mV) | EE (%) | AA (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Empty | Extract | Empty | Extract | Empty | Extract | Extract | Extract | |
| Liposomes | 111 ± 13 | 122 ± 23 | 0.36 | 0.24 | −39 ± 5 | −55 ± 6 | 88 ± 9 | 64 ± 4 |
| Glycerosomes | 108 ± 19 | 117 ± 15 | 0.32 | 0.30 | −41 ± 6 | −53 ± 5 | 85 ± 7 | 79 ± 7 |
| Gluglycerosomes | 121 ± 21 | 154 ± 22 | 0.35 | 0.30 | −37 ± 3 | −41 ± 4 | 88 ± 11 | 84 ± 6 |
| Gel-gluglycerosomes | 114 ± 16 | 163 ± 33 | 0.34 | 0.25 | −43 ± 7 | −39 ± 6 | 97 ± 4 | 83 ± 7 |
| Hyal-gluglycerosomes | 127 ± 24 | 154 ± 25 | 0.35 | 0.29 | −39 ± 4 | −50 ± 5 | 88 ± 13 | 81 ± 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allaw, M.; Manconi, M.; Aroffu, M.; Marongiu, F.; Porceddu, M.; Bacchetta, G.; Usach, I.; Rached, R.A.; Rajha, H.N.; Maroun, R.G.; et al. Extraction, Characterization and Incorporation of Hypericum scruglii Extract in Ad Hoc Formulated Phospholipid Vesicles Designed for the Treatment of Skin Diseases Connected with Oxidative Stress. Pharmaceutics 2020, 12, 1010. https://doi.org/10.3390/pharmaceutics12111010
Allaw M, Manconi M, Aroffu M, Marongiu F, Porceddu M, Bacchetta G, Usach I, Rached RA, Rajha HN, Maroun RG, et al. Extraction, Characterization and Incorporation of Hypericum scruglii Extract in Ad Hoc Formulated Phospholipid Vesicles Designed for the Treatment of Skin Diseases Connected with Oxidative Stress. Pharmaceutics. 2020; 12(11):1010. https://doi.org/10.3390/pharmaceutics12111010
Chicago/Turabian StyleAllaw, Mohamad, Maria Manconi, Matteo Aroffu, Francesca Marongiu, Marco Porceddu, Gianluigi Bacchetta, Iris Usach, Rita Abi Rached, Hiba N. Rajha, Richard G. Maroun, and et al. 2020. "Extraction, Characterization and Incorporation of Hypericum scruglii Extract in Ad Hoc Formulated Phospholipid Vesicles Designed for the Treatment of Skin Diseases Connected with Oxidative Stress" Pharmaceutics 12, no. 11: 1010. https://doi.org/10.3390/pharmaceutics12111010
APA StyleAllaw, M., Manconi, M., Aroffu, M., Marongiu, F., Porceddu, M., Bacchetta, G., Usach, I., Rached, R. A., Rajha, H. N., Maroun, R. G., Pedraz, J. L., Lopez-Mendez, T. B., Fadda, A. M., & Manca, M. L. (2020). Extraction, Characterization and Incorporation of Hypericum scruglii Extract in Ad Hoc Formulated Phospholipid Vesicles Designed for the Treatment of Skin Diseases Connected with Oxidative Stress. Pharmaceutics, 12(11), 1010. https://doi.org/10.3390/pharmaceutics12111010












