Pequi (Caryocar brasiliense Cambess)-Loaded Nanoemulsion, Orally Delivered, Modulates Inflammation in LPS-Induced Acute Lung Injury in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pequi Oil (Caryocar brasiliense Cambess) Extraction and Characterization
2.2. Preparation of Nanoemulsion Formulations
2.3. Physicochemical Characterization of the Formulations
2.4. Animals
2.5. Lipopolysaccharide(LPS)-Induced Pulmonary Disorder Model and Treatment Protocol
2.6. Assessment of Pulmonary Function and Airway Hyper-Reactivity (AHR)
2.7. Inflammatory Cell Analysis in the Airway Lumen
2.8. Quantification of Myeloperoxidase (MPO) in Pulmonary Tissue
2.9. Quantification of Inflammatory Mediators
2.10. Oxidative Stress Analysis
2.11. Statistical Analysis
3. Results
3.1. Physicochemical Characterization of the Nanoemulsion Containing Pequi Oil
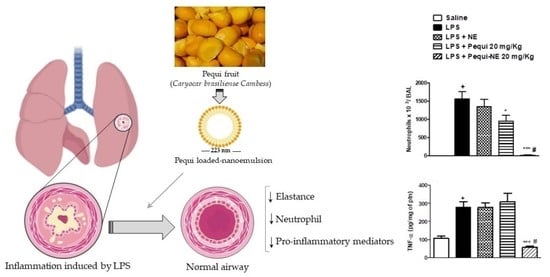
3.2. The Effect of Pequi Oil or Pequi-Loaded Nanoemulsion Treatment on LPS-Induced Pulmonary Inflammation
3.3. The Effect of Pequi Oil or Pequi-Loaded Nanoemulsion Treatment on LPS-Induced Pro-Inflammatory Cytokine Production
3.4. Effect of Pequi Oil or Pequi-Loaded Nanoemulsion Treatment on LPS-Induced Airway Hyper-Reactivity
3.5. The Effect of Pequi-Loaded Nanoemulsion or Oleic Acid-Loaded Nanoemulsion Treatment on LPS-Induced Airway Hyper-Reactivity and Pulmonary Inflammation
3.6. Effect of Pequi-Loaded Nanoemulsion or Oleic Acid-Loaded Nanoemulsion Treatment on Pulmonary Oxidative Markers Induced by LPS Exposure
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baradaran Rahimi, V.; Rakhshandeh, H.; Raucci, F.; Buono, B.; Shirazinia, R.; Samzadeh Kermani, A.; Maione, F.; Mascolo, N.; Askari, V.R. Anti-Inflammatory and Anti-Oxidant Activity of Portulaca oleracea Extract on LPS-Induced Rat Lung Injury. Molecules 2019, 24, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, L.; Zhao, X.; Zhang, H. HIPK1 Interference Attenuates Inflammation and Oxidative Stress of Acute Lung Injury via Autophagy. Med Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [Green Version]
- Biazotto, K.R.; de Souza Mesquita, L.M.; Neves, B.V.; Braga, A.R.C.; Tangerina, M.M.P.; Vilegas, W.; Mercadante, A.Z.; De Rosso, V.V. Brazilian Biodiversity Fruits: Discovering Bioactive Compounds from Underexplored Sources. J. Agric. Food Chem. 2019, 67, 1860–1876. [Google Scholar] [CrossRef]
- Roll, M.M.; Miranda-Vilela, A.L.; Longo, J.P.F.; Agostini-Costa, T.D.S.; Grisolia, C.K. The pequi pulp oil (Caryocar brasiliense Camb.) provides protection against aging-related anemia, inflammation and oxidative stress in Swiss mice, especially in females. Genet. Mol. Biol. 2018, 41, 858–869. [Google Scholar] [CrossRef] [Green Version]
- Traesel, G.K.; de Araujo, F.H.S.; Castro, L.H.A.; de Lima, F.F.; Menegati, S.; Justi, P.N.; Kassuya, C.A.L.; Cardoso, C.A.L.; Argandona, E.J.S.; Oesterreich, S.A. Safety Assessment of Oil from Pequi (Caryocar brasiliense Camb.): Evaluation of the Potential Genotoxic and Clastogenic Effects. J. Med. Food 2017, 20, 804–811. [Google Scholar] [CrossRef]
- Guedes, A.M.M.; Antoniassi, R.; Galdeano, M.C.; Grimaldi, R.; Carvalho, M.G.; Wilhelm, A.E.; Marangoni, A.G. Length-scale Specific Crystalline Structural Changes Induced by Molecular Randomization of Pequi Oil. J. Oleo. Sci. 2017, 66, 469–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pires, J.; Cargnin, S.T.; Costa, S.A.; Sinhorin, V.D.G.; Damazo, A.S.; Sinhorin, A.P.; Bicudo, R.C.; Cavalheiro, L.; Valladao, D.M.S.; Pohlmann, A.R.; et al. Healing of dermal wounds property of Caryocar brasiliense oil loaded polymeric lipid-core nanocapsules: Formulation and in vivo evaluation. Eur. J. Pharm. Sci. 2020, 150, 105356. [Google Scholar] [CrossRef]
- Silveira Serra, D.; Matias de Sousa, A.; Costa da Silva Andrade, L.; de Lima Gondim, F.; Evangelista de Avila Dos Santos, J.; Moura de Oliveira, M.L.; Torres Avila Pimenta, A. Effects of fixed oil of Caryocar coriaceum Wittm. Seeds on the respiratory system of rats in a short-term secondhand-smoke exposure model. J. Ethnopharmacol. 2020, 252, 112633. [Google Scholar] [CrossRef]
- Colombo, N.B.; Rangel, M.P.; Martins, V.; Hage, M.; Gelain, D.P.; Barbeiro, D.F.; Grisolia, C.K.; Parra, E.R.; Capelozzi, V.L. Caryocar brasiliense camb protects against genomic and oxidative damage in urethane-induced lung carcinogenesis. Braz. J. Med. Biol. Res. 2015, 48, 852–862. [Google Scholar] [CrossRef] [Green Version]
- Rathore, B.; Sunwoo, K.; Jangili, P.; Kim, J.; Kim, J.H.; Huang, M.; Xiong, J.; Sharma, A.; Yang, Z.; Qu, J.; et al. Nanomaterial designing strategies related to cell lysosome and their biomedical applications: A review. Biomaterials 2019, 211, 25–47. [Google Scholar] [CrossRef]
- Zahin, N.; Anwar, R.; Tewari, D.; Kabir, M.T.; Sajid, A.; Mathew, B.; Uddin, M.S.; Aleya, L.; Abdel-Daim, M.M. Nanoparticles and its biomedical applications in health and diseases: Special focus on drug delivery. Environ. Sci. Pollut. Res. 2019, 27, 19151–19168. [Google Scholar] [CrossRef]
- Barik, S.K.; Singh, B.N. Nanoemulsion-loaded hydrogel coatings for inhibition of bacterial virulence and biofilm formation on solid surfaces. Sci. Rep. 2019, 9, 6520. [Google Scholar] [CrossRef] [PubMed]
- Long, P.; Zhang, Q.; Xue, M.; Cao, G.; Li, C.; Chen, W.; Jin, F.; Li, Z.; Li, R.; Wang, X.; et al. Tomato lectin-modified nanoemulsion-encapsulated MAGE1-HSP70/SEA complex protein vaccine: Targeting intestinal M cells following peroral administration. Biomed. Pharmacother. 2019, 115, 108886. [Google Scholar] [CrossRef]
- Medeiros-de-Moraes, I.M.; Goncalves-de-Albuquerque, C.F.; Kurz, A.R.M.; Oliveira, F.M.J.; de Abreu, V.H.P.; Torres, R.C.; Carvalho, V.F.; Estato, V.; Bozza, P.T.; Sperandio, M.; et al. Omega-9 Oleic Acid, the Main Compound of Olive Oil, Mitigates Inflammation during Experimental Sepsis. Oxid. Med. Cell Longev. 2018, 2018, 6053492. [Google Scholar] [CrossRef] [Green Version]
- Bouchemal, K.; Briancon, S.; Perrier, E.; Fessi, H. Nano-emulsion formulation using spontaneous emulsification: Solvent, oil and surfactant optimisation. Int. J. Pharm. 2004, 280, 241–251. [Google Scholar] [CrossRef]
- de Oliveira, M.T.P.; de Sa Coutinho, D.; Tenorio de Souza, E.; Staniscuaski Guterres, S.; Pohlmann, A.R.; Silva, P.M.R.; Martins, M.A.; Bernardi, A. Orally delivered resveratrol-loaded lipid-core nanocapsules ameliorate LPS-induced acute lung injury via the ERK and PI3K/Akt pathways. Int. J. Nanomed. 2019, 14, 5215–5228. [Google Scholar] [CrossRef] [Green Version]
- Coutinho, D.S.; Anjos-Valotta, E.A.; do Nascimento, C.V.M.F.; Pires, A.L.A.; Napimoga, M.H.; Carvalho, V.F.; Torres, R.C.; e Silva, P.M.R.; Martins, M.A. 15-Deoxy-Delta-12,14-Prostaglandin J2 Inhibits Lung Inflammation and Remodeling in Distinct Murine Models of Asthma. Front. Immunol. 2017, 8, 740. [Google Scholar] [CrossRef] [Green Version]
- Amaral, L.F.; Moriel, P.; Foglio, M.A.; Mazzola, P.G. Caryocar brasiliense supercritical CO2 extract possesses antimicrobial and antioxidant properties useful for personal care products. BMC. Complement. Altern. Med. 2014, 14, 73. [Google Scholar] [CrossRef] [Green Version]
- Jorge, A.; Leitao, M.M.; Bernal, L.P.T.; Dos Santos, E.; Kuraoka-Oliveira, A.M.; Justi, P.; Argandona, E.; Kassuya, C.A.L. Analgesic and anti-inflammatory effects of Caryocar brasiliense. Antiinflamm Antiallergy Agents Med. Chem. 2019, 19, 313–322. [Google Scholar] [CrossRef]
- Torres, L.R.; Santana, F.C.; Torres-Leal, F.L.; Melo, I.L.; Yoshime, L.T.; Matos-Neto, E.M.; Seelaender, M.C.; Araujo, C.M.; Cogliati, B.; Mancini-Filho, J. Pequi (Caryocar brasiliense Camb.) almond oil attenuates carbon tetrachloride-induced acute hepatic injury in rats: Antioxidant and anti-inflammatory effects. Food Chem. Toxicol. 2016, 97, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.R.; Piazzini, V.; Guccione, C.; Risaliti, L.; Asprea, M.; Capecchi, G.; Bergonzi, M.C. Improving on Nature: The Role of Nanomedicine in the Development of Clinical Natural Drugs. Planta. Med. 2017, 83, 366–381. [Google Scholar] [CrossRef] [Green Version]
- Frank, L.A.; Contri, R.V.; Beck, R.C.; Pohlmann, A.R.; Guterres, S.S. Improving drug biological effects by encapsulation into polymeric nanocapsules. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 623–639. [Google Scholar] [CrossRef]
- Fiel, L.A.; Rebêlo, L.M.; de Melo Santiago, T.; Adorne, M.D.; Guterres, S.S.; Soares de Sousa, J.; Pohlmann, A.R. Diverse deformation properties of polymeric nanocapsules and lipid-core nanocapsules. Soft Matter 2011, 7, 7240–7247. [Google Scholar] [CrossRef]
- Korkmaz, B.; Jenne, D.E.; Gauthier, F. Relevance of the mouse model as a therapeutic approach for neutrophil proteinase 3-associated human diseases. Int. Immunopharmacol. 2013, 17, 1198–1205. [Google Scholar] [CrossRef]
- Gibbs, D.F.; Shanley, T.P.; Warner, R.L.; Murphy, H.S.; Varani, J.; Johnson, K.J. Role of Matrix Metalloproteinases in Models of Macrophage-Dependent Acute Lung Injury. Am. J. Respir. Cell Mol. Biol. 1999, 20, 1145–1154. [Google Scholar] [CrossRef]
- de Oliveira, F.F.; de Araujo, J.C.; Pereira, A.F.; Brito, G.A.; Gondim, D.V.; Ribeiro Rde, A.; de Menezes, I.R.; Vale, M.L. Antinociceptive and anti-inflammatory effects of Caryocar coriaceum Wittm fruit pulp fixed ethyl acetate extract on zymosan-induced arthritis in rats. J. Ethnopharmacol. 2015, 174, 452–463. [Google Scholar] [CrossRef]
- Davies, M.J. Myeloperoxidase-derived oxidation: Mechanisms of biological damage and its prevention. J. Clin. Biochem. Nutr. 2011, 48, 8–19. [Google Scholar] [CrossRef] [Green Version]
- de Figueiredo, P.R.L.; Oliveira, I.B.; Neto, J.B.S.; de Oliveira, J.A.; Ribeiro, L.B.; de Barros Viana, G.S.; Rocha, T.M.; Leal, L.; Kerntopf, M.R.; Felipe, C.F.B.; et al. Caryocar coriaceum Wittm. (Pequi) fixed oil presents hypolipemic and anti-inflammatory effects in vivo and in vitro. J. Ethnopharmacol. 2016, 191, 87–94. [Google Scholar] [CrossRef]
- Hunter, A.C.; Elsom, J.; Wibroe, P.P.; Moghimi, S.M. Polymeric particulate technologies for oral drug delivery and targeting: A pathophysiological perspective. Nanomedicine 2012, 8 (Suppl. 1), 5–20. [Google Scholar] [CrossRef]
- Tang, J.; Xu, N.; Ji, H.; Liu, H.; Wang, Z.; Wu, L. Eudragit nanoparticles containing genistein: Formulation, development, and bioavailability assessment. Int. J. Nanomed. 2011, 6, 2429–2435. [Google Scholar] [CrossRef] [Green Version]
- Nehoff, H.; Parayath, N.N.; Domanovitch, L.; Taurin, S.; Greish, K. Nanomedicine for drug targeting: Strategies beyond the enhanced permeability and retention effect. Int. J. Nanomed. 2014, 9, 2539–2555. [Google Scholar] [CrossRef] [Green Version]
- Paris, J.L.; de la Torre, P.; Victoria Cabanas, M.; Manzano, M.; Grau, M.; Flores, A.I.; Vallet-Regi, M. Vectorization of ultrasound-responsive nanoparticles in placental mesenchymal stem cells for cancer therapy. Nanoscale 2017, 9, 5528–5537. [Google Scholar] [CrossRef] [Green Version]
- Singh, I.; Pinsky, M.R. Chapter 15, Heart-Lung Interactions. In Mechanical Ventilation; Papadakos, P.J., Lachmann, B., Visser-Isles, L., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2008; pp. 173–184. [Google Scholar]
- Crimi, E.; Spanevello, A.; Neri, M.; Ind, P.W.; Rossi, G.A.; Brusasco, V. Dissociation between airway inflammation and airway hyperresponsiveness in allergic asthma. Am. J. Respir. Crit. Care Med. 1998, 157, 4–9. [Google Scholar] [CrossRef]
- Lefort, J.; Singer, M.; Leduc, D.; Renesto, P.; Nahori, M.A.; Huerre, M.; Créminon, C.; Chignard, M.; Vargaftig, B.B. Systemic Administration of Endotoxin Induces Bronchopulmonary Hyperreactivity Dissociated from TNF-α Formation and Neutrophil Sequestration into the Murine Lungs. J. Immunol. 1998, 161, 474–480. [Google Scholar]
- Faria-Machado, A.F.; Tres, A.; van Ruth, S.M.; Antoniassi, R.; Junqueira, N.T.; Lopes, P.S.; Bizzo, H.R. Discrimination of pulp oil and kernel oil from pequi (Caryocar brasiliense) by fatty acid methyl esters fingerprinting, using GC-FID and multivariate analysis. J. Agric. Food Chem. 2015, 63, 10064–10069. [Google Scholar] [CrossRef]
- Mariano, R.G.D.B.; Couri, S.; Freitas, S.P. Enzymatic technology to improve oil extraction from Caryocar brasiliense camb. (Pequi) Pulp. Rev. Bras. Frutic. 2009, 31, 637–643. [Google Scholar] [CrossRef]
- Zhou, H.; Li, F.; Niu, J.Y.; Zhong, W.Y.; Tang, M.Y.; Lin, D.; Cui, H.H.; Huang, X.H.; Chen, Y.Y.; Wang, H.Y.; et al. Ferroptosis was involved in the oleic acid-induced acute lung injury in mice. Sheng Li Xue Bao 2019, 71, 689–697. [Google Scholar]
- Schuster, D.P. ARDS: Clinical lessons from the oleic acid model of acute lung injury. Am. J. Respir. Crit. Care Med. 1994, 149, 245–260. [Google Scholar] [CrossRef]
- Yu, H.-P.; Liu, F.-C.; Umoro, A.; Lin, Z.-C.; Elzoghby, A.O.; Hwang, T.-L.; Fang, J.-Y. Oleic acid-based nanosystems for mitigating acute respiratory distress syndrome in mice through neutrophil suppression: How the particulate size affects therapeutic efficiency. J. Nanobiotechnology 2020, 18, 25. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Lee, Y.-H.; Chang, S.-H.; Tsai, Y.-F.; Fang, J.-Y.; Hwang, T.-L. Oleic acid-loaded nanostructured lipid carrier inhibit neutrophil activities in the presence of albumin and alleviates skin inflammation. Int. J. Nanomed. 2019, 14, 6539–6553. [Google Scholar] [CrossRef] [Green Version]
- Vale, A.F.; Ferreira, H.H.; Benetti, E.J.; Rebelo, A.C.S.; Figueiredo, A.C.R.; Barbosa, E.C.; Simoes, K. Antioxidant effect of the pequi oil (Caryocar brasiliense) on the hepatic tissue of rats trained by exhaustive swimming exercises. Braz. J. Biol. 2019, 79, 257–262. [Google Scholar] [CrossRef]
- Salim, S. Oxidative stress and psychological disorders. Curr. Neuropharmacol. 2014, 12, 140–147. [Google Scholar] [CrossRef] [Green Version]
- Kellner, M.; Noonepalle, S.; Lu, Q.; Srivastava, A.; Zemskov, E.; Black, S.M. ROS Signaling in the Pathogenesis of Acute Lung Injury (ALI) and Acute Respiratory Distress Syndrome (ARDS). Adv. Exp. Med. Biol. 2017, 967, 105–137. [Google Scholar] [CrossRef]
- de Oliveira, T.S.; Thomaz, D.V.; da Silva Neri, H.F.; Cerqueira, L.B.; Garcia, L.F.; Gil, H.P.V.; Pontarolo, R.; Campos, F.R.; Costa, E.A.; Dos Santos, F.C.A.; et al. Neuroprotective Effect of Caryocar brasiliense Camb. Leaves Is Associated with Anticholinesterase and Antioxidant Properties. Oxid. Med. Cell Longev. 2018, 2018, 9842908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilar, E.C.; Jascolka, T.L.; Teixeira, L.G.; Lages, P.C.; Ribeiro, A.C.; Vieira, E.L.; Peluzio, M.C.; Alvarez-Leite, J.I. Paradoxical effect of a pequi oil-rich diet on the development of atherosclerosis: Balance between antioxidant and hyperlipidemic properties. Braz. J. Med. Biol. Res. 2012, 45, 601–609. [Google Scholar] [CrossRef] [Green Version]









| Formulations | ||||
|---|---|---|---|---|
| BNE 5 | Pequi-NE 6 | Oleic Acid-NE 7 | ||
| Laser Diffraction (LD) 1 | Mean diameter (nm) | 157 ± 0.01 | 174 ± 0.01 | 163 ± 0.02 |
| SPAN 2 | 1.13 ± 0.01 | 1.51 ± 0.03 | 1.60 ± 0.02 | |
| Dynamic Light Scattering (DLS) 3 | Z-average diameter (nm) | 186 ± 0.01 | 223 ± 0.02 | 179 ± 0.08 |
| PDI 4 | 0.09 ± 0.02 | 0.11 ± 0.01 | 0.11 ± 0.01 | |
| Zeta Potential | Zeta potential (mV) | −6.72 ± 0.13 | −7.13 ± 0.08 | −13.6 ± 0.72 |
| pH | 5.44 ± 0.27 | 5.83 ± 0.12 | 5..51 ± 0.09 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Sá Coutinho, D.; Pires, J.; Gomes, H.; Raffin Pohlmann, A.; Stanisçuaski Guterres, S.; Rodrigues e Silva, P.M.; Martins, M.A.; Ferrarini, S.R.; Bernardi, A. Pequi (Caryocar brasiliense Cambess)-Loaded Nanoemulsion, Orally Delivered, Modulates Inflammation in LPS-Induced Acute Lung Injury in Mice. Pharmaceutics 2020, 12, 1075. https://doi.org/10.3390/pharmaceutics12111075
de Sá Coutinho D, Pires J, Gomes H, Raffin Pohlmann A, Stanisçuaski Guterres S, Rodrigues e Silva PM, Martins MA, Ferrarini SR, Bernardi A. Pequi (Caryocar brasiliense Cambess)-Loaded Nanoemulsion, Orally Delivered, Modulates Inflammation in LPS-Induced Acute Lung Injury in Mice. Pharmaceutics. 2020; 12(11):1075. https://doi.org/10.3390/pharmaceutics12111075
Chicago/Turabian Stylede Sá Coutinho, Diego, Jader Pires, Hyago Gomes, Adriana Raffin Pohlmann, Sílvia Stanisçuaski Guterres, Patrícia Machado Rodrigues e Silva, Marco Aurelio Martins, Stela Regina Ferrarini, and Andressa Bernardi. 2020. "Pequi (Caryocar brasiliense Cambess)-Loaded Nanoemulsion, Orally Delivered, Modulates Inflammation in LPS-Induced Acute Lung Injury in Mice" Pharmaceutics 12, no. 11: 1075. https://doi.org/10.3390/pharmaceutics12111075
APA Stylede Sá Coutinho, D., Pires, J., Gomes, H., Raffin Pohlmann, A., Stanisçuaski Guterres, S., Rodrigues e Silva, P. M., Martins, M. A., Ferrarini, S. R., & Bernardi, A. (2020). Pequi (Caryocar brasiliense Cambess)-Loaded Nanoemulsion, Orally Delivered, Modulates Inflammation in LPS-Induced Acute Lung Injury in Mice. Pharmaceutics, 12(11), 1075. https://doi.org/10.3390/pharmaceutics12111075