Polychemotherapy with Curcumin and Doxorubicin via Biological Nanoplatforms: Enhancing Antitumor Activity
Abstract
:1. Introduction
2. Curcumin: Structure and Pharmacokinetics
3. Antitumor Activity of Curcumin: In Vitro, In Vivo and Clinical Studies
4. Curcumin in Cancer Chemotherapy: Beyond Doxorubicin
5. Doxorubicin: Cancer Resistance and Side Effects
6. Curcumin in Combination with Doxorubicin
6.1. Anticancer Effects
6.1.1. Apoptosis Induction
6.1.2. Metastasis Inhibition
6.2. Effects on Resistance
6.2.1. Potential Mechanisms of DOX Resistance
6.2.2. Curcumin in Reversing DOX Resistance
6.3. Impact on Adverse Effects
7. Codelivery via Biological Nanovehicles
8. Conclusions and Remarks
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ROS | reactive oxygen species |
| EMT | epithelial-to-mesenchymal transition |
| miRNA | microRNA |
| PI3K | phosphatidylinositol 3-kinase |
| Akt | protein kinase-B |
| NF-κB | nuclear factor-kappaB |
| HCC | hepatocellular carcinoma |
| P-gp | P-glycoprotein |
| MDR1 | multidrug resistance 1 |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| TGF-β1 | transforming growth factor-beta 1 |
| DOX | doxorubicin |
| TFF3 | trefoil factor 3 |
| SphK1 | sphingosine kinase 1 |
| OS | osteosarcoma |
| GSH | glutathione |
| SOD | superoxide dismutase |
| MDH | malondialdehyde |
| ECM | extracellular matrix |
| TIMPs | tissue inhibitors of metalloproteinases |
| EMT | epithelial-to-mesenchymal transition |
| ZnCM-SD | curcumin-Zn solid dispersion |
| ABC | ATP-binding cassette |
| ABCB4 | ABC sub-family B member 4 |
| EGCG | epigallocatechin gallate |
| S100A8 | S100 calcium-binding protein A8 |
| AP-1 | activator protein-1 |
| CAT | catalase |
| TNF-α | tumor necrosis factor-α |
| IL-1β | interleukin-1β |
| CAR | carvacrol |
| PiC | mitochondrial phosphate carrier |
| MSNs | mesoporous silica nanoparticles |
| EE | encapsulation efficiency |
| VEGF | vascular endothelial growth factor |
| EPR | enhanced permeability and retention |
| MDR | multidrug resistance |
| GLUT-1 | glucose transporter-1 |
| BBB | blood-brain barrier |
| uPAR | urokinase plasminogen activator receptor |
| HO-1 | heme oxygenase-1 |
| NQO1 | NAD(P)H dehydrogenase [quinone] 1 |
References
- Zhang, Q.; Zhang, Z.Y.; Du, H.; Li, S.Z.; Tu, R.; Jia, Y.F.; Zheng, Z.; Song, X.M.; Du, R.L.; Zhang, X.D. DUB3 deubiquitinates and stabilizes NRF2 in chemotherapy resistance of colorectal cancer. Cell Death Differ. 2019, 26, 2300–2313. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lu, Y.; Xu, Y.; Hou, S.; Huang, J.; Wang, B.; Zhao, J.; Xia, S.; Fan, S.; Yu, X.; et al. Exosomal transfer of miR-501 confers doxorubicin resistance and tumorigenesis via targeting of BLID in gastric cancer. Cancer Lett. 2019, 459, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Song, X.; Li, Y.; Su, P.; Han, D.; Ma, T.; Guo, R.; Chen, B.; Zhao, W.; Sang, Y.; et al. circKDM4C suppresses tumor progression and attenuates doxorubicin resistance by regulating miR-548p/PBLD axis in breast cancer. Oncogene 2019, 38, 6850–6866. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Wu, G.; Su, C.; Dong, Y.; Zhang, K.; Li, J.; Sun, X.; Li, Y.; Chen, X.; Feng, C. pH-sensitive amphiphilic chitosan-quercetin conjugate for intracellular delivery of doxorubicin enhancement. Carbohydr. Polym. 2019, 223, 115072. [Google Scholar] [CrossRef]
- Soltantabar, P.; Calubaquib, E.L.; Mostafavi, E.; Biewer, M.C.; Stefan, M.C. Enhancement of Loading Efficiency by Coloading of Doxorubicin and Quercetin in Thermoresponsive Polymeric Micelles. Biomacromolecules 2020, 21, 1427–1436. [Google Scholar] [CrossRef]
- Gökçe Kütük, S.; Gökçe, G.; Kütük, M.; Gürses Cila, H.E.; Nazıroğlu, M. Curcumin enhances cisplatin-induced human laryngeal squamous cancer cell death through activation of TRPM2 channel and mitochondrial oxidative stress. Sci. Rep. 2019, 9, 17784. [Google Scholar] [CrossRef]
- Paciello, F.; Rita Fetoni, A.; Mezzogori, D.; Rolesi, R.; Di Pino, A.; Paludetti, G.; Grassi, C.; Troiani, D. The dual role of curcumin and ferulic acid in counteracting chemoresistance and cisplatin-induced ototoxicity. Sci. Rep. 2020, 10, 1063. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Huang, Y. Curcumin Derivative C086 Combined with Cisplatin Inhibits Proliferation of Osteosarcoma Cells. Med. Sci. Monit. 2020, 26, e924507. [Google Scholar] [CrossRef]
- Wang, W.; Shanmugam, M.K.; Xiang, P.; Yam, T.Y.A.; Kumar, V.; Chew, W.S.; Chang, J.K.; Ali, M.Z.B.; Reolo, M.J.Y.; Peh, Y.X.; et al. Sphingosine 1-Phosphate Receptor 2 Induces Otoprotective Responses to Cisplatin Treatment. Cancers 2020, 12, 211. [Google Scholar] [CrossRef] [Green Version]
- Aktaş, I.; Özmen, Ö.; Tutun, H.; Yalçın, A.; Türk, A. Artemisinin attenuates doxorubicin induced cardiotoxicity and hepatotoxicity in rats. Biotech. Histochem. 2020, 95, 121–128. [Google Scholar] [CrossRef]
- Wang, Y.; Chao, X.; Ahmad, F.U.D.; Shi, H.; Mehboob, H.; Hassan, W. Phoenix dactylifera Protects against Doxorubicin-Induced Cardiotoxicity and Nephrotoxicity. Cardiol. Res. Pr. 2019, 2019, 7395239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgakopoulos, P.; Kyriakidis, M.; Perpinia, A.; Karavidas, A.; Zimeras, S.; Mamalis, N.; Kouvela, M.; Charpidou, A. The Role of Metoprolol and Enalapril in the Prevention of Doxorubicin-induced Cardiotoxicity in Lymphoma Patients. Anticancer Res. 2019, 39, 5703–5707. [Google Scholar] [CrossRef] [PubMed]
- El-Gizawy, M.M.; Hosny, E.N.; Mourad, H.H.; Abd-El Razik, A.N. Curcumin nanoparticles ameliorate hepatotoxicity and nephrotoxicity induced by cisplatin in rats. Naunyn Schmiedebergs Arch. Pharm. 2020, 393, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Negrette-Guzmán, M. Combinations of the antioxidants sulforaphane or curcumin and the conventional antineoplastics cisplatin or doxorubicin as prospects for anticancer chemotherapy. Eur. J. Pharm. 2019, 859, 172513. [Google Scholar] [CrossRef]
- Navya, P.N.; Madhyastha, H.; Madhyastha, R.; Nakajima, Y.; Maruyama, M.; Srinivas, S.P.; Jain, D.; Amin, M.H.; Bhargava, S.K.; Daima, H.K. Single step formation of biocompatible bimetallic alloy nanoparticles of gold and silver using isonicotinylhydrazide. Mater. Sci. Eng. C 2019, 96, 286–294. [Google Scholar] [CrossRef]
- Sahoo, P.R.; Madhyastha, H.; Madhyastha, R.; Maruyama, M.; Nakajima, Y. Recent Progress in Nanotheranostic Medicine. Nanopharm. Princ. Appl. 2020, 3, 317–334. [Google Scholar]
- Sanjana, S.; Medha, M.; Meghna, M.; Shruthi, T.; Srinivas, S.; Madhyastha, H.; Navya, P.; Daima, H.K. Enzyme immobilization on quercetin capped gold and silver nanoparticles for improved performance. Mater. Today Proc. 2019, 10, 92–99. [Google Scholar] [CrossRef]
- Khan, M.M.; Madni, A.; Tahir, N.; Parveen, F.; Khan, S.; Jan, N.; Ali, A.; Abdurrahim, M.; Farooq, U.; Khan, M.I. Co-Delivery of Curcumin and Cisplatin to Enhance Cytotoxicity of Cisplatin Using Lipid-Chitosan Hybrid Nanoparticles. Int. J. Nanomed. 2020, 15, 2207–2217. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.D.; Li, J.Q.; Chen, F.Y.; Dong, W.; Wen, L.J.; Fei, W.D.; Zhang, X.; Yang, P.L.; Zhang, X.M.; Zheng, C.H. Co-Delivery of Curcumin and Paclitaxel by “Core-Shell” Targeting Amphiphilic Copolymer to Reverse Resistance in the Treatment of Ovarian Cancer. Int. J. Nanomed. 2019, 14, 9453–9467. [Google Scholar] [CrossRef] [Green Version]
- Zielińska, A.; Alves, H.; Marques, V.; Durazzo, A.; Lucarini, M.; Alves, T.F.; Morsink, M.; Willemen, N.; Eder, P.; Chaud, M.V. Properties, Extraction Methods, and Delivery Systems for Curcumin as a Natural Source of Beneficial Health Effects. Medicina 2020, 56, 336. [Google Scholar] [CrossRef]
- Alven, S.; Aderibigbe, B.A. Efficacy of Polymer-Based Nanocarriers for Co-Delivery of Curcumin and Selected Anticancer Drugs. Nanomaterials 2020, 10, 1556. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.R.; Siddiqua, T.J. Functional foods in cancer prevention and therapy: Recent epidemiological findings. In Functional Foods in Cancer Prevention and Therapy; Elsevier: Amsterdam, The Netherlands, 2020; pp. 405–433. [Google Scholar]
- Tang, F.; Ling, C. Curcumin ameliorates chronic obstructive pulmonary disease by modulating autophagy and endoplasmic reticulum stress through regulation of SIRT1 in a rat model. J. Int. Med. Res. 2019, 47, 4764–4774. [Google Scholar] [CrossRef] [PubMed]
- Voulgaropoulou, S.D.; van Amelsvoort, T.; Prickaerts, J.; Vingerhoets, C. The effect of curcumin on cognition in Alzheimer’s disease and healthy aging: A systematic review of pre-clinical and clinical studies. Brain Res. 2019, 1725, 146476. [Google Scholar] [CrossRef]
- Hirata, Y.; Ito, Y.; Takashima, M.; Yagyu, K.; Oh-Hashi, K.; Suzuki, H.; Ono, K.; Furuta, K.; Sawada, M. Novel Oxindole-Curcumin Hybrid Compound for Antioxidative Stress and Neuroprotection. Acs Chem. Neurosci. 2020, 11, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Motawi, T.K.; Sadik, N.A.H.; Hamed, M.A.; Ali, S.A.; Khalil, W.K.B.; Ahmed, Y.R. Potential therapeutic effects of antagonizing adenosine A(2A) receptor, curcumin and niacin in rotenone-induced Parkinson’s disease mice model. Mol. Cell Biochem. 2020, 465, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Tamddoni, A.; Mohammadi, E.; Sedaghat, F.; Qujeq, D.; As’Habi, A. The anticancer effects of curcumin via targeting the mammalian target of rapamycin complex 1 (mTORC1) signaling pathway. Pharmacol. Res. 2020, 156, 104798. [Google Scholar] [CrossRef]
- Baldi, A.; De Luca, A.; Maiorano, P.; D’Angelo, C.; Giordano, A. Curcumin as an Anticancer Agent in Malignant Mesothelioma: A Review. Int. J. Mol. Sci. 2020, 21, 1839. [Google Scholar] [CrossRef] [Green Version]
- Bagheri, H.; Ghasemi, F.; Barreto, G.E.; Rafiee, R.; Sathyapalan, T.; Sahebkar, A. Effects of curcumin on mitochondria in neurodegenerative diseases. Biofactors 2020, 46, 5–20. [Google Scholar] [CrossRef]
- Ba, P.; Xu, M.; Yu, M.; Li, L.; Duan, X.; Lv, S.; Fu, G.; Yang, J.; Yang, P.; Yang, C.; et al. Curcumin suppresses the proliferation and tumorigenicity of Cal27 by modulating cancer associated fibroblasts of TSCC. Oral. Dis. 2020. [Google Scholar] [CrossRef]
- Mani, J.; Fleger, J.; Rutz, J.; Maxeiner, S.; Bernd, A.; Kippenberger, S.; Zöller, N.; Chun, F.K.; Relja, B.; Juengel, E.; et al. Curcumin combined with exposure to visible light blocks bladder cancer cell adhesion and migration by an integrin dependent mechanism. Eur. Rev. Med. Pharm. Sci. 2019, 23, 10564–10574. [Google Scholar] [CrossRef]
- Wang, X.; Chang, X.; Zhan, H.; Zhang, Q.; Li, C.; Gao, Q.; Yang, M.; Luo, Z.; Li, S.; Sun, Y. Curcumin and Baicalin ameliorate ethanol-induced liver oxidative damage via the Nrf2/HO-1 pathway. J. Food Biochem. 2020, 44, e13425. [Google Scholar] [CrossRef] [PubMed]
- Al-Dossari, M.H.; Fadda, L.M.; Attia, H.A.; Hasan, I.H.; Mahmoud, A.M. Curcumin and Selenium Prevent Lipopolysaccharide/Diclofenac-Induced Liver Injury by Suppressing Inflammation and Oxidative Stress. Biol. Trace Elem. Res. 2020, 196, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Sudirman, S.; Lai, C.S.; Yan, Y.L.; Yeh, H.I.; Kong, Z.L. Histological evidence of chitosan-encapsulated curcumin suppresses heart and kidney damages on streptozotocin-induced type-1 diabetes in mice model. Sci. Rep. 2019, 9, 15233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Cui, L.S.; Zhou, B.; Zhang, L.L.; Liu, Z.H.; Zhang, L. Monocarbonyl curcumin analog A2 potently inhibits angiogenesis by inducing ROS-dependent endothelial cell death. Acta Pharm. Sin. 2019, 40, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Abo-Zaid, M.A.; Shaheen, E.S.; Ismail, A.H. Immunomodulatory effect of curcumin on hepatic cirrhosis in experimental rats. J. Food Biochem. 2020, 44, e13219. [Google Scholar] [CrossRef]
- Nayak, D.; Tripathi, N.; Kathuria, D.; Siddharth, S.; Nayak, A.; Bharatam, P.V.; Kundu, C. Quinacrine and curcumin synergistically increased the breast cancer stem cells death by inhibiting ABCG2 and modulating DNA damage repair pathway. Int. J. Biochem. Cell Biol. 2020, 119, 105682. [Google Scholar] [CrossRef]
- Li, Y.; Tian, L.; Sun, D.; Yin, D. Curcumin ameliorates atherosclerosis through upregulation of miR-126. J. Cell Physiol. 2019, 234, 21049–21059. [Google Scholar] [CrossRef]
- Ghelani, H.; Razmovski-Naumovski, V.; Chang, D.; Nammi, S. Chronic treatment of curcumin improves hepatic lipid metabolism and alleviates the renal damage in adenine-induced chronic kidney disease in Sprague-Dawley rats. BMC Nephrol. 2019, 20, 431. [Google Scholar] [CrossRef]
- Hadi, A.; Pourmasoumi, M.; Ghaedi, E.; Sahebkar, A. The effect of Curcumin/Turmeric on blood pressure modulation: A systematic review and meta-analysis. Pharm. Res. 2019, 150, 104505. [Google Scholar] [CrossRef]
- Liczbiński, P.; Michałowicz, J.; Bukowska, B. Molecular mechanism of curcumin action in signaling pathways: Review of the latest research. Phytother. Res. 2020. [Google Scholar] [CrossRef]
- Wahlström, B.; Blennow, G. A study on the fate of curcumin in the rat. Acta Pharmacol. Toxicol. 1978, 43, 86–92. [Google Scholar]
- Hsieh, C. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, 2859–2900. [Google Scholar]
- Sharma, R.A.; McLelland, H.R.; Hill, K.A.; Ireson, C.R.; Euden, S.A.; Manson, M.M.; Pirmohamed, M.; Marnett, L.J.; Gescher, A.J.; Steward, W.P. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin. Cancer Res. 2001, 7, 1894–1900. [Google Scholar] [PubMed]
- Sun, C.; Zhang, S.; Liu, C.; Liu, X. Curcumin Promoted miR-34a Expression and Suppressed Proliferation of Gastric Cancer Cells. Cancer Biother. Radiopharm. 2019, 34, 634–641. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, C.; Ren, Z.; Zhang, F.; Xu, J.; Zhang, X.; Zheng, H. Curcumin Affects Gastric Cancer Cell Migration, Invasion and Cytoskeletal Remodeling Through Gli1-β-Catenin. Cancer Manag. Res. 2020, 12, 3795–3806. [Google Scholar] [CrossRef]
- San, T.T.; Khaenam, P.; Prachayasittikul, V.; Sripa, B.; Kunkeaw, N.; Chan-On, W. Curcumin enhances chemotherapeutic effects and suppresses ANGPTL4 in anoikis-resistant cholangiocarcinoma cells. Heliyon 2020, 6, e03255. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Zhan, C.Z.; Wang, T.; You, H.; Yao, R. Curcumin Inhibits the Proliferation, Migration, Invasion, and Apoptosis of Diffuse Large B-Cell Lymphoma Cell Line by Regulating MiR-21/VHL Axis. Yonsei Med. J. 2020, 61, 20–29. [Google Scholar] [CrossRef]
- Zhou, C.; Hu, C.; Wang, B.; Fan, S.; Jin, W. Curcumin Suppresses Cell Proliferation, Migration, and Invasion Through Modulating miR-21-5p/SOX6 Axis in Hepatocellular Carcinoma. Cancer Biother. Radiopharm. 2020. [Google Scholar] [CrossRef]
- Gallardo, M.; Kemmerling, U.; Aguayo, F.; Bleak, T.C.; Muñoz, J.P.; Calaf, G.M. Curcumin rescues breast cells from epithelial-mesenchymal transition and invasion induced by anti-miR-34a. Int. J. Oncol. 2020, 56, 480–493. [Google Scholar] [CrossRef] [Green Version]
- Shi, N.; Yu, H.; Chen, T. Inhibition of esophageal cancer growth through the suppression of PI3K/AKT/mTOR signaling pathway. Oncol. Targets 2019, 12, 7637–7647. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Ma, Z.; Zhang, G.; Yang, X.; Du, Q.; Wang, W. CSNK2A1 Promotes Gastric Cancer Invasion Through the PI3K-Akt-mTOR Signaling Pathway. Cancer Manag. Res. 2019, 11, 10135–10143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Lin, R.; Liu, Z.; Yan, T.; Xia, Y.; Zhao, L.; Lin, F.; Zhang, X.; Li, C.; Wang, Y. Curcumin analog, WZ37, promotes G2/M arrest and apoptosis of HNSCC cells through Akt/mTOR inhibition. Toxicol. Vitr. 2020, 65, 104754. [Google Scholar] [CrossRef] [PubMed]
- Sak, K. Radiosensitizing Potential of Curcumin in Different Cancer Models. Nutr. Cancer 2020, 72, 1276–1289. [Google Scholar] [CrossRef] [PubMed]
- Bolat, Z.B.; Islek, Z.; Demir, B.N.; Yilmaz, E.N.; Sahin, F.; Ucisik, M.H. Curcumin- and Piperine-Loaded Emulsomes as Combinational Treatment Approach Enhance the Anticancer Activity of Curcumin on HCT116 Colorectal Cancer Model. Front. Bioeng. Biotechnol. 2020, 8, 50. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Tao, X.; Fu, Q.; Ge, C.; Li, R.; Li, Z.; Zhu, Y.; Tian, H.; Li, Q.; Liu, M.; et al. Curcumin inhibits cell proliferation and migration in NSCLC through a synergistic effect on the TLR4/MyD88 and EGFR pathways. Oncol. Rep. 2019, 42, 1843–1855. [Google Scholar] [CrossRef]
- Epelbaum, R.; Schaffer, M.; Vizel, B.; Badmaev, V.; Bar-Sela, G. Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr. Cancer 2010, 62, 1137–1141. [Google Scholar] [CrossRef]
- Greil, R.; Greil-Ressler, S.; Weiss, L.; Schönlieb, C.; Magnes, T.; Radl, B.; Bolger, G.T.; Vcelar, B.; Sordillo, P.P. A phase 1 dose-escalation study on the safety, tolerability and activity of liposomal curcumin (Lipocurc(™)) in patients with locally advanced or metastatic cancer. Cancer Chemother. Pharm. 2018, 82, 695–706. [Google Scholar] [CrossRef] [Green Version]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008, 14, 4491–4499. [Google Scholar] [CrossRef] [Green Version]
- Pastorelli, D.; Fabricio, A.S.C.; Giovanis, P.; D’Ippolito, S.; Fiduccia, P.; Soldà, C.; Buda, A.; Sperti, C.; Bardini, R.; Da Dalt, G.; et al. Phytosome complex of curcumin as complementary therapy of advanced pancreatic cancer improves safety and efficacy of gemcitabine: Results of a prospective phase II trial. Pharm. Res. 2018, 132, 72–79. [Google Scholar] [CrossRef]
- Guney Eskiler, G.; Sahin, E.; Deveci Ozkan, A.; Cilingir Kaya, O.T.; Kaleli, S. Curcumin induces DNA damage by mediating homologous recombination mechanism in triple negative breast cancer. Nutr. Cancer 2020, 72, 1057–1066. [Google Scholar] [CrossRef]
- Zhou, X.; Jiao, D.; Dou, M.; Zhang, W.; Lv, L.; Chen, J.; Li, L.; Wang, L.; Han, X. Curcumin inhibits the growth of triple-negative breast cancer cells by silencing EZH2 and restoring DLC1 expression. J. Cell. Mol. Med. 2020, 24, 10648–10662. [Google Scholar] [CrossRef]
- Tian, N.; Shangguan, W.; Zhou, Z.; Yao, Y.; Fan, C.; Cai, L. Lin28b is involved in curcumin-reversed paclitaxel chemoresistance and associated with poor prognosis in hepatocellular carcinoma. J. Cancer 2019, 10, 6074–6087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attia, Y.M.; El-Kersh, D.M.; Ammar, R.A.; Adel, A.; Khalil, A.; Walid, H.; Eskander, K.; Hamdy, M.; Reda, N.; Mohsen, N.E.; et al. Inhibition of aldehyde dehydrogenase-1 and p-glycoprotein-mediated multidrug resistance by curcumin and vitamin D3 increases sensitivity to paclitaxel in breast cancer. Chem. Biol. Interact. 2020, 315, 108865. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Fan, K.; Jin, Y.; Zhao, L.; Wang, Q.; Tang, Y.; Xu, H.; Liu, Z.; Wang, S.; Lin, J.; et al. PEGylated lipid bilayer coated mesoporous silica nanoparticles co-delivery of paclitaxel and curcumin leads to increased tumor site drug accumulation and reduced tumor burden. Eur. J. Pharm. Sci. 2019, 140, 105070. [Google Scholar] [CrossRef]
- Hiremath, C.G.; Heggnnavar, G.B.; Kariduraganavar, M.Y.; Hiremath, M.B. Co-delivery of paclitaxel and curcumin to foliate positive cancer cells using Pluronic-coated iron oxide nanoparticles. Prog. Biomater. 2019, 8, 155–168. [Google Scholar] [CrossRef] [Green Version]
- Maghmomeh, A.O.; El-Gayar, A.M.; El-Karef, A.; Abdel-Rahman, N. Arsenic trioxide and curcumin attenuate cisplatin-induced renal fibrosis in rats through targeting Hedgehog signaling. Naunyn Schmiedebergs Arch. Pharm. 2020, 393, 303–313. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Jiao, D.; Yang, S.; Li, P. Protective Effect of Astaxanthin on Ochratoxin A-Induced Kidney Injury to Mice by Regulating Oxidative Stress-Related NRF2/KEAP1 Pathway. Molecules 2020, 25, 1386. [Google Scholar] [CrossRef] [Green Version]
- Al Fayi, M.; Otifi, H.; Alshyarba, M.; Dera, A.A.; Rajagopalan, P. Thymoquinone and curcumin combination protects cisplatin-induced kidney injury, nephrotoxicity by attenuating NFκB, KIM-1 and ameliorating Nrf2/HO-1 signalling. J. Drug Target. 2020, 1–10. [Google Scholar] [CrossRef]
- Zheng, Z.H.; You, H.Y.; Feng, Y.J.; Zhang, Z.T. LncRNA KCNQ1OT1 is a key factor in the reversal effect of curcumin on cisplatin resistance in the colorectal cancer cells. Mol. Cell Biochem. 2020. [Google Scholar] [CrossRef]
- Ozawa-Umeta, H.; Kishimoto, A.; Imaizumi, A.; Hashimoto, T.; Asakura, T.; Kakeya, H.; Kanai, M. Curcumin β-D-glucuronide exhibits anti-tumor effects on oxaliplatin-resistant colon cancer with less toxicity in vivo. Cancer Sci. 2020, 111, 1785–1793. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Wu, S.; Xiang, B.; Li, L.; Lin, Y. Curcumin Attenuates Oxaliplatin-Induced Liver Injury and Oxidative Stress by Activating the Nrf2 Pathway. Drug Des. Dev. Ther. 2020, 14, 73–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, L.T.; Dong, S.Q.; Wang, S.S.; Chen, M.; Li, C.F.; Geng, D.; Zhu, J.X.; Liu, Q.; Cheng, J. Curcumin attenuates cognitive impairment by enhancing autophagy in chemotherapy. Neurobiol. Dis. 2020, 136, 104715. [Google Scholar] [CrossRef] [PubMed]
- Al-malky, H.S.; Al Harthi, S.E.; Osman, A.-M.M. Major obstacles to doxorubicin therapy: Cardiotoxicity and drug resistance. J. Oncol. Pharm. Pract. 2020, 26, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Morabito, A.; Gattuso, D.; Stani, S.C.; Fanelli, M.; Ferraù, F.; De Sio, L.; Castellana, M.A.; Lorusso, V.; Priolo, D.; Vitale, S. Safety and activity of the combination of pegylated liposomal doxorubicin and weekly docetaxel in advanced breast cancer. Breast. Cancer Res. Treat. 2004, 86, 249–258. [Google Scholar] [CrossRef]
- Gabizon, A.; Shmeeda, H.; Barenholz, Y. Pharmacokinetics of pegylated liposomal doxorubicin. Clin. Pharmacokinet. 2003, 42, 419–436. [Google Scholar] [CrossRef]
- Mohajeri, M.; Sahebkar, A. Protective effects of curcumin against doxorubicin-induced toxicity and resistance: A review. Crit. Rev. Oncol. Hematol. 2018, 122, 30–51. [Google Scholar] [CrossRef]
- Perry, J.K.; Kannan, N.; Grandison, P.M.; Mitchell, M.D.; Lobie, P.E. Are trefoil factors oncogenic? Trends Endocrinol. Metab. 2008, 19, 74–81. [Google Scholar] [CrossRef]
- Aamann, L.; Vestergaard, E.M.; Grønbæk, H. Trefoil factors in inflammatory bowel disease. World J. Gastroenterol. 2014, 20, 3223–3230. [Google Scholar] [CrossRef]
- Choudhary, A.; Smitha, C.N.; Suresh, D.K. Trefoils: An unexplored natural protective shield of oral cavity. J. Oral. Biol. Craniofac Res. 2015, 5, 226–231. [Google Scholar] [CrossRef] [Green Version]
- Poh, H.M.; Chiou, Y.S.; Chong, Q.Y.; Chen, R.M.; Rangappa, K.S.; Ma, L.; Zhu, T.; Kumar, A.P.; Pandey, V.; Lee, S.C.; et al. Inhibition of TFF3 Enhances Sensitivity-and Overcomes Acquired Resistance-to Doxorubicin in Estrogen Receptor-Positive Mammary Carcinoma. Cancers 2019, 11, 1528. [Google Scholar] [CrossRef] [Green Version]
- Gault, C.R.; Obeid, L.M. Still benched on its way to the bedside: Sphingosine kinase 1 as an emerging target in cancer chemotherapy. Crit. Rev. Biochem. Mol. Biol. 2011, 46, 342–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, K.; Zhang, Y.; D’Alessandro, A.; Nemkov, T.; Song, A.; Wu, H.; Liu, H.; Adebiyi, M.; Huang, A.; Wen, Y.E.; et al. Sphingosine-1-phosphate promotes erythrocyte glycolysis and oxygen release for adaptation to high-altitude hypoxia. Nat. Commun. 2016, 7, 12086. [Google Scholar] [CrossRef] [PubMed]
- Cuvillier, O.; Ader, I.; Bouquerel, P.; Brizuela, L.; Gstalder, C.; Malavaud, B. Hypoxia, therapeutic resistance, and sphingosine 1-phosphate. Adv. Cancer Res. 2013, 117, 117–141. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Su, C. Sphingosine kinase 1 contributes to doxorubicin resistance and glycolysis in osteosarcoma. Mol. Med. Rep. 2020, 22, 2183–2190. [Google Scholar] [CrossRef]
- Liu, C.; Xing, H.; Guo, C.; Yang, Z.; Wang, Y.; Wang, Y. MiR-124 reversed the doxorubicin resistance of breast cancer stem cells through STAT3/HIF-1 signaling pathways. Cell Cycle 2019, 18, 2215–2227. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, E.; Tang, Y.; Mao, J.; Shen, J.; Zheng, X.; Xie, S.; Zhang, S.; Wu, Y.; Liu, H.; et al. miR-223 overexpression inhibits doxorubicin-induced autophagy by targeting FOXO3a and reverses chemoresistance in hepatocellular carcinoma cells. Cell Death Dis. 2019, 10, 843. [Google Scholar] [CrossRef] [Green Version]
- Zhong, J.; Sun, P.; Xu, N.; Liao, M.; Xu, C.; Ding, Y.; Cai, J.; Zhang, Y.; Xie, W. Canagliflozin inhibits p-gp function and early autophagy and improves the sensitivity to the antitumor effect of doxorubicin. Biochem. Pharm. 2020, 175, 113856. [Google Scholar] [CrossRef]
- Le, Y.; Kan, A.; Li, Q.J.; He, M.K.; Chen, H.L.; Shi, M. NAP1L1 is a prognostic biomarker and contribute to doxorubicin chemotherapy resistance in human hepatocellular carcinoma. Cancer Cell Int. 2019, 19, 228. [Google Scholar] [CrossRef]
- Tan, X.; Liao, Z.; Zou, S.; Ma, L.; Wang, A. VASH2 Promotes Cell Proliferation and Resistance to Doxorubicin in Non-Small Cell Lung Cancer via AKT Signaling. Oncol. Res. 2020, 28, 3–11. [Google Scholar] [CrossRef]
- Ma, W.; Yang, L.; Liu, H.; Chen, P.; Ren, H.; Ren, P. PAXX is a novel target to overcome resistance to doxorubicin and cisplatin in osteosarcoma. Biochem. Biophys. Res. Commun. 2020, 521, 204–211. [Google Scholar] [CrossRef]
- Calcabrini, C.; Maffei, F.; Turrini, E.; Fimognari, C. Sulforaphane Potentiates Anticancer Effects of Doxorubicin and Cisplatin and Mitigates Their Toxic Effects. Front. Pharm. 2020, 11, 567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hu, C.; Kong, C.Y.; Song, P.; Wu, H.M.; Xu, S.C.; Yuan, Y.P.; Deng, W.; Ma, Z.G.; Tang, Q.Z. FNDC5 alleviates oxidative stress and cardiomyocyte apoptosis in doxorubicin-induced cardiotoxicity via activating AKT. Cell Death Differ. 2020, 27, 540–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, J.A.; Tang, Y.; Yu, J.Y.; Zhang, H.; Zhang, J.F.; Wang, C.Q.; Gu, J. miR-146a attenuates apoptosis and modulates autophagy by targeting TAF9b/P53 pathway in doxorubicin-induced cardiotoxicity. Cell Death Dis. 2019, 10, 668. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.M.; Abd El Fattah, M.A.; Ahmed, K.A.; Sayed, H.M. Protective effects of olmesartan and l-carnitine on doxorubicin-induced cardiotoxicity in rats. Can. J. Physiol. Pharm. 2020, 98, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Chu, L.; Liang, H.; Chen, J.; Liang, J.; Huang, Z.; Zhang, B.; Chen, X. Protective Effects of Dioscin Against Doxorubicin-Induced Hepatotoxicity Via Regulation of Sirt1/FOXO1/NF-κb Signal. Front. Pharm. 2019, 10, 1030. [Google Scholar] [CrossRef] [PubMed]
- Wali, A.F.; Rashid, S.; Rashid, S.M.; Ansari, M.A.; Khan, M.R.; Haq, N.; Alhareth, D.Y.; Ahmad, A.; Rehman, M.U. Naringenin Regulates Doxorubicin-Induced Liver Dysfunction: Impact on Oxidative Stress and Inflammation. Plants 2020, 9, 550. [Google Scholar] [CrossRef]
- Ibrahim, K.M.; Mantawy, E.M.; Elanany, M.M.; Abdelgawad, H.S.; Khalifa, N.M.; Hussien, R.H.; El-Agroudy, N.N.; El-Demerdash, E. Protection from doxorubicin-induced nephrotoxicity by clindamycin: Novel antioxidant, anti-inflammatory and anti-apoptotic roles. Naunyn Schmiedebergs Arch. Pharm. 2020, 393, 739–748. [Google Scholar] [CrossRef]
- Rafiee, Z.; Moaiedi, M.Z.; Gorji, A.V.; Mansouri, E. P-Coumaric Acid Mitigates Doxorubicin-Induced Nephrotoxicity Through Suppression of Oxidative Stress, Inflammation and Apoptosis. Arch. Med. Res. 2020, 51, 32–40. [Google Scholar] [CrossRef]
- Sikandar, A.; Farhat, K.; Afzal, A.; Ajmal, K.; Laeeq, M.; Khokhar, A. Protective Effects of Trimetazidine Against Doxorubicin-Induced Cardiotoxicity And Hepatotoxicity In Mice. J. Ayub Med. Coll Abbottabad 2020, 32, 304–309. [Google Scholar]
- Yu, X.; Ruan, Y.; Huang, X.; Dou, L.; Lan, M.; Cui, J.; Chen, B.; Gong, H.; Wang, Q.; Yan, M.; et al. Dexrazoxane ameliorates doxorubicin-induced cardiotoxicity by inhibiting both apoptosis and necroptosis in cardiomyocytes. Biochem. Biophys. Res. Commun. 2020, 523, 140–146. [Google Scholar] [CrossRef]
- Khames, A.; Khalaf, M.M.; Gad, A.M.; Abd El-Raouf, O.M.; Kandeil, M.A. Nicorandil combats doxorubicin-induced nephrotoxicity via amendment of TLR4/P38 MAPK/NFκ-B signaling pathway. Chem. Biol. Interact. 2019, 311, 108777. [Google Scholar] [CrossRef] [PubMed]
- Maris, J.M. Recent advances in neuroblastoma. N. Engl. J. Med. 2010, 362, 2202–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brodeur, G.M. Neuroblastoma: Biological insights into a clinical enigma. Nat. Rev. Cancer 2003, 3, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Sohara, Y.; Shimada, H.; DeClerck, Y.A. Mechanisms of bone invasion and metastasis in human neuroblastoma. Cancer Lett. 2005, 228, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Ara, T.; DeClerck, Y.A. Mechanisms of invasion and metastasis in human neuroblastoma. Cancer Metastasis Rev. 2006, 25, 645–657. [Google Scholar] [CrossRef]
- Namkaew, J.; Jaroonwitchawan, T.; Rujanapun, N.; Saelee, J.; Noisa, P. Combined effects of curcumin and doxorubicin on cell death and cell migration of SH-SY5Y human neuroblastoma cells. Vitr. Cell Dev. Biol. Anim. 2018, 54, 629–639. [Google Scholar] [CrossRef]
- Firouzi Amoodizaj, F.; Baghaeifar, S.; Taheri, E.; Farhoudi Sefidan Jadid, M.; Safi, M.; Seyyed Sani, N.; Hajazimian, S.; Isazadeh, A.; Shanehbandi, D. Enhanced anticancer potency of doxorubicin in combination with curcumin in gastric adenocarcinoma. J. Biochem. Mol. Toxicol. 2020, 34, e22486. [Google Scholar] [CrossRef]
- Sadzuka, Y.; Nagamine, M.; Toyooka, T.; Ibuki, Y.; Sonobe, T. Beneficial effects of curcumin on antitumor activity and adverse reactions of doxorubicin. Int. J. Pharm. 2012, 432, 42–49. [Google Scholar] [CrossRef]
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillère, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013, 342, 971–976. [Google Scholar] [CrossRef] [Green Version]
- Margalit, O.; Simon, A.J.; Yakubov, E.; Puca, R.; Yosepovich, A.; Avivi, C.; Jacob-Hirsch, J.; Gelernter, I.; Harmelin, A.; Barshack, I. Zinc supplementation augments in vivo antitumor effect of chemotherapy by restoring p53 function. Int. J. Cancer 2012, 131, E562–E568. [Google Scholar] [CrossRef]
- Fang, A.P.; Chen, P.Y.; Wang, X.Y.; Liu, Z.Y.; Zhang, D.M.; Luo, Y.; Liao, G.C.; Long, J.A.; Zhong, R.H.; Zhou, Z.G. Serum copper and zinc levels at diagnosis and hepatocellular carcinoma survival in the Guangdong Liver Cancer Cohort. Int. J. Cancer 2019, 144, 2823–2832. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Mei, X.; Ye, Y.; Xue, T.; Wang, J.; Sun, W.; Lin, C.; Xue, R.; Zhang, J.; Xu, D. Zn(II)-curcumin solid dispersion impairs hepatocellular carcinoma growth and enhances chemotherapy by modulating gut microbiota-mediated zinc homeostasis. Pharm. Res. 2019, 150, 104454. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Niu, H.; Liu, Y.; Wang, L.; Zhang, N.; Zhang, G.; Liu, R.; Han, M. LOX inhibition downregulates MMP-2 and MMP-9 in gastric cancer tissues and cells. J. Cancer 2019, 10, 6481–6490. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.Y.; Ho, Y.C.; Lin, C.W.; Yang, W.E.; Yu, Y.L.; Tsai, M.C.; Yang, S.F.; Su, S.C. Salvianolic acid A suppresses MMP-2 expression and restrains cancer cell invasion through ERK signaling in human nasopharyngeal carcinoma. J. Ethnopharmacol. 2020, 252, 112601. [Google Scholar] [CrossRef] [PubMed]
- Gialeli, C.; Theocharis, A.D.; Karamanos, N.K. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. Febs J. 2011, 278, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Caley, M.P.; Martins, V.L.; O’Toole, E.A. Metalloproteinases and wound healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Mohan, C.D.; Deivasigamani, A.; Jung, Y.Y.; Rangappa, S.; Basappa, S.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Garg, M.; et al. Brusatol suppresses STAT3-driven metastasis by downregulating epithelial-mesenchymal transition in hepatocellular carcinoma. J. Adv. Res. 2020, 26, 83–94. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.-T.; Wang, L.; Wang, H.; Tang, F.-R.; Cai, W.-Q.; Sethi, G.; Xin, H.-W.; Ma, Z. Insights into biological role of LncRNAs in epithelial-mesenchymal transition. Cells 2019, 8, 1178. [Google Scholar] [CrossRef] [Green Version]
- Baek, S.H.; Ko, J.H.; Lee, J.H.; Kim, C.; Lee, H.; Nam, D.; Lee, J.; Lee, S.G.; Yang, W.M.; Um, J.Y. Ginkgolic acid inhibits invasion and migration and tgf-β-induced emt of lung cancer cells through pi3k/akt/mtor inactivation. J. Cell. Physiol. 2017, 232, 346–354. [Google Scholar] [CrossRef]
- Dai, X.; Ahn, K.S.; Wang, L.Z.; Kim, C.; Deivasigamni, A.; Arfuso, F.; Um, J.-Y.; Kumar, A.P.; Chang, Y.-C.; Kumar, D. Ascochlorin enhances the sensitivity of doxorubicin leading to the reversal of epithelial-to-mesenchymal transition in hepatocellular carcinoma. Mol. Cancer Ther. 2016, 15, 2966–2976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.N.; Ahn, D.H.; Kang, N.; Yeo, C.D.; Kim, Y.K.; Lee, K.Y.; Kim, T.J.; Lee, S.H.; Park, M.S.; Yim, H.W.; et al. TGF-β induced EMT and stemness characteristics are associated with epigenetic regulation in lung cancer. Sci. Rep. 2020, 10, 10597. [Google Scholar] [CrossRef] [PubMed]
- Marques da Fonseca, L.; Jacques da Silva, L.R.; Santos Dos Reis, J.; Rodrigues da Costa Santos, M.A.; de Sousa Chaves, V.; Monteiro da Costa, K.; Sa-Diniz, J.N.; Freire de Lima, C.G.; Morrot, A.; Nunes Franklim, T.; et al. Piperine Inhibits TGF-β Signaling Pathways and Disrupts EMT-Related Events in Human Lung Adenocarcinoma Cells. Medicines 2020, 7, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Liu, Q.; Chen, S.; Liu, Y.; Huang, Y.; Chen, P.; Li, X.; Gao, G.; Xu, K.; Fan, S.; et al. APLNR is involved in ATRA-induced growth inhibition of nasopharyngeal carcinoma and may suppress EMT through PI3K-Akt-mTOR signaling. Faseb J. 2019, 33, 11959–11972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Liu, S.; Zhu, Y. A-kinase-interacting protein 1 promotes EMT and metastasis via PI3K/Akt/IKKβ pathway in cervical cancer. Cell Biochem. Funct. 2020, 38, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Lai, Y.A.; Lin, Y.C.; Ma, J.W.; Huang, L.F.; Yang, N.S.; Ho, C.T.; Kuo, S.C.; Way, T.D. Curcumin suppresses doxorubicin-induced epithelial-mesenchymal transition via the inhibition of TGF-β and PI3K/AKT signaling pathways in triple-negative breast cancer cells. J. Agric. Food Chem. 2013, 61, 11817–11824. [Google Scholar] [CrossRef]
- Ween, M.P.; Armstrong, M.A.; Oehler, M.K.; Ricciardelli, C. The role of ABC transporters in ovarian cancer progression and chemoresistance. Crit. Rev. Oncol. Hematol. 2015, 96, 220–256. [Google Scholar] [CrossRef]
- Van der Bliek, A.M.; Baas, F.; Ten Houte de Lange, T.; Kooiman, P.M.; Van der Velde-Koerts, T.; Borst, P. The human mdr3 gene encodes a novel P-glycoprotein homologue and gives rise to alternatively spliced mRNAs in liver. Embo J. 1987, 6, 3325–3331. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Wang, Q.; Chen, Z.; Li, X.; Wu, Z.; Hu, C.; Liao, D.; Zhang, W.; Chen, Z.S. The PI3K subunits, P110α and P110β are potential targets for overcoming P-gp and BCRP-mediated MDR in cancer. Mol. Cancer 2020, 19, 10. [Google Scholar] [CrossRef] [Green Version]
- Khonkarn, R.; Daowtak, K.; Okonogi, S. Chemotherapeutic Efficacy Enhancement in P-gp-Overexpressing Cancer Cells by Flavonoid-Loaded Polymeric Micelles. Aaps Pharmscitech. 2020, 21, 121. [Google Scholar] [CrossRef]
- Nicolas, E.; Ramus, C.; Berthier, S.; Arlotto, M.; Bouamrani, A.; Lefebvre, C.; Morel, F.; Garin, J.; Ifrah, N.; Berger, F.; et al. Expression of S100A8 in leukemic cells predicts poor survival in de novo AML patients. Leukemia 2011, 25, 57–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spijkers-Hagelstein, J.A.; Schneider, P.; Hulleman, E.; de Boer, J.; Williams, O.; Pieters, R.; Stam, R.W. Elevated S100A8/S100A9 expression causes glucocorticoid resistance in MLL-rearranged infant acute lymphoblastic leukemia. Leukemia 2012, 26, 1255–1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.Y.; Zhang, M.Y.; Zhou, Q.; Wu, S.Y.; Zhao, Y.; Gu, W.Y.; Pan, J.; Cen, J.N.; Chen, Z.X.; Guo, W.G.; et al. High expression of S100A8 gene is associated with drug resistance to etoposide and poor prognosis in acute myeloid leukemia through influencing the apoptosis pathway. Oncol. Targets 2016, 9, 4887–4899. [Google Scholar] [CrossRef] [Green Version]
- Wen, C.; Fu, L.; Huang, J.; Dai, Y.; Wang, B.; Xu, G.; Wu, L.; Zhou, H. Curcumin reverses doxorubicin resistance via inhibition the efflux function of ABCB4 in doxorubicin-resistant breast cancer cells. Mol. Med. Rep. 2019, 19, 5162–5168. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; Liu, T.; Li, J.; Guan, Q.; Wang, H.; Yan, S.; Li, Z.; Zuo, D.; Zhang, W.; Wu, Y. YAN, a novel microtubule inhibitor, inhibits P-gp and MRP1 function and induces mitotic slippage followed by apoptosis in multidrug-resistant A549/Taxol cells. Toxicol. Vitr. 2020, 69, 104971. [Google Scholar] [CrossRef]
- Li, H.; Krstin, S.; Wink, M. Modulation of multidrug resistant in cancer cells by EGCG, tannic acid and curcumin. Phytomedicine 2018, 50, 213–222. [Google Scholar] [CrossRef]
- Yang, L.; Li, D.; Tang, P.; Zuo, Y. Curcumin increases the sensitivity of K562/DOX cells to doxorubicin by targeting S100 calcium-binding protein A8 and P-glycoprotein. Oncol. Lett. 2020, 19, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.; Sun, Y.; Dong, X. Zwitterionic Polymer Micelles with Dual Conjugation of Doxorubicin and Curcumin: Synergistically Enhanced Efficacy against Multidrug-Resistant Tumor Cells. Langmuir 2020, 36, 2383–2395. [Google Scholar] [CrossRef]
- Huang, J.; Chen, Y.; Li, J.; Zhang, K.; Chen, J.; Chen, D.; Feng, B.; Song, H.; Feng, J.; Wang, R.; et al. Notch-1 Confers Chemoresistance in Lung Adenocarcinoma to Taxanes through AP-1/microRNA-451 Mediated Regulation of MDR-1. Mol. Nucleic Acids 2016, 5, e375. [Google Scholar] [CrossRef] [Green Version]
- Meng, Q.; Liang, C.; Hua, J.; Zhang, B.; Liu, J.; Zhang, Y.; Wei, M.; Yu, X.; Xu, J.; Shi, S. A miR-146a-5p/TRAF6/NF-kB p65 axis regulates pancreatic cancer chemoresistance: Functional validation and clinical significance. Theranostics 2020, 10, 3967–3979. [Google Scholar] [CrossRef]
- Dhandapani, K.M.; Mahesh, V.B.; Brann, D.W. Curcumin suppresses growth and chemoresistance of human glioblastoma cells via AP-1 and NFkappaB transcription factors. J. Neurochem. 2007, 102, 522–538. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Tripathy, D.; Shete, S.; Ashfaq, R.; Haley, B.; Perkins, S.; Beitsch, P.; Khan, A.; Euhus, D.; Osborne, C.; et al. HER-2 gene amplification can be acquired as breast cancer progresses. Proc. Natl. Acad Sci. USA 2004, 101, 9393–9398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, L.; Ma, X.M.; Wang, T.T.; Yang, Y.; Zhang, N.; Zeng, N.; Bai, Z.G.; Yin, J.; Zhang, J.; Ding, G.Q.; et al. Psoralen Suppresses Cisplatin-Mediated Resistance and Induces Apoptosis of Gastric Adenocarcinoma by Disruption of the miR196a-HOXB7-HER2 Axis. Cancer Manag. Res. 2020, 12, 2803–2827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meiyanto, E.; Putri, D.D.; Susidarti, R.A.; Murwanti, R.; Sardjiman, R.A.; Fitriasari, A.; Husnaa, U.; Purnomo, H.; Kawaichi, M. Curcumin and its analogues (PGV-0 and PGV-1) enhance sensitivity of resistant MCF-7 cells to doxorubicin through inhibition of HER2 and NF-kB activation. Asian Pac. J. Cancer Prev. 2014, 15, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Klippstein, R.; Bansal, S.S.; Al-Jamal, K.T. Doxorubicin enhances curcumin’s cytotoxicity in human prostate cancer cells in vitro by enhancing its cellular uptake. Int. J. Pharm. 2016, 514, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Notarbartolo, M.; Poma, P.; Perri, D.; Dusonchet, L.; Cervello, M.; D’Alessandro, N. Antitumor effects of curcumin, alone or in combination with cisplatin or doxorubicin, on human hepatic cancer cells. Analysis of their possible relationship to changes in NF-kB activation levels and in IAP gene expression. Cancer Lett. 2005, 224, 53–65. [Google Scholar] [CrossRef]
- El-Far, A.H.; Darwish, N.H.E.; Mousa, S.A. Senescent Colon and Breast Cancer Cells Induced by Doxorubicin Exhibit Enhanced Sensitivity to Curcumin, Caffeine, and Thymoquinone. Integr. Cancer 2020, 19. [Google Scholar] [CrossRef] [Green Version]
- Renu, K.; Abilash, V.G.; Tirupathi Pichiah, P.B.; Arunachalam, S. Molecular mechanism of doxorubicin-induced cardiomyopathy–An update. Eur. J. Pharmacol. 2018, 818, 241–253. [Google Scholar] [CrossRef]
- Alexander, J.L.; Wilson, I.D.; Teare, J.; Marchesi, J.R.; Nicholson, J.K.; Kinross, J.M. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 356–365. [Google Scholar] [CrossRef]
- Wallace, B.D.; Wang, H.; Lane, K.T.; Scott, J.E.; Orans, J.; Koo, J.S.; Venkatesh, M.; Jobin, C.; Yeh, L.-A.; Mani, S. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science 2010, 330, 831–835. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.-H.; Ko, J.-L.; Liao, J.-M.; Huang, S.-S.; Lin, M.-Y.; Lee, L.-H.; Chang, L.-Y.; Ou, C.-C. D-methionine alleviates cisplatin-induced mucositis by restoring the gut microbiota structure and improving intestinal inflammation. Ther. Adv. Med. Oncol. 2019, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montassier, E.; Gastinne, T.; Vangay, P.; Al-Ghalith, G.; Bruley des Varannes, S.; Massart, S.; Moreau, P.; Potel, G.; de La Cochetiere, M.; Batard, E. Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment. Pharmacol. Ther. 2015, 42, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, Q.; Qi, H.; Wang, C.; Wang, C.; Zhang, J.; Dong, L. Doxorubicin-induced systemic inflammation is driven by upregulation of toll-like receptor TLR4 and endotoxin leakage. Cancer Res. 2016, 76, 6631–6642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carr, J.S.; King, S.; Dekaney, C.M. Depletion of enteric bacteria diminishes leukocyte infiltration following doxorubicin-induced small intestinal damage in mice. PLoS ONE 2017, 12, e0173429. [Google Scholar] [CrossRef]
- Wu, R.; Mei, X.; Wang, J.; Sun, W.; Xue, T.; Lin, C.; Xu, D. Zn(ii)-Curcumin supplementation alleviates gut dysbiosis and zinc dyshomeostasis during doxorubicin-induced cardiotoxicity in rats. Food Funct. 2019, 10, 5587–5604. [Google Scholar] [CrossRef]
- Mantawy, E.M.; El-Bakly, W.M.; Esmat, A.; Badr, A.M.; El-Demerdash, E. Chrysin alleviates acute doxorubicin cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. Eur. J. Pharmacol. 2014, 728, 107–118. [Google Scholar] [CrossRef]
- Benzer, F.; Kandemir, F.M.; Ozkaraca, M.; Kucukler, S.; Caglayan, C. Curcumin ameliorates doxorubicin-induced cardiotoxicity by abrogation of inflammation, apoptosis, oxidative DNA damage, and protein oxidation in rats. J. Biochem. Mol. Toxicol. 2018, 32, e22030. [Google Scholar] [CrossRef]
- Hou, N.; Mai, Y.; Qiu, X.; Yuan, W.; Li, Y.; Luo, C.; Liu, Y.; Zhang, G.; Zhao, G.; Luo, J.D. Carvacrol Attenuates Diabetic Cardiomyopathy by Modulating the PI3K/AKT/GLUT4 Pathway in Diabetic Mice. Front. Pharm. 2019, 10, 998. [Google Scholar] [CrossRef] [Green Version]
- Jamhiri, M.; Safi Dahaj, F.; Astani, A.; Hejazian, S.H.; Hafizibarjin, Z.; Ghobadi, M.; Moradi, A.; Khoradmehr, A.; Safari, F. Carvacrol Ameliorates Pathological Cardiac Hypertrophy in Both In-vivo and In-vitro Models. Iran J. Pharm. Res. 2019, 18, 1380–1394. [Google Scholar] [CrossRef]
- Jafarinezhad, Z.; Rafati, A.; Ketabchi, F.; Noorafshan, A.; Karbalay-Doust, S. Cardioprotective effects of curcumin and carvacrol in doxorubicin-treated rats: Stereological study. Food Sci. Nutr. 2019, 7, 3581–3588. [Google Scholar] [CrossRef] [Green Version]
- Kwong, J.Q.; Davis, J.; Baines, C.P.; Sargent, M.A.; Karch, J.; Wang, X.; Huang, T.; Molkentin, J.D. Genetic deletion of the mitochondrial phosphate carrier desensitizes the mitochondrial permeability transition pore and causes cardiomyopathy. Cell Death Differ. 2014, 21, 1209–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alcalá, S.; Klee, M.; Fernández, J.; Fleischer, A.; Pimentel-Muiños, F.X. A high-throughput screening for mammalian cell death effectors identifies the mitochondrial phosphate carrier as a regulator of cytochrome c release. Oncogene 2008, 27, 44–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junkun, L.; Erfu, C.; Tony, H.; Xin, L.; Sudeep, K.C.; Mingliang, Z.; Yanqin, W.; XiangQian, Q. Curcumin Downregulates Phosphate Carrier and Protects against Doxorubicin Induced Cardiomyocyte Apoptosis. Biomed. Res. Int. 2016, 2016, 1980763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, H.; Luo, Y.; Qiao, Y.; Zhang, Z.; Yin, D.; Yao, J.; You, J.; He, M. Curcumin attenuates doxorubicin-induced cardiotoxicity via suppressing oxidative stress and preventing mitochondrial dysfunction mediated by 14-3-3γ. Food Funct. 2018, 9, 4404–4418. [Google Scholar] [CrossRef]
- Yadav, Y.C.; Pattnaik, S.; Swain, K. Curcumin loaded mesoporous silica nanoparticles: Assessment of bioavailability and cardioprotective effect. Drug. Dev. Ind. Pharm. 2019, 45, 1889–1895. [Google Scholar] [CrossRef]
- Mohammed, H.S.; Hosny, E.N.; Khadrawy, Y.A.; Magdy, M.; Attia, Y.S.; Sayed, O.A.; AbdElaal, M. Protective effect of curcumin nanoparticles against cardiotoxicity induced by doxorubicin in rat. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165665. [Google Scholar] [CrossRef]
- Jain, A.; Rani, V. Mode of treatment governs curcumin response on doxorubicin-induced toxicity in cardiomyoblasts. Mol. Cell. Biochem. 2018, 442, 81–96. [Google Scholar] [CrossRef]
- Aksu, E.H.; Kandemir, F.M.; Yıldırım, S.; Küçükler, S.; Dörtbudak, M.B.; Çağlayan, C.; Benzer, F. Palliative effect of curcumin on doxorubicin-induced testicular damage in male rats. J. Biochem. Mol. Toxicol. 2019, 33, e22384. [Google Scholar] [CrossRef]
- Saad, S.Y.; Najjar, T.A.; Al-Rikabi, A.C. The preventive role of deferoxamine against acute doxorubicin-induced cardiac, renal and hepatic toxicity in rats. Pharmacol. Res. 2001, 43, 211–218. [Google Scholar] [CrossRef]
- Rashid, S.; Ali, N.; Nafees, S.; Ahmad, S.T.; Arjumand, W.; Hasan, S.K.; Sultana, S. Alleviation of doxorubicin-induced nephrotoxicity and hepatotoxicity by chrysin in Wistar rats. Toxicol. Mech. Methods 2013, 23, 337–345. [Google Scholar] [CrossRef]
- Benzer, F.; Kandemir, F.M.; Kucukler, S.; Comaklı, S.; Caglayan, C. Chemoprotective effects of curcumin on doxorubicin-induced nephrotoxicity in wistar rats: By modulating inflammatory cytokines, apoptosis, oxidative stress and oxidative DNA damage. Arch. Physiol. Biochem. 2018, 124, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Noone, D.G.; Iijima, K.; Parekh, R. Idiopathic nephrotic syndrome in children. Lancet 2018, 392, 61–74. [Google Scholar] [CrossRef]
- Fan, H.Y.; Wang, X.K.; Li, X.; Ji, K.; Du, S.H.; Liu, Y.; Kong, L.L.; Xu, J.C.; Yang, G.Q.; Chen, D.Q.; et al. Curcumin, as a pleiotropic agent, improves doxorubicin-induced nephrotic syndrome in rats. J. Ethnopharmacol. 2020, 250, 112502. [Google Scholar] [CrossRef] [PubMed]
- Li, C.L.; Liu, X.H.; Qiao, Y.; Ning, L.N.; Li, W.J.; Sun, Y.S.; Liu, D.S.; Gao, W.; Ma, C.M. Allicin alleviates inflammation of diabetic macroangiopathy via the Nrf2 and NF-kB pathway. Eur. J. Pharm. 2020, 876, 173052. [Google Scholar] [CrossRef]
- Hannan, M.A.; Dash, R.; Sohag, A.A.M.; Haque, M.N.; Moon, I.S. Neuroprotection Against Oxidative Stress: Phytochemicals Targeting TrkB Signaling and the Nrf2-ARE Antioxidant System. Front. Mol. Neurosci. 2020, 13, 116. [Google Scholar] [CrossRef]
- Ungvari, Z.; Tarantini, S.; Nyúl-Tóth, Á.; Kiss, T.; Yabluchanskiy, A.; Csipo, T.; Balasubramanian, P.; Lipecz, A.; Benyo, Z.; Csiszar, A. Nrf2 dysfunction and impaired cellular resilience to oxidative stressors in the aged vasculature: From increased cellular senescence to the pathogenesis of age-related vascular diseases. Geroscience 2019, 41, 727–738. [Google Scholar] [CrossRef]
- Imbaby, S.; Ewais, M.; Essawy, S.; Farag, N. Cardioprotective effects of curcumin and nebivolol against doxorubicin-induced cardiac toxicity in rats. Hum. Exp. Toxicol. 2014, 33, 800–813. [Google Scholar] [CrossRef]
- Wang, L.; Song, Y.; Parikh, A.; Joyce, P.; Chung, R.; Liu, L.; Afinjuomo, F.; Hayball, J.D.; Petrovsky, N.; Barclay, T.G.; et al. Doxorubicin-Loaded Delta Inulin Conjugates for Controlled and Targeted Drug Delivery: Development, Characterization, and In Vitro Evaluation. Pharmaceutics 2019, 11. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zhang, N.; Hao, Y.; Wang, Y.; Jia, S.; Zhang, H. Enhancement of curcumin antitumor efficacy and further photothermal ablation of tumor growth by single-walled carbon nanotubes delivery system in vivo. Drug Deliv. 2019, 26, 1017–1026. [Google Scholar] [CrossRef] [Green Version]
- Mohammadinejad, R.; Dehshahri, A.; Madamsetty, V.S.; Zahmatkeshan, M.; Tavakol, S.; Makvandi, P.; Khorsandi, D.; Pardakhty, A.; Ashrafizadeh, M.; Afshar, E.G. In vivo gene delivery mediated by non-viral vectors for cancer therapy. J. Control. Release 2020, 325, 249–275. [Google Scholar] [CrossRef]
- Vandita, K.; Shashi, B.; Santosh, K.G.; Pal, K.I. Enhanced apoptotic effect of curcumin loaded solid lipid nanoparticles. Mol. Pharm. 2012, 9, 3411–3421. [Google Scholar] [CrossRef] [PubMed]
- Puglia, C.; Frasca, G.; Musumeci, T.; Rizza, L.; Puglisi, G.; Bonina, F.; Chiechio, S. Curcumin loaded NLC induces histone hypoacetylation in the CNS after intraperitoneal administration in mice. Eur. J. Pharm. Biopharm. 2012, 81, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.W.; Chun, M.K.; Kim, O.; Subedi, R.K.; Ahn, S.G.; Yoon, J.H.; Choi, H.K. Doxorubicin-loaded solid lipid nanoparticles to overcome multidrug resistance in cancer therapy. Nanomedicine 2010, 6, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.L.; Rauth, A.M.; Bendayan, R.; Manias, J.L.; Ramaswamy, M.; Liu, Z.; Erhan, S.Z.; Wu, X.Y. A new polymer-lipid hybrid nanoparticle system increases cytotoxicity of doxorubicin against multidrug-resistant human breast cancer cells. Pharm. Res. 2006, 23, 1574–1585. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, Q.; Liu, W.; Li, Y.; Tang, H.; Liu, X.; Yang, X. Codelivery of doxorubicin and curcumin with lipid nanoparticles results in improved efficacy of chemotherapy in liver cancer. Int. J. Nanomed. 2015, 10, 257–270. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Chen, Q.; Li, Y.; Tang, H.; Liu, W.; Yang, X. Doxorubicin and curcumin co-delivery by lipid nanoparticles for enhanced treatment of diethylnitrosamine-induced hepatocellular carcinoma in mice. Eur. J. Pharm. Biopharm. 2015, 93, 27–36. [Google Scholar] [CrossRef]
- Dong, H.; Hu, J.; Wang, L.; Qi, M.; Lu, N.; Tan, X.; Yang, M.; Bai, X.; Zhan, X.; Han, B. SOX4 is activated by C-MYC in prostate cancer. Med. Oncol. 2019, 36, 92. [Google Scholar] [CrossRef]
- Cai, H.; Gong, L.; Liu, J.; Zhou, Q.; Zheng, Z. Diosgenin inhibits tumor angiogenesis through regulating GRP78-mediated HIF-1α and VEGF/VEGFR signaling pathways. Pharmazie 2019, 74, 680–684. [Google Scholar] [CrossRef]
- Xiong, X.-B.; Falamarzian, A.; Garg, S.M.; Lavasanifar, A. Engineering of amphiphilic block copolymers for polymeric micellar drug and gene delivery. J. Control. Release 2011, 155, 248–261. [Google Scholar]
- Li, P.-Y.; Lai, P.-S.; Hung, W.-C.; Syu, W.-J. Poly (L-lactide)-vitamin E TPGS nanoparticles enhanced the cytotoxicity of doxorubicin in drug-resistant MCF-7 breast cancer cells. Biomacromolecules 2010, 11, 2576–2582. [Google Scholar]
- Vandenplas, Y.; Bacarea, A.; Marusteri, M.; Bacarea, V.; Constantin, M.; Manolache, M. Efficacy and safety of APT198K for the treatment of infantile colic: A pilot study. J. Comp. Eff. Res. 2017, 6, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Xu, Q.; Wang, N.; Yang, Y.; Liu, J.; Yu, G.; Yang, X.; Xu, H.; Wang, H. A complex micellar system co-delivering curcumin with doxorubicin against cardiotoxicity and tumor growth. Int. J. Nanomed. 2018, 13, 4549–4561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karavasili, C.; Andreadis, D.A.; Katsamenis, O.L.; Panteris, E.; Anastasiadou, P.; Kakazanis, Z.; Zoumpourlis, V.; Markopoulou, C.K.; Koutsopoulos, S.; Vizirianakis, I.S. Synergistic antitumor potency of a self-assembling peptide hydrogel for the local co-delivery of doxorubicin and curcumin in the treatment of head and neck cancer. Mol. Pharm. 2019, 16, 2326–2341. [Google Scholar] [CrossRef] [PubMed]
- Mueckler, M.; Thorens, B. The SLC2 (GLUT) family of membrane transporters. Mol. Asp. Med. 2013, 34, 121–138. [Google Scholar] [CrossRef] [Green Version]
- Szablewski, L. Expression of glucose transporters in cancers. Biochim. Biophys. Acta (Bba) Rev. Cancer 2013, 1835, 164–169. [Google Scholar] [CrossRef]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Yeh, W.-L.; Lin, C.-J.; Fu, W.-M. Enhancement of glucose transporter expression of brain endothelial cells by vascular endothelial growth factor derived from glioma exposed to hypoxia. Mol. Pharmacol. 2008, 73, 170–177. [Google Scholar] [CrossRef]
- Boado, R.J.; Black, K.L.; Pardridge, W.M. Gene expression of GLUT3 and GLUT1 glucose transporters in human brain tumors. Mol. Brain Res. 1994, 27, 51–57. [Google Scholar] [CrossRef]
- Sarisozen, C.; Dhokai, S.; Tsikudo, E.G.; Luther, E.; Rachman, I.M.; Torchilin, V.P. Nanomedicine based curcumin and doxorubicin combination treatment of glioblastoma with scFv-targeted micelles: In vitro evaluation on 2D and 3D tumor models. Eur. J. Pharm. Biopharm. 2016, 108, 54–67. [Google Scholar] [CrossRef]
- Huang, J.; Shen, F.; Huang, H.; Ling, C.; Zhang, G. Th1high in tumor microenvironment is an indicator of poor prognosis for patients with NSCLC. Oncotarget 2017, 8, 13116–13125. [Google Scholar] [CrossRef] [Green Version]
- Thakkar, S.; Sharma, D.; Kalia, K.; Tekade, R.K. Tumor microenvironment targeted nanotherapeutics for cancer therapy and diagnosis: A review. Acta Biomater. 2020, 101, 43–68. [Google Scholar] [CrossRef] [PubMed]
- Sesarman, A.; Tefas, L.; Sylvester, B.; Licarete, E.; Rauca, V.; Luput, L.; Patras, L.; Porav, S.; Banciu, M.; Porfire, A. Co-delivery of curcumin and doxorubicin in PEGylated liposomes favored the antineoplastic C26 murine colon carcinoma microenvironment. Drug Deliv. Transl. Res. 2019, 9, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.; Maitra, A. Dextran-doxorubicin/chitosan nanoparticles for solid tumor therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Kataoka, K. Preclinical and clinical studies of anticancer agent-incorporating polymer micelles. Cancer Sci. 2009, 100, 572–579. [Google Scholar] [CrossRef]
- Gupta, M.; Agrawal, G.P.; Vyas, S.P. Polymeric nanomedicines as a promising vehicle for solid tumor therapy and targeting. Curr. Mol. Med. 2013, 13, 179–204. [Google Scholar] [CrossRef]
- Maeda, H.; Bharate, G.Y.; Daruwalla, J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur. J. Pharm. Biopharm. 2009, 71, 409–419. [Google Scholar] [CrossRef]
- Deng, M.; Nair, L.S.; Nukavarapu, S.P.; Kumbar, S.G.; Jiang, T.; Krogman, N.R.; Singh, A.; Allcock, H.R.; Laurencin, C.T. Miscibility and in vitro osteocompatibility of biodegradable blends of poly[(ethyl alanato)(p-phenyl phenoxy)phosphazene] and poly(lactic acid-glycolic acid). Biomaterials 2008, 29, 337–349. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Song, Y.; Song, W.; Liu, Y.; Liu, Z.; Zhang, D.; Tang, Z.; Bai, O. Co-delivery of Doxorubicin and Curcumin with Polypeptide Nanocarrier for Synergistic Lymphoma Therapy. Sci. Rep. 2020, 10, 7832. [Google Scholar] [CrossRef]
- He, L.; Qing, F.; Li, M.; Lan, D. Paclitaxel/IR1061-Co-Loaded Protein Nanoparticle for Tumor-Targeted and pH/NIR-II-Triggered Synergistic Photothermal-Chemotherapy. Int. J. Nanomed. 2020, 15, 2337–2349. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Yang, Q.; Weng, Q.; Wang, S. pH sensitive doxorubicin-loaded nanoparticle based on Radix pseudostellariae protein-polysaccharide conjugate and its improvement on HepG2 cellular uptake of doxorubicin. Food Chem. Toxicol. 2020, 136, 111099. [Google Scholar] [CrossRef]
- Abdalla, M.O.; Karna, P.; Sajja, H.K.; Mao, H.; Yates, C.; Turner, T.; Aneja, R. Enhanced noscapine delivery using uPAR-targeted optical-MR imaging trackable nanoparticles for prostate cancer therapy. J. Control. Release 2011, 149, 314–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Persson, M.; Juhl, K.; Rasmussen, P.; Brandt-Larsen, M.; Madsen, J.; Ploug, M.; Kjær, A. uPAR targeted radionuclide therapy with 177Lu-DOTA-AE105 inhibits dissemination of metastatic prostate cancer. Mol. Pharm. 2014, 11, 2796–2806. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zheng, K.; Hu, P.; Chen, Z.; Zhou, S.; Chen, J.; Yuan, C.; Chen, S.; Zheng, W.; Ma, E. A novel tumor targeting drug carrier for optical imaging and therapy. Theranostics 2014, 4, 642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, J.-T.; Ge, M.; Peng, S.; Zhong, J.; Li, Y.; Weng, Z.; Wu, L.; Zheng, L. Dynamic imine bonds based shape memory polymers with permanent shape reconfigurability for 4D printing. Acs Appl. Mater. Interfaces 2019, 11, 40642–40651. [Google Scholar] [CrossRef] [PubMed]
- Kratz, F.; Müller, I.A.; Ryppa, C.; Warnecke, A. Prodrug strategies in anticancer chemotherapy. Chem. Med. Chem. 2008, 3, 20–53. [Google Scholar] [CrossRef] [PubMed]
- Khandare, J.J.; Chandna, P.; Wang, Y.; Pozharov, V.P.; Minko, T. Novel polymeric prodrug with multivalent components for cancer therapy. J. Pharm. Exp. 2006, 317, 929–937. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yang, C.; Wang, W.; Liu, J.; Liu, Q.; Huang, F.; Chu, L.; Gao, H.; Li, C.; Kong, D.; et al. Co-delivery of doxorubicin and curcumin by pH-sensitive prodrug nanoparticle for combination therapy of cancer. Sci. Rep. 2016, 6, 21225. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Li, J.; Li, Y.; Song, L.; Li, D.; Peng, L.; Wan, Y.; Hua, S. Nanomicelles loaded with doxorubicin and curcumin for alleviating multidrug resistance in lung cancer. Int. J. Nanomed. 2016, 11, 5757–5770. [Google Scholar] [CrossRef] [Green Version]
- Pramanik, D.; Majeti, B.K.; Mondal, G.; Karmali, P.P.; Sistla, R.; Ramprasad, O.G.; Srinivas, G.; Pande, G.; Chaudhuri, A. Lipopeptide with a RGDK tetrapeptide sequence can selectively target genes to proangiogenic α5β1 integrin receptor and mouse tumor vasculature. J. Med. Chem. 2008, 51, 7298–7302. [Google Scholar] [CrossRef]
- Barui, S.; Saha, S.; Mondal, G.; Haseena, S.; Chaudhuri, A. Simultaneous delivery of doxorubicin and curcumin encapsulated in liposomes of pegylated RGDK-lipopeptide to tumor vasculature. Biomaterials 2014, 35, 1643–1656. [Google Scholar] [CrossRef]
- Reddy, K.L.; Sharma, P.K.; Singh, A.; Kumar, A.; Shankar, K.R.; Singh, Y.; Garg, N.; Krishnan, V. Amine-functionalized, porous silica-coated NaYF(4):Yb/Er upconversion nanophosphors for efficient delivery of doxorubicin and curcumin. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, N.G.; Panda, S.S.; Fayad, W.; El-Manawaty, M.A.; Srour, A.M.; Girgis, A.S. Novel Curcumin Inspired Antineoplastic 1-Sulfonyl-4-Piperidones: Design, Synthesis and Molecular Modeling Studies. Anticancer Agents Med. Chem. 2019, 19, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, J.; Shi, Z.; Yang, Y.; Xie, X.; Lee, S.M.; Wang, Y.; Leong, K.W.; Chen, M. pH-sensitive polymeric nanoparticles for co-delivery of doxorubicin and curcumin to treat cancer via enhanced pro-apoptotic and anti-angiogenic activities. Acta Biomater. 2017, 58, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Fumoto, S.; Miyamoto, H.; Chen, Y.; Kuroda, N.; Nishida, K. One-step formation of lipid-polyacrylic acid-calcium carbonate nanoparticles for co-delivery of doxorubicin and curcumin. J. Drug Target. 2017, 25, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Tefas, L.R.; Sylvester, B.; Tomuta, I.; Sesarman, A.; Licarete, E.; Banciu, M.; Porfire, A. Development of antiproliferative long-circulating liposomes co-encapsulating doxorubicin and curcumin, through the use of a quality-by-design approach. Drug Des. Dev. Ther. 2017, 11, 1605–1621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, J.D.; ZhuGe, D.L.; Tong, M.Q.; Lin, M.T.; Xu, X.F.; Tang, X.; Zhao, Y.Z.; Xu, H.L. pH-sensitive polymeric nanoparticles of mPEG-PLGA-PGlu with hybrid core for simultaneous encapsulation of curcumin and doxorubicin to kill the heterogeneous tumour cells in breast cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 302–313. [Google Scholar] [CrossRef] [Green Version]
- Yousef, S.; Alsaab, H.O.; Sau, S.; Iyer, A.K. Development of asialoglycoprotein receptor directed nanoparticles for selective delivery of curcumin derivative to hepatocellular carcinoma. Heliyon 2018, 4, e01071. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Zhou, C.; Zou, Y.; Jin, Y.; Han, S.; Liu, Q.; Hu, X.; Wang, L.; Ma, Y.; Liu, Y. Multi pH-sensitive polymer-drug conjugate mixed micelles for efficient co-delivery of doxorubicin and curcumin to synergistically suppress tumor metastasis. Biomater. Sci. 2020, 8, 5029–5046. [Google Scholar] [CrossRef]
- Hong, W.; Shi, H.; Qiao, M.; Zhang, Z.; Yang, W.; Dong, L.; Xie, F.; Zhao, C.; Kang, L. pH-sensitive micelles for the intracellular co-delivery of curcumin and Pluronic L61 unimers for synergistic reversal effect of multidrug resistance. Sci. Rep. 2017, 7, 42465. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Li, X.; Liao, Q.; Liu, Y.; Xi, K.; Huang, W.; Jia, X. Water-dispersible PEG-curcumin/amine-functionalized covalent organic framework nanocomposites as smart carriers for in vivo drug delivery. Nat. Commun. 2018, 9, 2785. [Google Scholar] [CrossRef] [Green Version]
- Rastegar, R.; Akbari Javar, H.; Khoobi, M.; Dehghan Kelishadi, P.; Hossein Yousefi, G.; Doosti, M.; Hossien Ghahremani, M.; Shariftabrizi, A.; Imanparast, F.; Gholibeglu, E.; et al. Evaluation of a novel biocompatible magnetic nanomedicine based on beta-cyclodextrin, loaded doxorubicin-curcumin for overcoming chemoresistance in breast cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 207–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.T.; Ye, Q.B.; Yang, Q.J.; Wang, G.H. Hierarchical bioresponsive nanocarriers for codelivery of curcumin and doxorubicin. Colloids Surf. B Biointerfaces 2019, 180, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Sesarman, A.; Muntean, D.; Abrudan, B.; Tefas, L.; Sylvester, B.; Licarete, E.; Rauca, V.; Luput, L.; Patras, L.; Banciu, M.; et al. Improved pharmacokinetics and reduced side effects of doxorubicin therapy by liposomal co-encapsulation with curcumin. J. Liposome Res. 2019, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yu, L.; Guo, R.; Dong, A.; Lin, C.; Zhang, J. A Modular Coassembly Approach to All-In-One Multifunctional Nanoplatform for Synergistic Codelivery of Doxorubicin and Curcumin. Nanomaterial 2018, 8, 167. [Google Scholar] [CrossRef] [Green Version]
- Rejinold, N.S.; Yoo, J.; Jon, S.; Kim, Y.C. Curcumin as a Novel Nanocarrier System for Doxorubicin Delivery to MDR Cancer Cells: In vitro and in vivo Evaluation. Acs Appl. Mater. Interfaces 2018, 10, 28458–28470. [Google Scholar] [CrossRef]
- Yan, T.; Li, D.; Li, J.; Cheng, F.; Cheng, J.; Huang, Y.; He, J. Effective co-delivery of doxorubicin and curcumin using a glycyrrhetinic acid-modified chitosan-cystamine-poly(ε-caprolactone) copolymer micelle for combination cancer chemotherapy. Colloids Surf. B Biointerfaces 2016, 145, 526–538. [Google Scholar] [CrossRef]
- Sabzi, A.; Rahmani, A.; Edalati, M.; Kahroba, H.; Dadpour, M.R.; Salehi, R.; Zarebkohan, A. Targeted co-delivery of curcumin and doxorubicin by citric acid functionalized Poly (ε-caprolactone) based micelle in MDA-MB-231 cell. Colloids Surf. B Biointerfaces 2020, 194, 111225. [Google Scholar] [CrossRef]
- Kumari, M.; Purohit, M.P.; Patnaik, S.; Shukla, Y.; Kumar, P.; Gupta, K.C. Curcumin loaded selenium nanoparticles synergize the anticancer potential of doxorubicin contained in self-assembled, cell receptor targeted nanoparticles. Eur. J. Pharm. Biopharm. 2018, 130, 185–199. [Google Scholar] [CrossRef]
- Guorgui, J.; Wang, R.; Mattheolabakis, G.; Mackenzie, G.G. Curcumin formulated in solid lipid nanoparticles has enhanced efficacy in Hodgkin’s lymphoma in mice. Arch. Biochem. Biophys. 2018, 648, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Fathy Abd-Ellatef, G.E.; Gazzano, E.; Chirio, D.; Hamed, A.R.; Belisario, D.C.; Zuddas, C.; Peira, E.; Rolando, B.; Kopecka, J.; Assem Said Marie, M.; et al. Curcumin-Loaded Solid Lipid Nanoparticles Bypass P-Glycoprotein Mediated Doxorubicin Resistance in Triple Negative Breast Cancer Cells. Pharmaceutics 2020, 12, 96. [Google Scholar] [CrossRef] [Green Version]
- Harini, L.; Srivastava, S.; Gnanakumar, G.P.; Karthikeyan, B.; Ross, C.; Krishnakumar, V.; Kannan, V.R.; Sundar, K.; Kathiresan, T. An ingenious non-spherical mesoporous silica nanoparticle cargo with curcumin induces mitochondria-mediated apoptosis in breast cancer (MCF-7) cells. Oncotarget 2019, 10, 1193–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
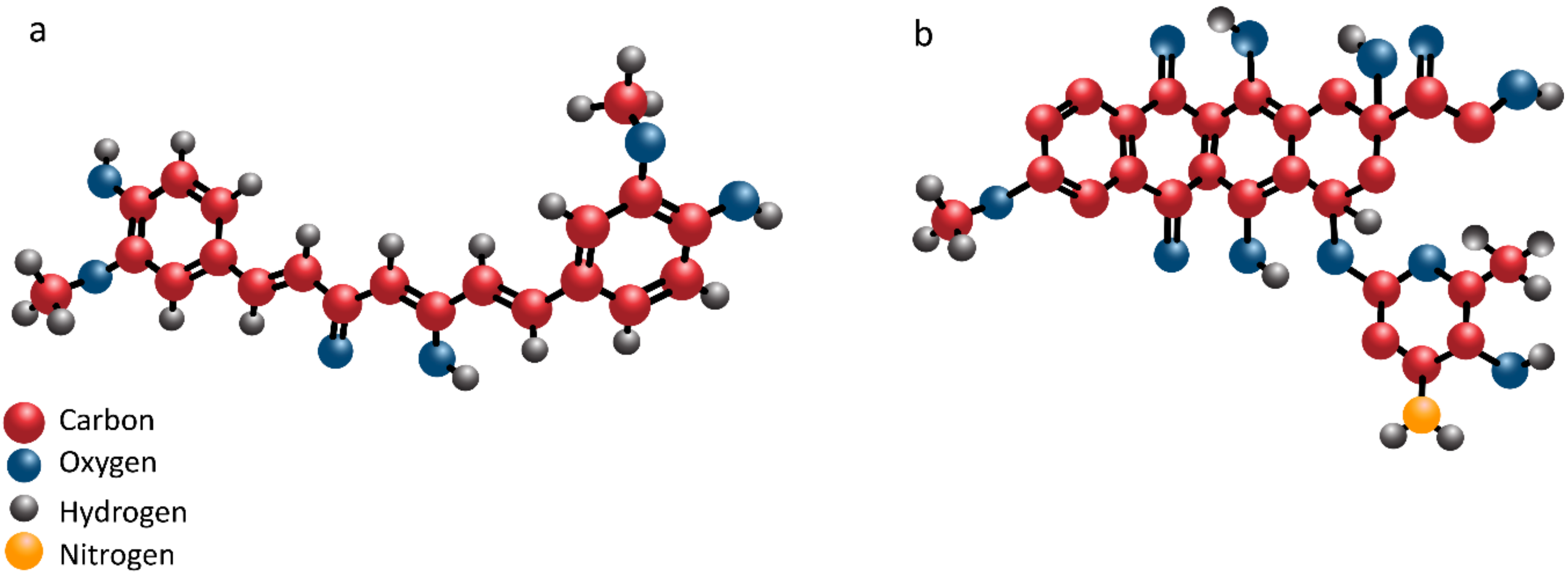
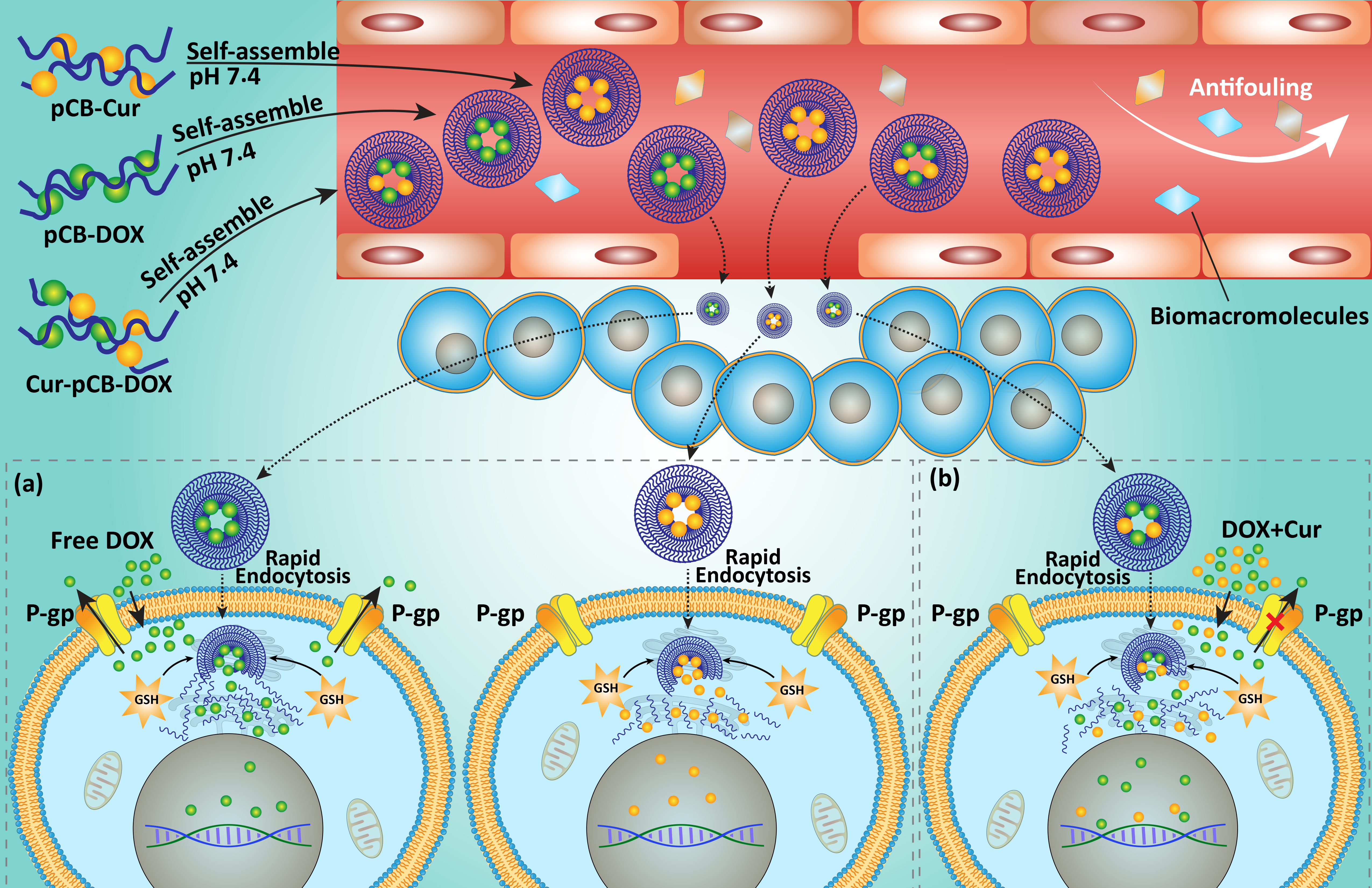
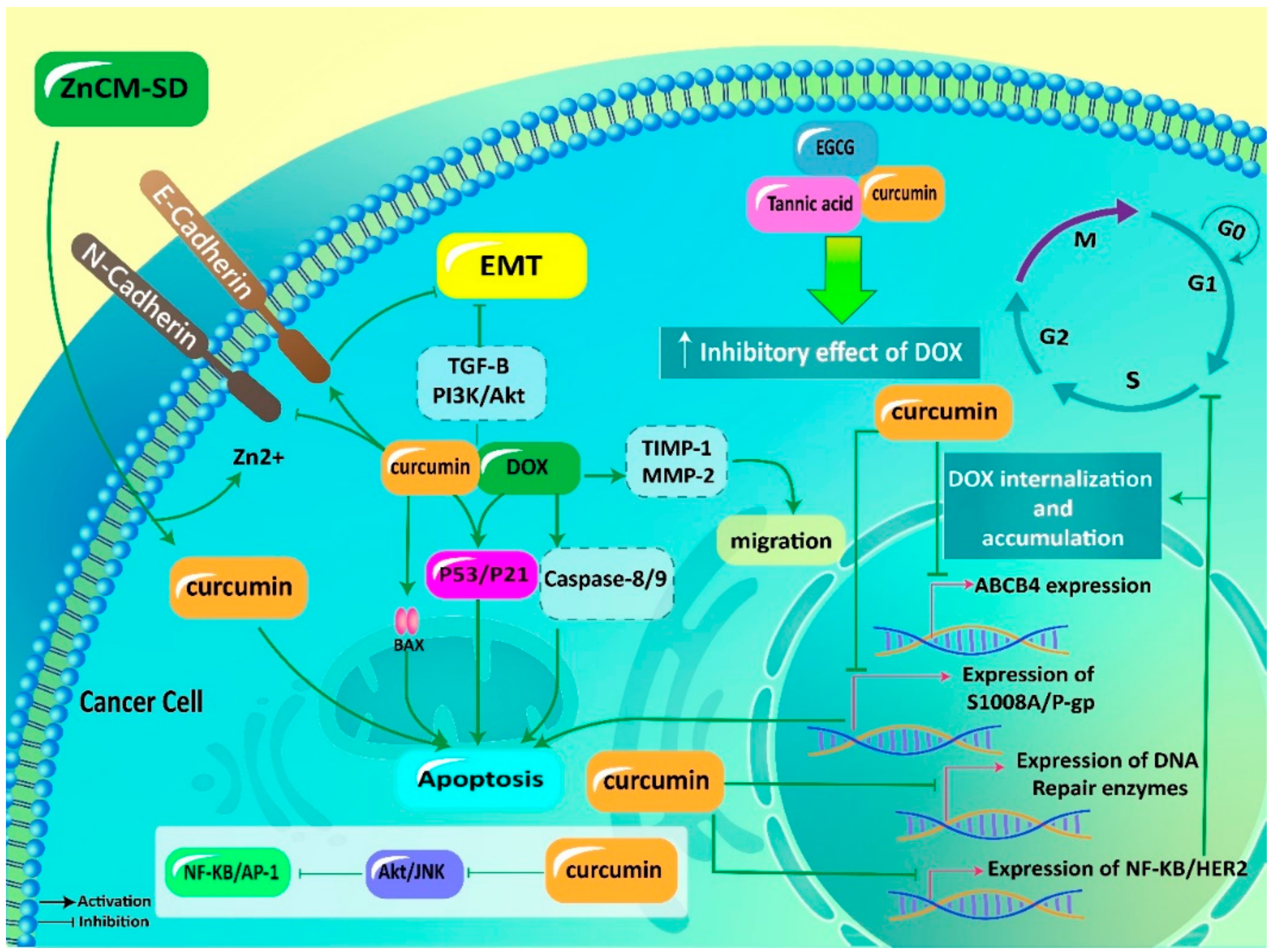



| Cancer Type | In vitro/In vivo | Cell Line/Animal Model | Effect on Doxorubicin Efficacy | Dose | Experiment Duration | Remarks | Refs |
|---|---|---|---|---|---|---|---|
| Glioblastoma | In vitro | T98G (world health organization (WHO) grade IV), U87MG (WHO grade III), and T67 (WHO grade III) human glioma and C6 rat glioma cell lines | Enhancement | 20, 40 and 60 μmol/L | 48 h | Promoting inhibitory effect of DOX on cancer cells Reversing chemoresistance Downregulation of AP-1 and NF-κB Apoptosis induction Inhibiting JNK and Akt signaling pathways | [142] |
| Breast cancer | In vitro | MCF-7 cells | Enhancement | 6-110 μM | 24 h | Downregulation of HER2 and NF-κB Subsequent sensitivity into DOX chemotherapy | [145] |
| Breast cancer | In vitro | MCF-7 and MDA-MB-231 cells | Enhancement | 0-100 μM | 72 h | Downregulation of ABCB4 Increasing DOX accumulation and cytotoxicity | [135] |
| Chronic myeloid leukemia | In vitro | Human CML cell lines K562 and K562/DOX | Enhancement | 0.5, 1 and 2 μM | 48 h | Promoting sensitivity into DOX chemotherapy via P-gp and S100A8 downregulation | [138] |
| Hepatic cancer | In vitro | HA22T/VGH cells | Enhancement | 10, 15, 20 and 25 μM | 72 h | Downregulation of c-Myc and COX-2 Decreasing expression of Bcl-2, Bcl-Xl and c-IAP-2 levels Apoptosis induction | [147] |
| Colon cancer Breast cancer | In vitro | Human colon HCT116 and breast cancer MCF7 cell lines | Enhancement | 15 μM | - | Apoptosis induction Exerting antiproliferative activity Enhancing DOX efficacy | [148] |
| Neuroblastoma | In vitro | SH-SY5Y cells | Enhancement | 5-50 μM | 24 h | Disrupting cancer invasion Downregulation of MMP-2 Upregulation of TIMP-1 Enhancing DOX sensitivity | [107] |
| Gastric adenocarcinoma | In vitro | AGS cells | Enhancement | 0-30 μg/mL | 24, 48, 72 and 96 h | Apoptosis stimulation via Bcl-2 downregulation and Bax and caspase-9 upregulation Suppressing tumor spheroid formation, proliferation and metastasis | [108] |
| In vitro/In vivo | Cell Line/Animal Model | Dose | Experiment Duration | Administration Route | Remarks | Refs |
|---|---|---|---|---|---|---|
| In vivo | Wistar rats | 100 and 200 mg/kg | 7 days | Oral gavage | Reducing oxidative stress and MDH levels Promoting sperm motility and viability Preventing necrosis and degeneration | [169] |
| In vivo | Rat | 100 and 200 mg/kg | 7 days | Oral administration | Preventing apoptosis via caspase-3 downregulation Ameliorating inflammation via TNF-α and NF-κB downregulation Inhibiting DNA damage | [172] |
| In vivo | DOX-mediated cardiotoxicity in rat | 100 mg/kg of curcumin 50 mg/kg CAR | 22 days | Intraperitoneal administration | Reducing volume of myocardium and vessels Decreasing number of cardiomyocyte nuclei Improving heart function Decreasing connective tissue volume | [161] |
| In vivo | Rat | 100 and 200 mg/kg | 7 days | Oral administration | Alleviation of oxidative stress, inflammation, apoptosis and DNA damage Downregulation of TNF-α and NF-κB | [158] |
| In vivo | Rat | 100 and 200 mg/kg of curcumin 1 and 2 mg/kg of nebivolol | 30 days | Intraperitoneal administration | Enhancing survival rate Improving body weight, heart index and ECG parameters Preventing oxidative damage and apoptosis | [178] |
| Nanocarrier | In vitro/In vivo | Cell Line/Animal Model | Encapsulation Efficiency (%) | Particle Size (nm) | Zeta Potential (mV) | Remarks | Refs |
|---|---|---|---|---|---|---|---|
| Liposome | In vitro | B16F10 cells | 100 (DOX) 86 (curcumin) | 190–230 | 2–4 | Surface modification of liposomes with RGDK promotes their cellular uptake Enhancing antitumor activity by 2–3 folds | [221] |
| Polymeric nanoparticles | In vitro | HepG 2 and HeLa cells | 18.35 (DOX) 91 (curcumin) | 183.5 | −0.68 | Possessing pH sensitivity capability Being inactive at blood circulation Activation at tumor site Enhancing antitumor activity | [218] |
| Polymeric nanoparticles | In vitro | HUVEC cells and MCF-7/ADR cells | 92 | 115–135 | 0.41 | High drug loading Hemodynamic stability Intracellular accumulation Suppressing cancer progression | [235] |
| Polymeric nanoparticles | In vitro In vivo | NIH-3T3 (mouse embryonic fibroblast cells), HeLa (human cervical cancer cells), NCI-H460 (Human lung carcinoma cells), and HFL1 (Human normal lung cells) | 23–53 | 180–220 | 10–15 | Apoptosis stimulation in resistant cancer cells DNA fragmentation Enhancing bioavailability and antitumor activity | [236] |
| Micelle | In vitro In vivo | H9C2 cells tumor-bearing mice | 90.6–99.8 | 90.6–120 | −0.13 to −2.34 | High encapsulation efficiency and drug loading Sustained drug release Elevating DOX accumulation in cancer cells Providing a delay in cancer growth | [193] |
| Micelle | In vitro | U87MG cells | - | 14.4 to 14.8 | −4.2 to −4.4 | Apoptosis induction via caspase-3 and caspase-7 upregulation Deep internalization via GLUT-1 Decreasing cancer viability | [200] |
| Polymeric micelle | In vitro | HepG2 and HUVEC cells | 90.9 (DOX) 70.7 (curcumin) | 80–110 | −0.5 to +10 | Redox-responsive drug release Internalization through endocytosis Providing a synergistic effect and enhancing antitumor activity | [237] |
| Polymeric micelles | In vitro | MCF-7 cells | - | 164.2–190 | - | Enhanced cellular uptake due to EPR effect Elevating cytotoxicity against cancer cells | [139] |
| Micelle | In vitro | MDA-MB-231 cells | 94.69 (DOX) 99.97 (curcumin) | 60 | −16.4 | Exerting synergistic effect Apoptosis induction | [238] |
| Selenium nanoparticles | In vitro In vivo | HCT116 cells Tumor-bearing mice | - | 202–240 | −31 to −37 | Increasing ROS levels Disrupting mitochondrial homeostasis Triggering apoptosis and cell cycle arrest Suppressing metastasis via EMT and NF-κB downregulation Autophagy inhibition Promoting antitumor activity | [239] |
| Solid lipid nanoparticles | In vivo | Hodgkin’s lymphoma in mice | - | 125.2 | −19.4 | Promoting curcumin bioavailability Reducing cancer growth and viability Apoptosis induction via XIAP and Mcl-1 downregulation Ameliorating inflammation by reducing pro-inflammatory cytokines including IL-6 and TNF-α | [240] |
| Solid lipid nanoparticles | In vitro In vivo | Human MCF-7 cells, human TNBC MDA-MB-231 and murine mammary cancer JC cells Tumor xenograft | - | - | - | Enhancing cytotoxicity against cancer cells by 5-10 folds Inhibition of NF-κB signaling pathway and subsequent decrease in P-gp expression Increasing accumulation of antitumor agents in cancer cells | [241] |
| Mesoporous silica nanoparticles | In vitro | MCF-7 cells | - | - | - | Localization in cytoplasmic vesicles Triggering apoptosis via capase-6, -9 and -12 upregulation Activation of PTEN and CHOP for stimulating apoptosis Disrupting mitochondrial homeostasis Autophagy induction by nanoparticles | [242] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashrafizadeh, M.; Zarrabi, A.; Hashemi, F.; Zabolian, A.; Saleki, H.; Bagherian, M.; Azami, N.; Bejandi, A.K.; Hushmandi, K.; Ang, H.L.; et al. Polychemotherapy with Curcumin and Doxorubicin via Biological Nanoplatforms: Enhancing Antitumor Activity. Pharmaceutics 2020, 12, 1084. https://doi.org/10.3390/pharmaceutics12111084
Ashrafizadeh M, Zarrabi A, Hashemi F, Zabolian A, Saleki H, Bagherian M, Azami N, Bejandi AK, Hushmandi K, Ang HL, et al. Polychemotherapy with Curcumin and Doxorubicin via Biological Nanoplatforms: Enhancing Antitumor Activity. Pharmaceutics. 2020; 12(11):1084. https://doi.org/10.3390/pharmaceutics12111084
Chicago/Turabian StyleAshrafizadeh, Milad, Ali Zarrabi, Farid Hashemi, Amirhossein Zabolian, Hossein Saleki, Morteza Bagherian, Negar Azami, Atefe Kazemzade Bejandi, Kiavash Hushmandi, Hui Li Ang, and et al. 2020. "Polychemotherapy with Curcumin and Doxorubicin via Biological Nanoplatforms: Enhancing Antitumor Activity" Pharmaceutics 12, no. 11: 1084. https://doi.org/10.3390/pharmaceutics12111084
APA StyleAshrafizadeh, M., Zarrabi, A., Hashemi, F., Zabolian, A., Saleki, H., Bagherian, M., Azami, N., Bejandi, A. K., Hushmandi, K., Ang, H. L., Makvandi, P., Khan, H., & Kumar, A. P. (2020). Polychemotherapy with Curcumin and Doxorubicin via Biological Nanoplatforms: Enhancing Antitumor Activity. Pharmaceutics, 12(11), 1084. https://doi.org/10.3390/pharmaceutics12111084









