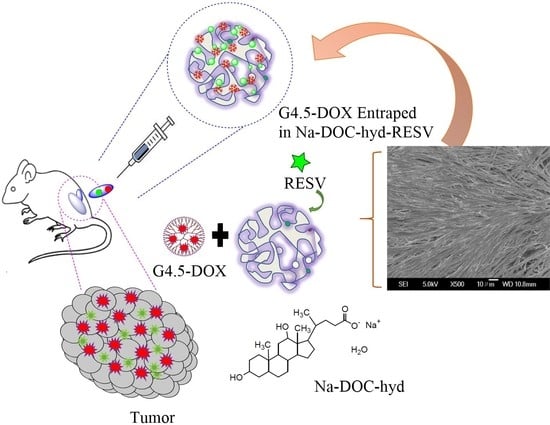
Bioinspired Composite, pH-Responsive Sodium Deoxycholate Hydrogel and Generation 4.5 Poly(amidoamine) Dendrimer Improves Cancer Treatment Efficacy via Doxorubicin and Resveratrol Co-Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of In Situ Sodium Deoxycholate Gelling Systems
2.3. Characterization Na-DOC-Hyd
2.3.1. Rheological Study of Na-DOC-Hyd
2.3.2. Studies on Na-DOC-Hyd Morphology
2.3.3. Na-DOC-Hyd X-ray Diffraction Studies
2.3.4. Na-DOC-Hyd Fourier Transform Infrared (FTIR) Studies
2.3.5. Swelling Ratio of Na-DOC-Hyd
2.3.6. Degradation of Na-DOC-Hyd
2.4. Drug Loading and Releasing Studies
2.4.1. Doxorubicin Loading and Releasing of G4.5 PAMAM Dendrimer
2.4.2. Co-Encapsulation of RESV and G4.5-DOX in Na-DOC-Hyd
2.5. Drug Release Studies of G4.5-DOX and Na-DOC-Hyd-RESV+G4.5-DOX
2.6. In Vitro Cytotoxicity Test (MTT Assay)
2.7. Cellular Uptake Studies
2.8. Animal Experiment
2.9. Histopathological Studies
2.10. Statistical Analysis
3. Results and Discussions
3.1. Preparation and Characterizations of Na-DOC-Hyd
3.1.1. Rheological Study of the Na-DOC-Hydrogel
3.1.2. Microstructural Study of Na-DOC-Hyd Using XRD
3.1.3. Assessment of Hydrogen Bonding in the Gelation of Na-DOC Using FTIR
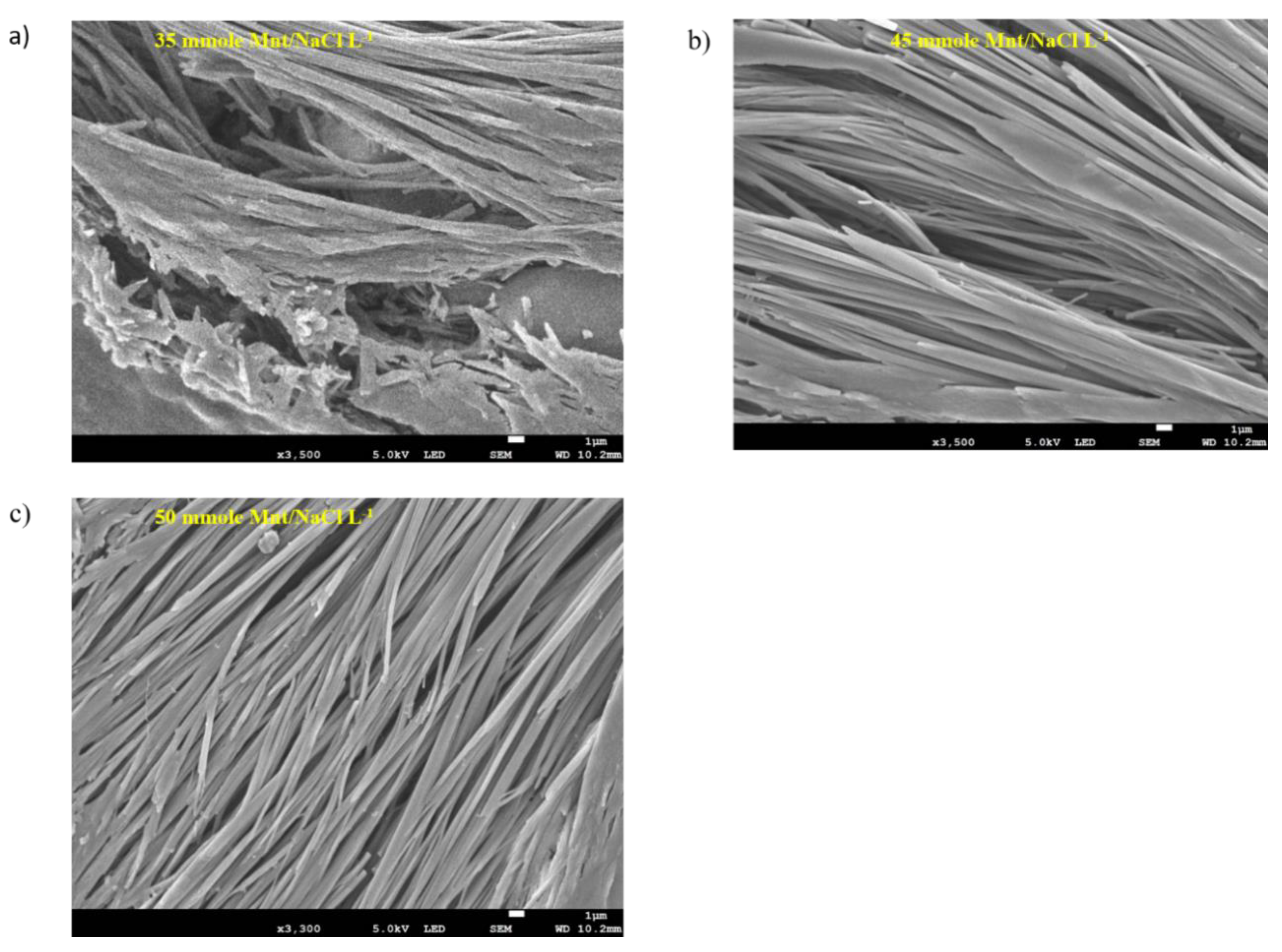
3.1.4. Na-DOC-Hyd Morphology Using FESEM
3.1.5. Swelling Study of Na-DOC-Hyd
3.1.6. Degradation of Na-DOC-Hyd
3.2. Drug Loading and Release Studies
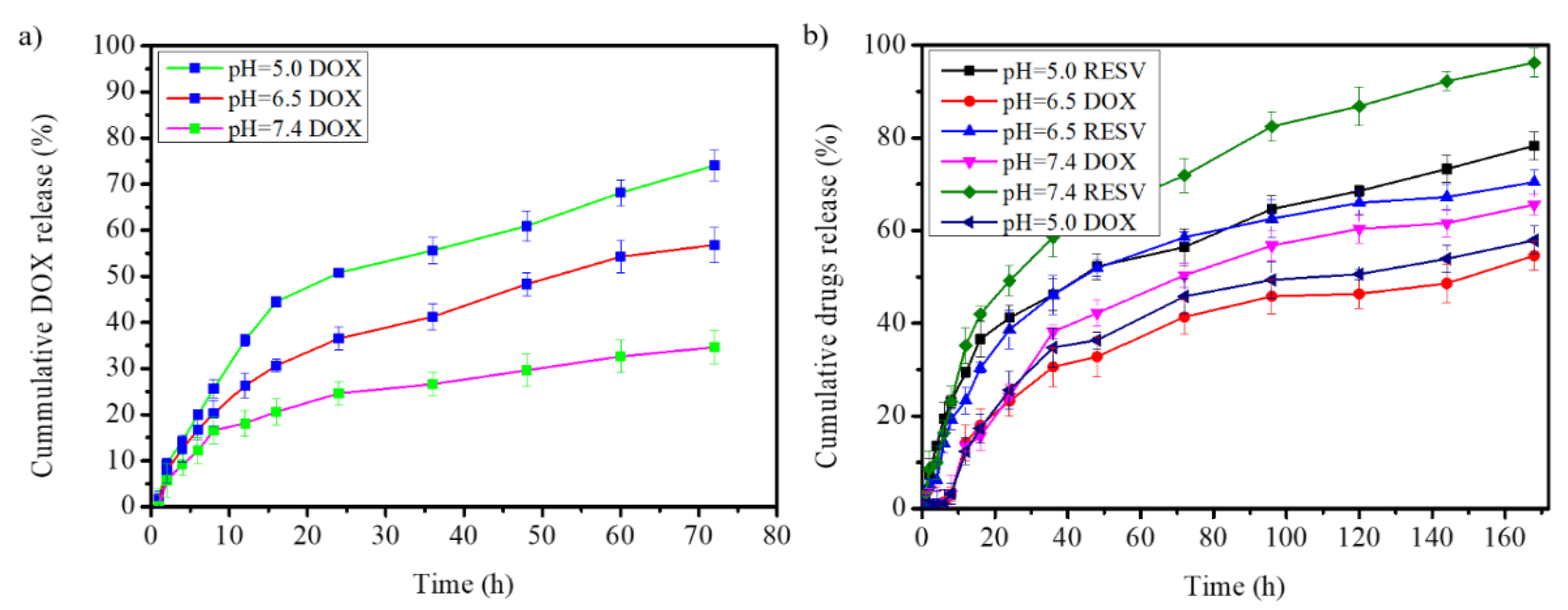
3.2.1. Doxorubicin Loading and Releasing from G4.5 PAMAM Dendrimer
3.2.2. Drugs Release from Na-DOC-Hyd-RESV+G4.5-DOX
3.3. In Vitro Cytotoxicity Test (MTT Assay)
3.4. Cellular Uptake Studies
3.5. In Vivo Antitumor Study of Na-DOC-Hyd-RESV+G4.5-DOX
3.6. Histological Analysis of the Internal Organs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Da Silva, C.; Camps, M.G.; Li, T.M.; Zerrillo, L.; Löwik, C.W.; Ossendorp, F.; Cruz, L.J. Effective chemoimmunotherapy by co-delivery of doxorubicin and immune adjuvants in biodegradable nanoparticles. Theranostics 2019, 9, 6485–6500. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Woodle, M.C.; Mixson, A.J. Advances in delivery systems for doxorubicin. J. Nanomedicien Nanotechnol. 2018, 9, 9. [Google Scholar] [CrossRef]
- Cheng, C.; Zhang, X.; Meng, Y.; Chen, L.; Zhang, Q.-Q. Development of a dual drug-loaded hydrogel delivery system for enhanced cancer therapy: In situ formation, degradation and synergistic antitumor efficiency. J. Mater. Chem. B 2017, 5, 8487–8497. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yung, B.C.; Kim, W.J.; Chen, X. Combination of nitric oxide and drug delivery systems: Tools for overcoming drug resistance in chemotherapy. J. Control. Release 2017, 263, 223–230. [Google Scholar] [CrossRef]
- Kundur, S.; Prayag, A.; Selvakumar, P.; Nguyen, H.; McKee, L.; Cruz, C.; Srinivasan, A.; Shoyele, S.; Lakshmikuttyamma, A. Synergistic anticancer action of quercetin and curcumin against triple-negative breast cancer cell lines. J. Cell. Physiol. 2018, 234, 11103–11118. [Google Scholar] [CrossRef]
- Meng, J.; Guo, F.; Xu, H.; Liang, W.; Wang, C.; Yang, X.-D. Combination therapy using co-encapsulated resveratrol and paclitaxel in liposomes for drug resistance reversal in breast cancer cells in vivo. Sci. Rep. 2016, 6, 22390. [Google Scholar] [CrossRef]
- Song, Y.-F.; Liu, D.-Z.; Cheng, Y.; Liu, M.; Ye, W.-L.; Zhang, B.-L.; Liu, X.-Y.; Zhou, S.-Y. Dual subcellular compartment delivery of doxorubicin to overcome drug resistant and enhance antitumor activity. Sci. Rep. 2015, 5, 16125. [Google Scholar] [CrossRef]
- Al-Harthi, S.E.; AlArabi, O.M.; Ramadan, W.S.; Alaama, M.N.; Al-Kreathy, H.M.; Damanhouri, Z.A.; Khan, L.M.; Osman, A.-M.M. Amelioration of doxorubicin-induced cardiotoxicity by resveratrol. Mol. Med. Rep. 2014, 10, 1455–1460. [Google Scholar] [CrossRef] [Green Version]
- Remesh, A. Toxicities of anticancer drugs and its management. Int. J. Basic Clin. Pharmacol. 2012, 1, 2–12. [Google Scholar] [CrossRef] [Green Version]
- Pugazhendhi, A.; Edison, T.N.J.I.; Velmurugan, B.K.; Jacob, J.A.; Karuppusamy, I. Toxicity of Doxorubicin (Dox) to different experimental organ systems. Life Sci. 2018, 200, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, C.; Wang, W.; Liu, J.; Liu, Q.; Huang, F.; Chu, L.; Gao, H.; Li, C.; Kong, D.; et al. Co-delivery of doxorubicin and curcumin by pH-sensitive prodrug nanoparticle for combination therapy of cancer. Sci. Rep. 2016, 6, 21225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolat, Z.B.; Islek, Z.; Demir, B.N.; Yilmaz, E.N.; Sahin, F.; Ucisik, M.H. Curcumin- and Piperine-loaded emulsomes as combinational treatment approach enhance the anticancer activity of Curcumin on HCT116 colorectal cancer model. Front. Bioeng. Biotechnol. 2020, 8, 50. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.H.; Shin, Y.J.; Won, A.J.; Lee, B.M.; Choi, W.S.; Jung, J.H.; Chung, H.Y.; Kim, H.S. Resveratrol enhances chemosensitivity of doxorubicin in multidrug-resistant human breast cancer cells via increased cellular influx of doxorubicin. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2014, 1840, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Fu, L.; Huang, J.; Dai, Y.; Wang, B.; Xu, G.; Wu, L.; Zhou, H. Curcumin reverses doxorubicin resistance via inhibition the efflux function of ABCB4 in doxorubicin-resistant breast cancer cells. Mol. Med. Rep. 2019, 19, 5162–5168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, G.; Mishra, S.; Suman, S.; Shukla, Y. Resveratrol improves the anticancer effects of doxorubicin in vitro and in vivo models: A mechanistic insight. Phytomedicine 2016, 23, 233–242. [Google Scholar] [CrossRef]
- Oktem, G.; Uysal, A.; Oral, O.; Sezer, E.D.; Olukman, M.; Erol, A.; Akgur, S.A.; Bilir, A. Resveratrol attenuates doxorubicin-induced cellular damage by modulating nitric oxide and apoptosis. Exp. Toxicol. Pathol. 2012, 64, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.-H.; Sethi, G.; Um, J.-Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The role of resveratrol in cancer therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef] [Green Version]
- Vervandier-Fasseur, D.; Latruffe, N. The potential use of resveratrol for cancer prevention. Molecules 2019, 24, 4506. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.; Wu, X.-N.; Chen, J.; Wang, W.-X.; Lu, Z.-F. Resveratrol reverses multidrug resistance in human breast cancer doxorubicin-resistant cells. Exp. Ther. Med. 2014, 7, 1611–1616. [Google Scholar] [CrossRef]
- Abedi-Gaballu, F.; Dehghan, G.; Ghaffari, M.; Yekta, R.; Abbaspour-Ravasjani, S.; Baradaran, B.; Dolatabadi, J.E.N.; Hamblin, M.R. PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Appl. Mater. Today 2018, 12, 177–190. [Google Scholar] [CrossRef]
- Chauhan, A.S. Dendrimers for Drug Delivery. Molecules 2018, 23, 938–946. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Liang, H.; Liu, J.; Wang, Z. Poly (amidoamine) (PAMAM) dendrimer mediated delivery of drug and pDNA/siRNA for cancer therapy. Int. J. Pharm. 2018, 546, 215–225. [Google Scholar] [CrossRef]
- Bastien, E.; Schneider, R.; Hackbarth, S.; Dumas, D.; Jasniewski, J.; Röder, B.; Bezdetnaya, L.; Lassalle, H.-P. PAMAM G4.5-chlorin e6 dendrimeric nanoparticles for enhanced photodynamic effects. Photochem. Photobiol. Sci. 2015, 14, 2203–2212. [Google Scholar] [CrossRef]
- Fakhari, A.; Subramony, J.A. Engineered in-situ depot-forming hydrogels for intratumoral drug delivery. J. Control. Release 2015, 220, 465–475. [Google Scholar] [CrossRef]
- Li, X.; Fan, R.; Wang, Y.; Wu, M.; Tong, A.; Shi, J.; Xiang, M.; Zhou, L.; Guo, G. In situ gel-forming dual drug delivery system for synergistic combination therapy of colorectal peritoneal carcinomatosis. RSC Adv. 2015, 5, 101494–101506. [Google Scholar] [CrossRef]
- Mendonça, P.V.; Matos, A.; Sousa, A.F.; Serra, A.C.; Simões, S.; Coelho, J.F.J. Increasing the bile acid sequestration performance of cationic hydrogels by using an advanced/controlled polymerization technique. Pharm. Res. 2017, 34, 1934–1943. [Google Scholar] [CrossRef]
- Stojančević, M.; Pavlović, N.; Golocorbin-Kon, S.; Mikov, M. Application of bile acids in drug formulation and delivery. Front. Life Sci. 2013, 7, 112–122. [Google Scholar] [CrossRef]
- Pavlović, N.; Goločorbin-Kon, S.; Ðanić, M.; Stanimirov, B.; Al-Salami, H.; Stankov, K.; Mikov, M. Bile acids and their derivatives as potential modifiers of drug release and pharmacokinetic profiles. Front. Pharmacol. 2018, 9, 1283. [Google Scholar] [CrossRef]
- Cunningham, A.J.; Zhu, X. Polymers made of bile acids: From soft to hard biomaterials. Can. J. Chem. 2016, 94, 659–666. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Wang, R.; Shang, Y.; Liu, H. Effects of polymers on the properties of hydrogels constructed using sodium deoxycholate and amino acid. RSC Adv. 2018, 8, 8699–8708. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xin, X.; Shen, J.; Tang, W.; Ren, Y.; Wang, L. Biodegradable, multiple stimuli-responsive sodium deoxycholate–amino acids–NaCl mixed systems for dye delivery. RSC Adv. 2014, 4, 62262–62271. [Google Scholar] [CrossRef]
- Maity, M.; Maitra, U. Supramolecular gels from conjugates of bile acids and amino acids and their applications. Eur. J. Org. Chem. 2017, 2017, 1713–1720. [Google Scholar] [CrossRef]
- Wei, X.; Gong, C.; Gou, M.; Fu, S.; Guo, Q.; Shi, S.; Luo, F.; Guo, G.; Qiu, L.; Qian, Z. Biodegradable poly(epsilon-caprolactone)-poly(ethylene glycol) copolymers as drug delivery system. Int. J. Pharm. 2009, 381, 1–18. [Google Scholar] [CrossRef]
- Wu, J.; Sailor, M.J. Chitosan hydrogel-capped porous SiO2 as a pH responsive nano-valve for triggered release of insulin. Adv. Funct. Mater. 2009, 19, 733–741. [Google Scholar] [CrossRef]
- Addisu, K.D.; Hsu, W.-H.; Hailemeskel, B.Z.; Andrgie, A.T.; Chou, H.-Y.; Yuh, C.-H.; Lai, J.-Y.; Tsai, H.-C. Mixed lanthanide oxide nanoparticles coated with alginate-polydopamine as multifunctional nanovehicles for dual modality: Targeted imaging and chemotherapy. ACS Biomater. Sci. Eng. 2019, 5, 5453–5469. [Google Scholar] [CrossRef]
- Hailemeskel, B.Z.; Hsu, W.-H.; Addisu, K.D.; Andrgie, A.T.; Chou, H.-Y.; Lai, J.-Y.; Tsai, H.-C. Diselenide linkage containing triblock copolymer nanoparticles based on Bi(methoxyl poly(ethylene glycol))-poly(epsilon-carprolactone): Selective intracellular drug delivery in cancer cells. Mater. Sci. Eng. C 2019, 103, 109803. [Google Scholar] [CrossRef]
- Hanurry, E.Y.; Mekonnen, T.W.; Andrgie, A.T.; Darge, H.F.; Birhan, Y.S.; Hsu, W.-H.; Chou, H.-Y.; Cheng, C.-C.; Lai, J.-Y.; Tsai, H.-C. Biotin-Decorated PAMAM G4.5 dendrimer nanoparticles to enhance the delivery, anti-proliferative, and apoptotic effects of chemotherapeutic drug in cancer cells. Pharmaceutics 2020, 12, 443. [Google Scholar] [CrossRef]
- Mekonnen, T.W.; Birhan, Y.S.; Andrgie, A.T.; Hanurry, E.Y.; Darge, H.F.; Chou, H.-Y.; Lai, J.-Y.; Tsai, H.-C.; Yang, J.M.; Chang, Y.-H. Encapsulation of gadolinium ferrite nanoparticle in generation 4.5 poly(amidoamine) dendrimer for cancer theranostics applications using low frequency alternating magnetic field. Colloids Surf. B Biointerfaces 2019, 184, 110531. [Google Scholar] [CrossRef]
- Jiang, L.; Ding, Y.; Xue, X.; Zhou, S.; Li, C.; Zhang, X.; Jiang, X. Entrapping multifunctional dendritic nanoparticles into a hydrogel for local therapeutic delivery and synergetic immunochemotherapy. Nano Res. 2018, 11, 6062–6073. [Google Scholar] [CrossRef]
- Hanurry, E.Y.; Hsu, W.-H.; Darge, H.F.; Birhan, Y.S.; Mekonnen, T.W.; Andrgie, A.T.; Chou, H.-Y.; Cheng, C.-C.; Lai, J.-Y.; Tsai, H.-C. In vitro siRNA delivery via diethylenetriamine- and tetraethylenepentamine-modified carboxyl group-terminated Poly(amido)amine generation 4.5 dendrimers. Mater. Sci. Eng. C 2020, 106, 110245. [Google Scholar] [CrossRef]
- Darge, H.F.; Andrgie, A.T.; Hanurry, E.Y.; Birhan, Y.S.; Mekonnen, T.W.; Chou, H.-Y.; Hsu, W.-H.; Lai, J.-Y.; Lin, S.-Y.; Tsai, H.-C. Localized controlled release of bevacizumab and doxorubicin by thermo-sensitive hydrogel for normalization of tumor vasculature and to enhance the efficacy of chemotherapy. Int. J. Pharm. 2019, 572, 118799. [Google Scholar] [CrossRef]
- Shokry, D.; Waters, L.J.; Parkes, G.M.; Mitchell, J.C.; Snowden, M.J. Formation of a bile salt-drug hydrogel to predict human intestinal absorption. J. Pharm. Sci. 2019, 108, 279–287. [Google Scholar] [CrossRef] [Green Version]
- Coello, A.; Meijide, F.; Núñez, E.R.; Tato, J.V. Aggregation behavior of bile salts in aqueous solution. J. Pharm. Sci. 1996, 85, 9–15. [Google Scholar] [CrossRef]
- McNeel, K.E.; Das, S.; Siraj, N.; Negulescu, I.I.; Warner, I.M. Sodium deoxycholate hydrogels: Effects of modifications on gelation, drug release, and nanotemplating. J. Phys. Chem. B 2015, 119, 8651–8659. [Google Scholar] [CrossRef]
- Sun, X.; Zhiping, D.; Li, E.; Xin, X.; Tang, N.; Wang, L.; Yuan, J. Rheological properties of the gels of biological surfactant sodium deoxycholate/amino acids/halide salts systems. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 345–353. [Google Scholar] [CrossRef]
- Zhang, M.; Waldron, K.C.; Zhu, X. Formation of molecular hydrogels from a bile acid derivative and selected carboxylic acids. RSC Adv. 2016, 6, 35436–35440. [Google Scholar] [CrossRef]
- Xu, L.; Xu, G.; Liu, T.; Chen, Y.; Gong, H. The comparison of rheological properties of aqueous welan gum and xanthan gum solutions. Carbohydr. Polym. 2013, 92, 516–522. [Google Scholar] [CrossRef]
- Yan, C.; Pochan, D.J. Rheological properties of peptide-based hydrogels for biomedical and other applications. Chem. Soc. Rev. 2010, 39, 3528–3540. [Google Scholar] [CrossRef] [Green Version]
- McNeel, K.E.; Siraj, N.; Negulescu, I.; Warner, I.M. Sodium deoxycholate/TRIS-based hydrogels for multipurpose solute delivery vehicles: Ambient release, drug release, and enantiopreferential release. Talanta 2018, 177, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Tanaka, S.; Ogura, A.; Akashi, M. Tough, thin hydrogel membranes with giant crystalline domains composed of precisely synthesized macromolecules. Macromolecules 2005, 38, 4861–4867. [Google Scholar] [CrossRef]
- Nurunnabi, M.; Khatun, Z.; Revuri, V.; Nafiujjaman, M.; Cha, S.; Cho, S.; Huh, K.M.; Lee, Y.-K. Design and strategies for bile acid mediated therapy and imaging. RSC Adv. 2016, 6, 73986–74002. [Google Scholar] [CrossRef]
- Wang, N.; Hao, J. Self-assembly fibrillar network gels of simple surfactants in organic solvents. Langmuir 2011, 27, 1713–1717. [Google Scholar] [CrossRef]
- Yuan, Z.; Lu, W.; Liu, W.; Hao, J. Gel phase originating from molecular quasi-crystallization and nanofiber growth of sodium laurate–water system. Soft Matter 2008, 4, 1639. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 1–17. [Google Scholar] [CrossRef]
- Serrano-Aroca, Á. Enhancement of hydrogels’ properties for biomedical applications: Latest achievements. In Hydrogels; Intech Open Limited: London, UK, 2018. [Google Scholar]
- Bogdanova, L.R.; Gnezdilov, O.I.; Idiyatullin, B.Z.; Kurbanov, R.K.; Zuev, Y.F.; Us’Yarov, O.G. Micellization in sodium deoxycholate solutions. Colloid J. 2012, 74, 1–6. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Li, J.; Yang, B.; Wang, C.; Fang, W.; Ji, F.; Wen, Y.; Yao, F. Stable and pH-responsive polyamidoamine based unimolecular micelles capped with a zwitterionic polymer shell for anticancer drug delivery. RSC Adv. 2016, 6, 17728–17739. [Google Scholar] [CrossRef]
- Chen, J.-M.; Bai, J.-Y.; Yang, K.-X. Effect of resveratrol on doxorubicin resistance in breast neoplasm cells by modulating PI3K/Akt signaling pathway. IUBMB Life 2018, 70, 491–500. [Google Scholar] [CrossRef] [Green Version]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Aluyen, J.K.; Ton, Q.N.; Tran, T.; Yang, A.E.; Gottlieb, H.B.; Bellanger, R.A. Resveratrol: Potential as anticancer agent. J. Diet. Suppl. 2012, 9, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Motevalli, S.M.; Eltahan, A.S.; Liu, L.; Magrini, A.; Rosato, N.; Guo, W.; Bottini, M.; Liang, X.-J. Co-encapsulation of curcumin and doxorubicin in albumin nanoparticles blocks the adaptive treatment tolerance of cancer cells. Biophys. Rep. 2019, 5, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Taymaz-Nikerel, H.; Karabekmez, M.E.; Eraslan, S.; Kırdar, B. Doxorubicin induces an extensive transcriptional and metabolic rewiring in yeast cells. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharmacogenet. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef]
- Mahbub, A.A.; Le Maitre, C.L.; Haywood-Small, S.L.; Cross, N.; Jordanmahy, N. Polyphenols act synergistically with doxorubicin and etoposide in leukaemia cell lines. Cell Death Discov. 2015, 1, 15043. [Google Scholar] [CrossRef]
- Stella, G.; Kolling, S.; Benvenuti, S.; Bortolotto, C. Lung-seeking metastases. Cancers 2019, 11, 1010. [Google Scholar] [CrossRef] [Green Version]
- Zacharias, M.; Brcic, L.; Eidenhammer, S.; Popper, H. Bulk tumour cell migration in lung carcinomas might be more common than epithelial-mesenchymal transition and be differently regulated. BMC Cancer 2018, 18, 717. [Google Scholar] [CrossRef] [Green Version]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mekonnen, T.W.; Andrgie, A.T.; Darge, H.F.; Birhan, Y.S.; Hanurry, E.Y.; Chou, H.-Y.; Lai, J.-Y.; Tsai, H.-C.; Yang, J.M.; Chang, Y.-H. Bioinspired Composite, pH-Responsive Sodium Deoxycholate Hydrogel and Generation 4.5 Poly(amidoamine) Dendrimer Improves Cancer Treatment Efficacy via Doxorubicin and Resveratrol Co-Delivery. Pharmaceutics 2020, 12, 1069. https://doi.org/10.3390/pharmaceutics12111069
Mekonnen TW, Andrgie AT, Darge HF, Birhan YS, Hanurry EY, Chou H-Y, Lai J-Y, Tsai H-C, Yang JM, Chang Y-H. Bioinspired Composite, pH-Responsive Sodium Deoxycholate Hydrogel and Generation 4.5 Poly(amidoamine) Dendrimer Improves Cancer Treatment Efficacy via Doxorubicin and Resveratrol Co-Delivery. Pharmaceutics. 2020; 12(11):1069. https://doi.org/10.3390/pharmaceutics12111069
Chicago/Turabian StyleMekonnen, Tefera Worku, Abegaz Tizazu Andrgie, Haile Fentahun Darge, Yihenew Simegniew Birhan, Endiries Yibru Hanurry, Hsiao-Ying Chou, Juin-Yih Lai, Hsieh-Chih Tsai, Jen Ming Yang, and Yen-Hsiang Chang. 2020. "Bioinspired Composite, pH-Responsive Sodium Deoxycholate Hydrogel and Generation 4.5 Poly(amidoamine) Dendrimer Improves Cancer Treatment Efficacy via Doxorubicin and Resveratrol Co-Delivery" Pharmaceutics 12, no. 11: 1069. https://doi.org/10.3390/pharmaceutics12111069
APA StyleMekonnen, T. W., Andrgie, A. T., Darge, H. F., Birhan, Y. S., Hanurry, E. Y., Chou, H.-Y., Lai, J.-Y., Tsai, H.-C., Yang, J. M., & Chang, Y.-H. (2020). Bioinspired Composite, pH-Responsive Sodium Deoxycholate Hydrogel and Generation 4.5 Poly(amidoamine) Dendrimer Improves Cancer Treatment Efficacy via Doxorubicin and Resveratrol Co-Delivery. Pharmaceutics, 12(11), 1069. https://doi.org/10.3390/pharmaceutics12111069