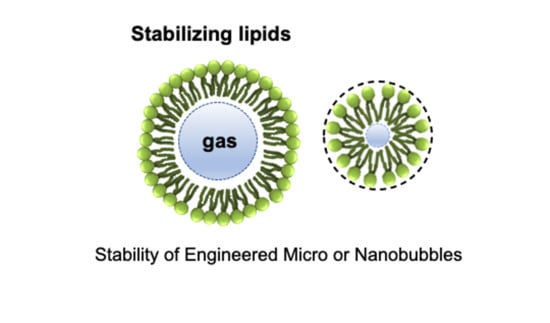
Stability of Engineered Micro or Nanobubbles for Biomedical Applications
Abstract
:1. Introduction
2. Bubble Structure
2.1. Shell
2.2. Core Gas
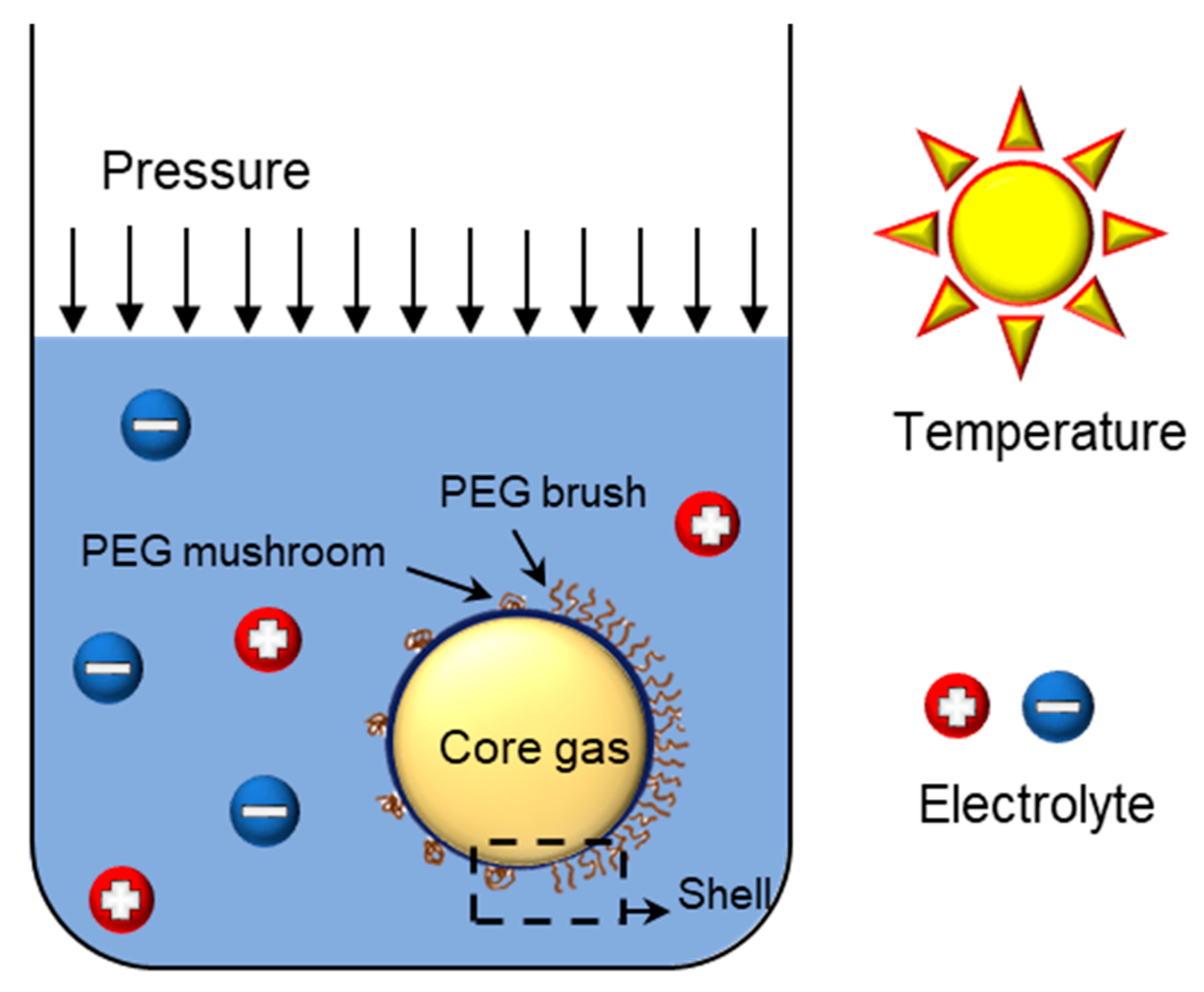
3. Stocking Condition
3.1. Zeta Potential, Electrolyte
3.2. Pressure, Temperature
3.3. Initial Bubble Concentration
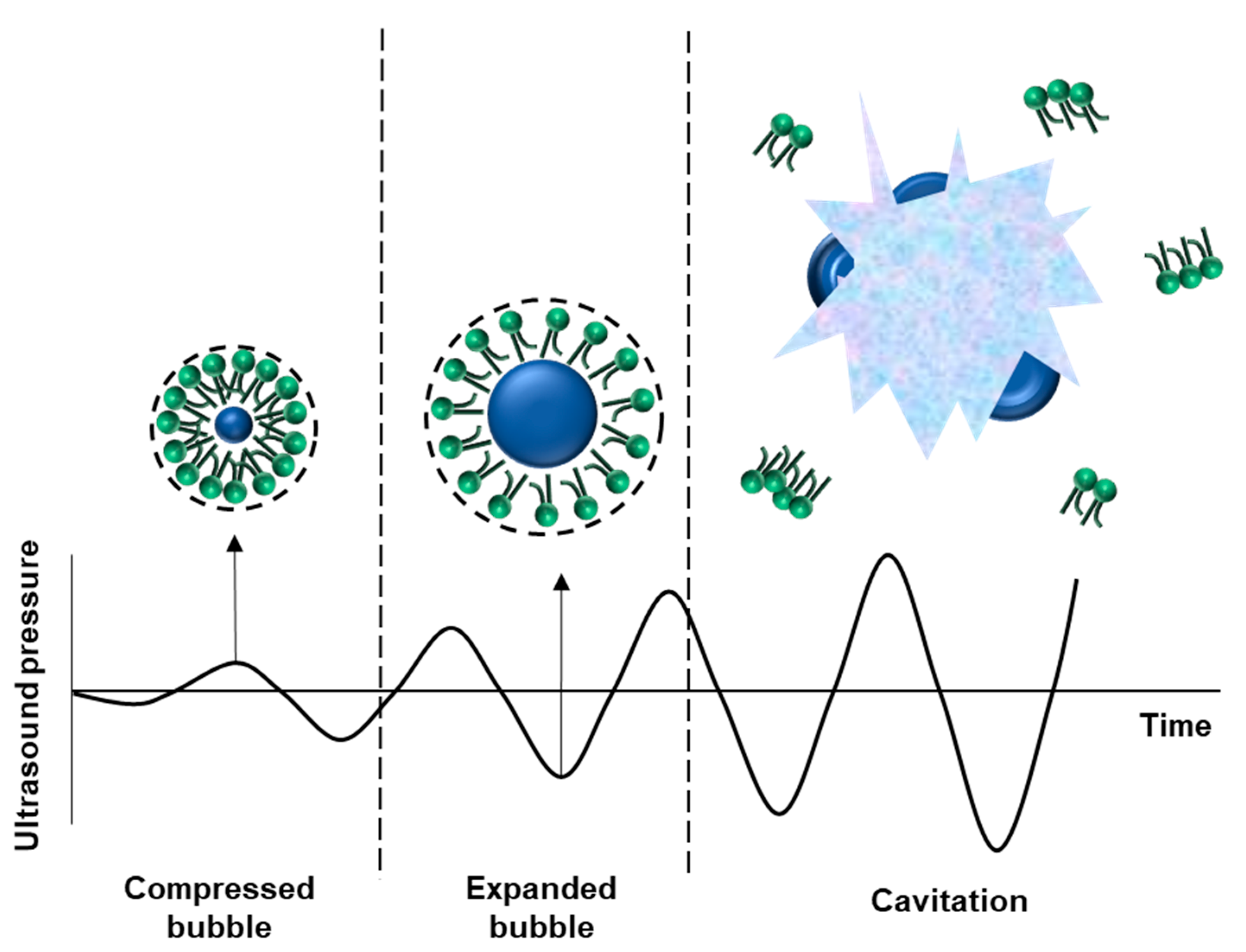
4. Bubble Stability in the Ultrasonication Field
- The cell membrane potential changes favorably to ensure the inclusion effect, while there is a regular mechanical load exerted on the cell membrane by the vibration of the bubble by stable cavitation.
- The volume of the vibrating bubble changes as it changes from its stable state to inertial cavitation. Accordingly, the gap between the vascular endothelial cells is temporarily increased, the cohesive force is weakened, the diffusion of reactants is strengthened, and absorption into the tissue is increased.
- Temporary pores, based on ultrasonic treatment caused by inertial cavitation, are created in vascular endothelial cells, and the inclusion of large molecules in the cell increases.
5. In Vivo Application
- Destruction by phagocytosis by the body’s immune system.
- Central gas elution and destruction by physical impact.
6. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Shin, S.; Han, D.; Park, M.C.; Mun, J.Y.; Choi, J.; Chun, H.; Kim, S.; Hong, J.W. Separation of extracellular nanovesicles and apoptotic bodies from cancer cell culture broth using tunable microfluidic systems. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.; Tung, S.H.; Wang, N.S.; Reipa, V. Small-angle neutron scattering measurement of silicon nanoparticle size. Nanotechnology 2008, 19, 085715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebina, K.; Shi, K.; Hirao, M.; Hashimoto, J.; Kawato, Y.; Kaneshiro, S.; Morimoto, T.; Koizumi, K.; Yoshikawa, H. Oxygen and air nanobubble water solution promote the growth of plants, fishes, and mice. PLoS ONE 2013, 8, e65339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.F.; Zhou, J.; Chen, X.R.; Yu, J. Drug-loaded nanobubbles for ultrasound-mediated antitumor treatment. J. Biol. Regul. Homeost. Agents 2018, 32, 923–929. [Google Scholar]
- Khan, M.S.; Hwang, J.; Lee, K.; Choi, Y.; Kim, K.; Koo, H.J.; Hong, J.W.; Choi, J. Oxygen-carrying micro/nanobubbles: Composition, synthesis techniques and potential prospects in photo-triggered theranostics. Molecules 2018, 23, 2210. [Google Scholar] [CrossRef] [Green Version]
- Kwan, J.J.; Kaya, M.; Borden, M.A.; Dayton, P.A. Theranostic oxygen delivery using ultrasound and microbubbles. Theranostics 2012, 2, 1174. [Google Scholar] [CrossRef] [Green Version]
- Schutt, E.G.; Klein, D.H.; Mattrey, R.M.; Riess, J.G. Injectable microbubbles as contrast agents for diagnostic ultrasound imaging: The key role of perfluorochemicals. Angew. Chem. Int. Ed. Engl. 2003, 42, 3218–3235. [Google Scholar] [CrossRef]
- Kuo, T.T.; Wang, C.H.; Wang, J.Y.; Chiou, H.J.; Fan, C.H.; Yeh, C.K. Concurrent osteosarcoma theranostic strategy using contrast-enhanced ultrasound and drug-loaded bubbles. Pharmaceutics 2019, 11, 223. [Google Scholar] [CrossRef] [Green Version]
- Bjanes, T.; Kotopoulis, S.; Murvold, E.T.; Kamceva, T.; Gjertsen, B.T.; Gilja, O.H.; Schjøtt, J.; Riedel, B.; McCormack, E. Ultrasound- and microbubble-assisted gemcitabine delivery to pancreatic cancer cells. Pharmaceutics 2020, 12, 141. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, K.; Yonezawa, K.; Fumoto, S.; Miura, Y.; Hagimori, M.; Nishida, K.; Kawakami, S. Application of direct sonoporation from a defined surface area of the peritoneum: Evaluation of transfection characteristics in mice. Pharmaceutics 2019, 11, 244. [Google Scholar] [CrossRef] [Green Version]
- Graham, K.; Unger, E. Overcoming tumor hypoxia as a barrier to radiotherapy, chemotherapy and immunotherapy in cancer treatment. Int. J. Nanomed. 2018, 13, 6049–6058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tagalakis, A.D.; He, L.; Saraiva, L.; Gustafsson, K.T.; Hart, S.L. Receptor-targeted liposome-peptide nanocomplexes for siRNA delivery. Biomaterials 2011, 32, 6302–6315. [Google Scholar] [CrossRef]
- Kim, Y.R.; Hwang, J.; Koh, H.J.; Jang, K.; Lee, J.D.; Choi, J.; Yang, C.S. The targeted delivery of the c-Src peptide complexed with schizophyllan to macrophages inhibits polymicrobial sepsis and ulcerative colitis in mice. Biomaterials 2016, 89, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, F.; Rawool, N.M.; Merton, D.A.; Liu, J.B.; Goldberg, B.B. Contrast enhanced vascular three-dimensional ultrasound imaging. Ultrasonics 2002, 40, 117–122. [Google Scholar] [CrossRef]
- Abenojar, E.C.; Bederman, I.; Leon, A.C.; Zhu, J.; Hadley, J.; Kolios, M.C.; Exner, A.A. Theoretical and experimental gas volume quantification of micro- and nanobubble ultrasound contrast agents. Pharmaceutics 2020, 12, 208. [Google Scholar] [CrossRef] [Green Version]
- Vanhille, C. Numerical simulations of stable cavitation bubble generation and primary Bjerknes forces in a three-dimensional nonlinear phased array focused ultrasound field. Ultrason. Sonochem. 2020, 63, 104972. [Google Scholar] [CrossRef]
- Unger, E.C.; Matsunaga, T.O.; McCreery, T.; Schumann, P.; Sweitzer, R.; Quigley, R. Therapeutic applications of microbubbles. Eur. J. Radiol. 2002, 42, 160–168. [Google Scholar] [CrossRef]
- De Matos, M.B.C.; Deckers, R.; van Elburg, B.; Lajoinie, G.; de Miranda, B.S.; Versluis, M.; Schiffelers, R.; Kok, R.J. Ultrasound-sensitive liposomes for triggered macromolecular drug delivery: Formulation and in vitro characterization. Front. Pharmacol. 2019, 10, 1463. [Google Scholar] [CrossRef]
- Negishi, Y.; Yamane, M.; Kurihara, N.; Endo-Takahashi, Y.; Sashida, S.; Takagi, N.; Suzuki, R.; Maruyama, K. Enhancement of blood-brain barrier permeability and delivery of antisense oligonucleotides or plasmid DNA to the brain by the combination of bubble liposomes and high-intensity focused ultrasound. Pharmaceutics 2015, 7, 344–362. [Google Scholar] [CrossRef] [Green Version]
- LeWitt, P.A.; Lipsman, N.; Kordower, J.H. Focused ultrasound opening of the blood-brain barrier for treatment of Parkinson’s disease. Mov. Disord. 2019, 34, 1274–1278. [Google Scholar] [CrossRef]
- Omata, D.; Maruyama, T.; Unga, J.; Hagiwara, F.; Munakata, L.; Kageyama, S.; Shimaa, T.; Suzukia, Y.; Maruyamab, K.; Suzukia, R. Effects of encapsulated gas on stability of lipid-based microbubbles and ultrasound-triggered drug delivery. J. Control. Release 2019, 311–312, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Matsuki, N.; Ichiba, S.; Ishikawa, T.; Nagano, O.; Takeda, M.; Ujike, Y.; Yamaguchi, T. Blood oxygenation using microbubble suspensions. Eur. Biophys. J. Biophy. 2012, 41, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Stride, E.; Edirisinghe, M. Novel microbubble preparation technologies. Soft Matter. 2008, 4, 2350–2359. [Google Scholar] [CrossRef]
- Chong, W.K.; Papadopoulou, V.; Dayton, P.A. Imaging with ultrasound contrast agents: Current status and future. Abdom. Radiol. 2018, 43, 762–772. [Google Scholar] [CrossRef]
- Plesset, M.S.; Sadhal, S.S. On the stability of gas-bubbles in liquid-gas solutions. Appl. Sci. Res. 1982, 38, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Dressaire, E.; Bee, R.; Bell, D.C.; Lips, A.; Stone, H.A. Interfacial polygonal nanopatterning of stable microbubbles. Science 2008, 320, 1198–1201. [Google Scholar] [CrossRef]
- Rovers, T.A.; Sala, G.; van der Linden, E.; Meinders, M.B. Effect of temperature and pressure on the stability of protein microbubbles. ACS Appl. Mater Interfaces 2016, 8, 333–340. [Google Scholar] [CrossRef]
- Koppolu, S.; Chitnis, P.V.; Mamou, J.; Allen, J.S.; Ketterling, J.A. Correlation of rupture dynamics to the nonlinear backscatter response from polymer-shelled ultrasound contrast agents. IEEE Trans. Ultrason. Ferroelectr Freq. Control. 2015, 62, 494–501. [Google Scholar] [CrossRef] [Green Version]
- Goyal, G.; Hwang, J.; Aviral, J.; Seo, Y.; Jo, Y.; Son, J.; Choi, J. Green synthesis of silver nanoparticles using beta-glucan, and their incorporation into doxorubicin-loaded water-in-oil nanoemulsions for antitumor and antibacterial applications. J. Ind. Eng. Chem. 2017, 47, 179–186. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, B.; Li, M.; Zhou, M.; Li, F.; Huang, X.; Pan, M.; Xue, L.; Yan, F. Preparation and characterization of a novel silicon-modified nanobubble. PLoS ONE 2017, 12, e0178031. [Google Scholar] [CrossRef] [Green Version]
- Borden, M.A.; Dayton, P.; Zhao, S.K.; Ferrara, K.W. Physico-chemical properties of the microbubble lipid shell—Composition, microstructure & properties of targeted ultrasound contrast agents. Ultrasonics 2004, 1, 20–23. [Google Scholar]
- Cox, D.J.; Thomas, J.L. Ultrasound-induced dissolution of lipid-coated and uncoated gas bubbles. Langmuir 2010, 26, 14774–14781. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.J.; Thomas, J.L. Temperature-dependent biphasic shrinkage of lipid-coated bubbles in ultrasound. Langmuir 2013, 29, 4485–4491. [Google Scholar] [CrossRef] [PubMed]
- Ekemen, Z.; Chang, H.; Ahmad, Z.; Bayram, C.; Rong, Z.; Denkbas, E.B.; Stride, E.; Vadgama, P.; Edirisinghe, M. Fabrication of biomaterials via controlled protein bubble generation and manipulation. Biomacromolecules 2011, 12, 4291–4300. [Google Scholar] [CrossRef]
- Yang, H.; Shen, X.; Yan, J.; Xie, X.; Chen, Z.; Li, T.; Li, S.; Qin, X.; Wu, C.; Liu, Y. Charge-reversal-functionalized PLGA nanobubbles as theranostic agents for ultrasonic-imaging-guided combination therapy. Biomater. Sci. 2018, 6, 2426–2439. [Google Scholar] [CrossRef]
- Bosca, F.; Bielecki, P.A.; Exner, A.A.; Barge, A. Porphyrin-loaded pluronic nanobubbles: A new US-activated agent for future theranostic applications. Bioconjug. Chem. 2018, 29, 234–240. [Google Scholar] [CrossRef]
- Seo, Y.; Kim, J.E.; Jeong, Y.; Lee, K.H.; Hwang, J.; Hong, J.; Park, H.; Choi, J. Engineered nanoconstructs for the multiplexed and sensitive detection of high-risk pathogens. Nanoscale 2016, 8, 1944–1951. [Google Scholar] [CrossRef]
- Seo, Y.; Hwang, J.; Lee, E.; Kim, Y.J.; Lee, K.; Park, C.; Choi, Y.; Jeon, H.; Choi, J. Engineering copper nanoparticles synthesized on the surface of carbon nanotubes for anti-microbial and anti-biofilm applications. Nanoscale 2018, 10, 15529–15544. [Google Scholar] [CrossRef]
- Hernandez, C.; Nieves, L.; de Leon, A.C.; Advincula, R.; Exner, A.A. Role of surface tension in gas nanobubble stability under ultrasound. ACS Appl. Mater. Interfaces 2018, 10, 9949–9956. [Google Scholar] [CrossRef]
- Ljunggren, S.; Eriksson, J.C. The lifetime of a colloid-sized gas bubble in water and the cause of the hydrophobic attraction. Colloid Surf. A 1997, 129, 151–155. [Google Scholar] [CrossRef]
- Johnson, B.D.; Cooke, R.C. Generation of stabilized microbubbles in seawater. Science 1981, 213, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Kabalnov, A.; Klein, D.; Pelura, T.; Schutt, E.; Weers, J. Dissolution of multicomponent microbubbles in the bloodstream: 1. Theory. Ultrasound Med. Biol. 1998, 24, 739–749. [Google Scholar] [CrossRef]
- Krafft, M.P. Fluorocarbons and fluorinated amphiphiles in drug delivery and biomedical research. Adv. Drug Deliv. Rev. 2001, 47, 209–228. [Google Scholar] [CrossRef]
- Riess, J.G. Oxygen carriers (“blood substitutes”)—Raison d’Etre, chemistry, and some physiology. Chem. Rev. 2001, 101, 2797–2919. [Google Scholar] [CrossRef] [PubMed]
- Krafft, M.P.; Riess, J.G. Perfluorocarbons: Life sciences and biomedical uses—Dedicated to the memory of Professor Guy Ourisson, a true renaissance man. J. Polym. Sci. Pol. Chem. 2007, 45, 1185–1198. [Google Scholar] [CrossRef]
- Haiss, F.; Jolivet, R.; Wyss, M.T.; Reichold, J.; Braham, N.B.; Scheffold, F.; Krafft, M.P.; Weber, B. Improved in vivo two-photon imaging after blood replacement by perfluorocarbon. J. Physiol. 2009, 587, 3153–3158. [Google Scholar] [CrossRef]
- Szijjarto, C.; Rossi, S.; Waton, G.; Krafft, M.P. Effects of perfluorocarbon gases on the size and stability characteristics of phospholipid-coated microbubbles: Osmotic effect versus interfacial film stabilization. Langmuir 2012, 28, 1182–1189. [Google Scholar] [CrossRef]
- German, S.R.; Edwards, M.A.; Chen, Q.; White, H.S. Laplace pressure of individual H2 nanobubbles from pressure-addition electrochemistry. Nano Lett. 2016, 16, 6691–6694. [Google Scholar] [CrossRef]
- Chauhan, S.; Kaur, M.; Kumar, K.; Chauhan, M.S. Study of the effect of electrolyte and temperature on the critical micelle concentration of dodecyltrimethylammonium bromide in aqueous medium. J. Chem. 2014, 78, 175–181. [Google Scholar] [CrossRef]
- Fumoto, S.; Kawakami, S.; Ito, Y.; Shigeta, K.; Yamashita, F.; Hashida, M. Enhanced hepatocyte-selective in vivo gene expression by stabilized galactosylated liposome/plasmid DNA complex using sodium chloride for complex formation. Mol. Ther. 2004, 10, 719–729. [Google Scholar] [CrossRef]
- Ogawa, K.; Fuchigami, Y.; Hagimori, M.; Fumoto, S.; Miura, Y.; Kawakami, S. Efficient gene transfection to the brain with ultrasound irradiation in mice using stabilized bubble lipopolyplexes prepared by the surface charge regulation method. Int. J. Nanomed. 2018, 13, 2309–2320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, A.K.; Thareja, S. In vitro and in vivo characterization of pharmaceutical nanocarriers used for drug delivery. Artif. Cells Nanomed. Biotechnol. 2019, 47, 524–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamamoto, S.; Takemura, T.; Suzuki, K.; Nishimura, T. Effects of pH on nano-bubble stability and transport in saturated porous media. J. Contam. Hydrol. 2018, 208, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Liu, S.; Enari, M.; Oshita, S.; Yamazaki, K.; Gohara, K. Effect of NaCl on the lifetime of micro- and nanobubbles. Nanomaterials 2016, 6, 31. [Google Scholar] [CrossRef]
- Kelsall, G.H.; Tang, S.Y.; Yurdakul, S.; Smith, A.L. Electrophoretic behaviour of bubbles in aqueous electrolytes. J. Chem. Soc. Faraday Trans. 1996, 92, 3887–3893. [Google Scholar] [CrossRef]
- Takahashi, M. Zeta potential of microbubbles in aqueous solutions: Electrical properties of the gas-water interface. J. Phys. Chem. B 2005, 109, 21858–21864. [Google Scholar] [CrossRef]
- Shekhar, H.; Smith, N.J.; Raymond, J.L.; Holland, C.K. Effect of temperature on the size distribution, shell properties, and stability of Definity®. Ultrasound Med. Biol. 2018, 44, 434–446. [Google Scholar] [CrossRef]
- Unga, J.; Kageyama, S.; Suzuki, R.; Omata, D.; Maruyama, K. Scale-up production, characterization and toxicity of a freeze-dried lipid-stabilized microbubble formulation for ultrasound imaging and therapy. J. Liposome Res. 2020, 30, 297–304. [Google Scholar] [CrossRef]
- Unga, J.; Omata, D.; Kudo, N.; Ueno, S.; Munakata, L.; Shima, T.; Maruyama, K.; Suzuki, R. Development and evaluation of stability and ultrasound response of DSPC-DPSG-based freeze-dried microbubbles. J. Liposome Res. 2019, 29, 368–374. [Google Scholar] [CrossRef]
- Ojha, T.; Pathak, V.; Drude, N.; Weiler, M.; Rommel, D.; Rutten, S.; Geinitz, B.; van Steenbergen, M.J.; Storm, G.; Kiessling, F.; et al. Shelf-life evaluation and lyophilization of PBCA-based polymeric microbubbles. Pharmaceutics 2019, 11, 433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weijs, J.H.; Seddon, J.R.; Lohse, D. Diffusive shielding stabilizes bulk nanobubble clusters. ChemPhysChem 2012, 13, 2197–2204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammi, A.Y.; Cleveland, R.O.; Mamou, J.; Wang, G.I.; Bridal, S.L.; O’Brien, W.D., Jr. Ultrasonic contrast agent shell rupture detected by inertial cavitation and rebound signals. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2006, 53, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Tezel, A.; Mitragotri, S. Interactions of inertial cavitation bubbles with stratum corneum lipid bilayers during low-frequency sonophoresis. Biophys. J. 2003, 85, 3502–3512. [Google Scholar] [CrossRef] [Green Version]
- Marmottant, P.; Hilgenfeldt, S. Controlled vesicle deformation and lysis by single oscillating bubbles. Nature 2003, 423, 153–156. [Google Scholar] [CrossRef]
- Sponer, J. Dependence of the cavitation threshold on the ultrasonic frequency. Czech J. Phys. 1990, 40, 1123–1132. [Google Scholar] [CrossRef]
- Pouliopoulos, A.N.; Li, C.Q.; Tinguely, M.; Garbin, V.; Tang, M.X.; Choi, J.J. Rapid short-pulse sequences enhance the spatiotemporal uniformity of acoustically driven microbubble activity during flow conditions. J. Acoust. Soc. Am. 2016, 140, 2469–2480. [Google Scholar] [CrossRef]
- Sirsi, S.; Feshitan, J.; Kwan, J.; Homma, S.; Borden, M. Effect of microbubble size on fundamental mode high frequency ultrasound imaging in mice. Ultrasound Med. Biol. 2010, 36, 935–948. [Google Scholar] [CrossRef] [Green Version]
- Caskey, C.F.; Stieger, S.M.; Qin, S.; Dayton, P.A.; Ferrara, K.W. Direct observations of ultrasound microbubble contrast agent interaction with the microvessel wall. J. Acoust. Soc. Am. 2007, 122, 1191–1200. [Google Scholar] [CrossRef]
- Duan, L.; Yang, L.; Jin, J.; Yang, F.; Liu, D.; Hu, K.; Wang, Q.; Yue, Y.; Gu, N. Micro/nano-bubble-assisted ultrasound to enhance the EPR effect and potential theranostic applications. Theranostics 2020, 10, 462–483. [Google Scholar] [CrossRef]
- Garg, S.; Thomas, A.A.; Borden, M.A. The effect of lipid monolayer in-plane rigidity on in vivo microbubble circulation persistence. Biomaterials 2013, 34, 6862–6870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turecek, P.L.; Bossard, M.J.; Schoetens, F.; Ivens, I.A. PEGylation of biopharmaceuticals: A review of chemistry and nonclinical safety information of approved drugs. J. Pharm. Sci. 2016, 105, 460–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.G.; Lv, F.M.; Wang, J.; Cao, S.J.; Liu, Z.P.; Liu, Y.; Lu, W.Y. RGD-modified PEGylated paclitaxel nanocrystals with enhanced stability and tumor-targeting capability. Int. J. Pharm. 2019, 556, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Kenworthy, A.K.; Hristova, K.; Needham, D.; McIntosh, T.J. Range and magnitude of the steric pressure between bilayers containing phospholipids with covalently attached poly (ethylene glycol). Biophys. J. 1995, 68, 1921–1936. [Google Scholar] [CrossRef] [Green Version]
- Needham, D.; Kim, D.H. PEG-covered lipid surfaces: Bilayers and monolayers. Colloids Surf. B Biointerfaces 2000, 18, 183–195. [Google Scholar] [CrossRef]
- Blume, G.; Cevc, G.; Crommelin, M.D.; Bakker-Woudenberg, I.A.; Kluft, C.; Storm, G. Specific targeting with poly(ethylene glycol)-modified liposomes: Coupling of homing devices to the ends of the polymeric chains combines effective target binding with long circulation times. Biochim. Biophys. Acta 1993, 1149, 180–184. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, B.; Yoon, S.; Choi, Y.; Jang, J.; Park, S.; Choi, J. Stability of Engineered Micro or Nanobubbles for Biomedical Applications. Pharmaceutics 2020, 12, 1089. https://doi.org/10.3390/pharmaceutics12111089
Park B, Yoon S, Choi Y, Jang J, Park S, Choi J. Stability of Engineered Micro or Nanobubbles for Biomedical Applications. Pharmaceutics. 2020; 12(11):1089. https://doi.org/10.3390/pharmaceutics12111089
Chicago/Turabian StylePark, Beomjin, Semi Yoon, Yonghyun Choi, Jaehee Jang, Soomin Park, and Jonghoon Choi. 2020. "Stability of Engineered Micro or Nanobubbles for Biomedical Applications" Pharmaceutics 12, no. 11: 1089. https://doi.org/10.3390/pharmaceutics12111089
APA StylePark, B., Yoon, S., Choi, Y., Jang, J., Park, S., & Choi, J. (2020). Stability of Engineered Micro or Nanobubbles for Biomedical Applications. Pharmaceutics, 12(11), 1089. https://doi.org/10.3390/pharmaceutics12111089