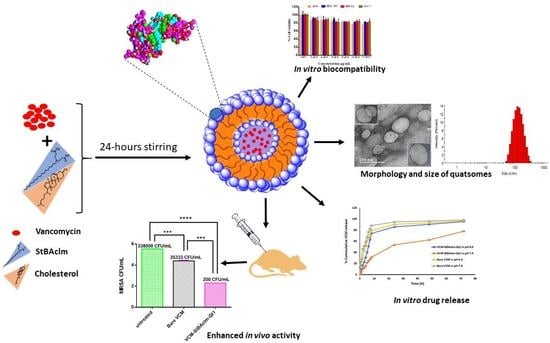
Formulation of pH-Responsive Quatsomes from Quaternary Bicephalic Surfactants and Cholesterol for Enhanced Delivery of Vancomycin against Methicillin Resistant Staphylococcus aureus
Abstract
Highlights
- A novel surfactant (StBAclm) was synthesized, and its structure confirmed.
- Vancomycin-loaded pH-responsive quatsomes (VCM-StBAclm-Qt1) were prepared from StBAclm.
- The in vitro drug results showed a faster VCM release from the quatsomes at pH 6.0 compared to pH 7.4 and enhanced in vitro antibacterial activity against MRSA as compared to bare VCM.
- There was an enhanced bacterial killing kinetics and high perforation of MRSA membrane cell wall by the quatsomes.
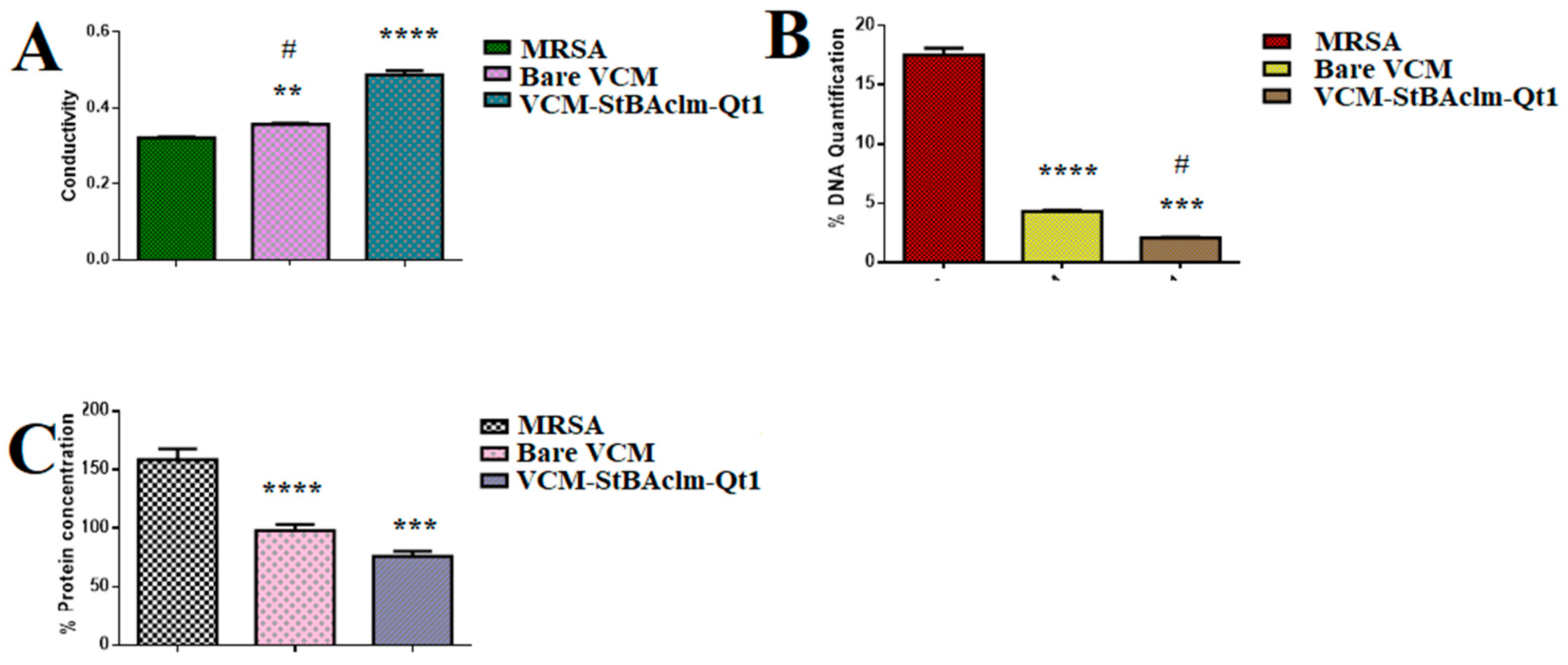
- A higher electrical conductivity, reduced DNA, and protein concentration were achieved by the quatsomes.
- There was an enhanced in vivo antibacterial activity of the drug quatsomes against MRSA compared to bare VCM in a mice skin infection model.
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation (1H NMR, 13C NMR, FT-IR and HR-MS Spectra)
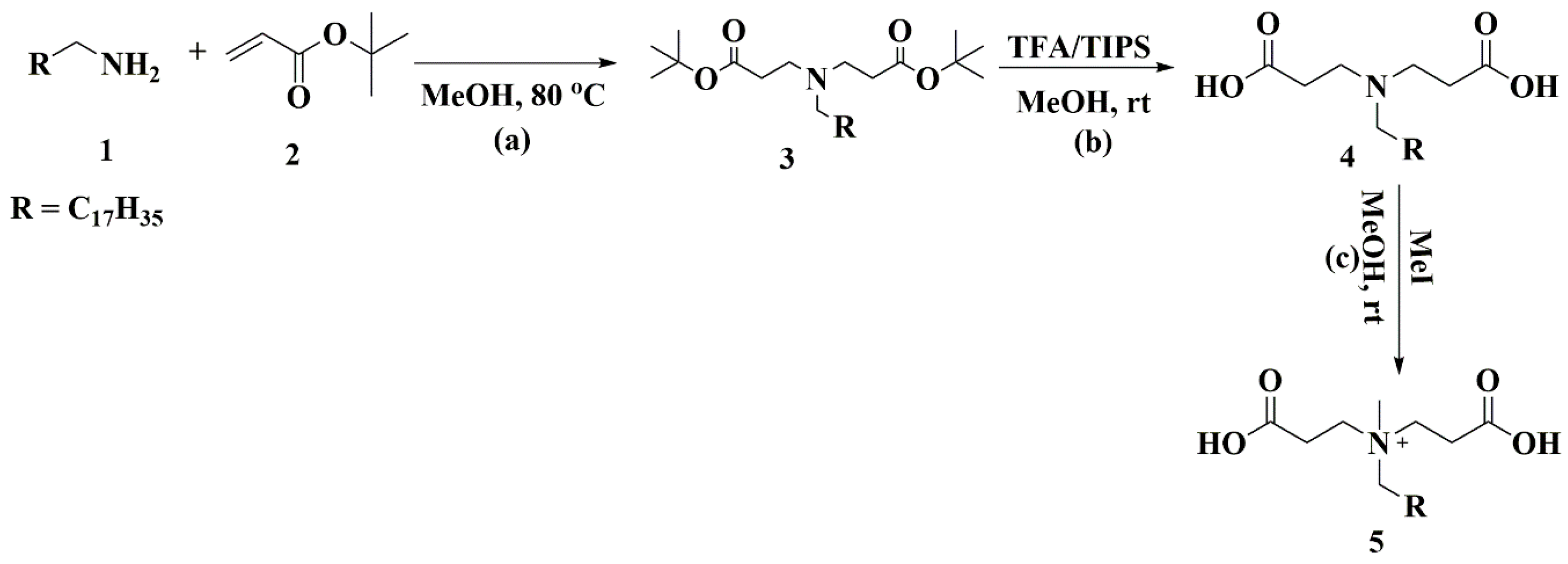
2.3. Synthesis and Characterization of the Surfactant
2.4. Formulation of VCM-Loaded Quatsomes (VCM-StBAclm-Qt)
2.5. Characterization of the VCM-StBAclm-Qt Quatsomes
2.5.1. Mean Hydrodynamic Diameter (MHD), Polydispersity Index (PDI), Zeta Potential (ζ), and Morphology
2.5.2. Drug Entrapment Efficiency (DEE%) and Drug Loading Capacity (DLC%)
2.6. Molecular Modeling Simulations (MDS)
2.6.1. Vancomycin, CHol, and Surfactant Simulation Quatsomes
2.6.2. VCM, CHol, and Surfactant Self-Assembly Complexation Simulation
2.6.3. Post-Dynamic Analysis and Binding-Free Energy Calculations
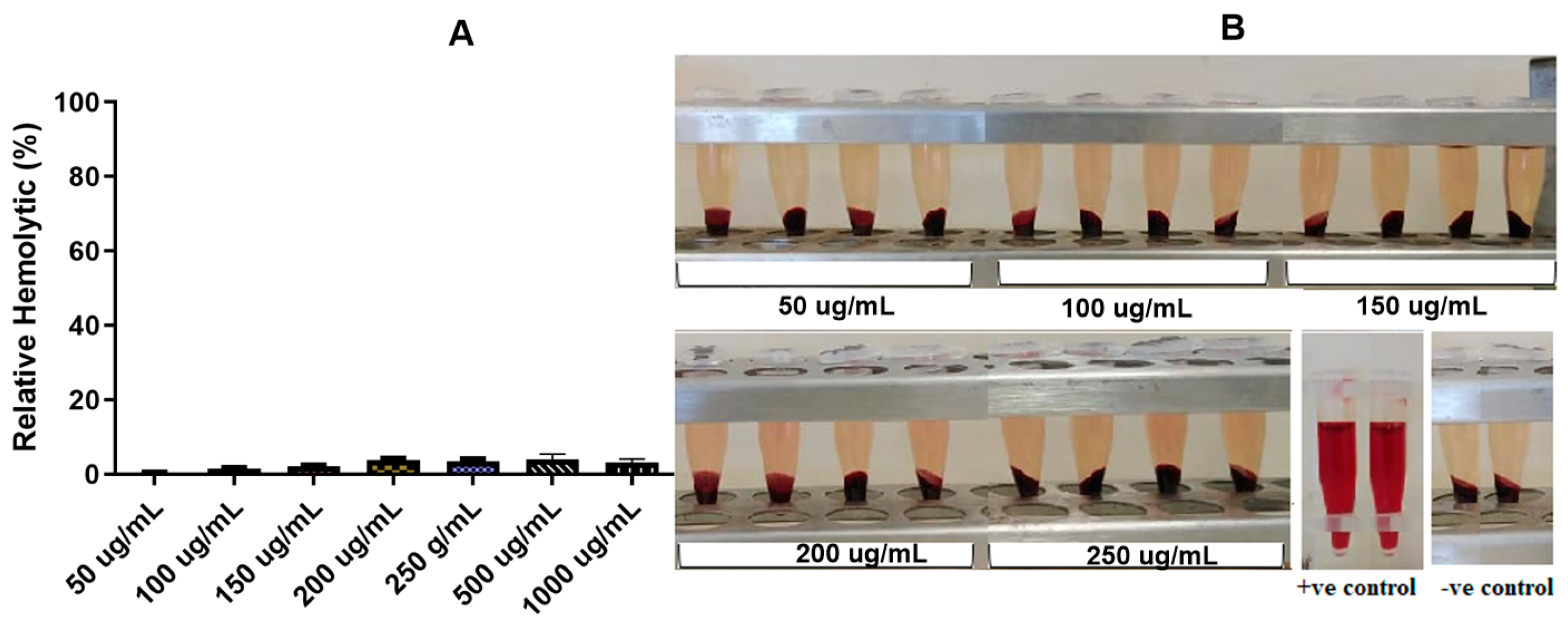
2.7. In Vitro Hemolytic Activity Assay of the VCM-StBAclm-Qt1 Quatsomes
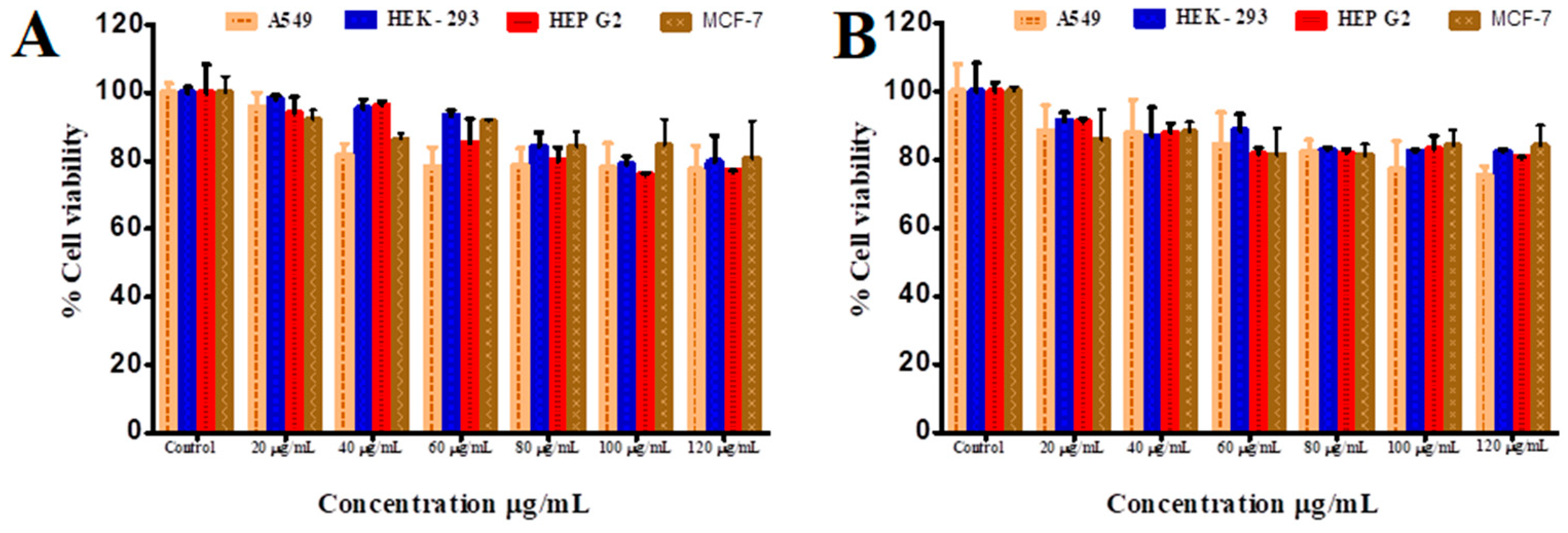
2.8. In Vitro Cytotoxicity of StBAclm and VCM-StBAclm-Qt against Different Cell Lines
2.9. In Vitro Drug Release of VCM from VCM-loaded StBAclm-Qt Quatsomes
2.10. Evaluation of In Vitro Antibacterial Activities on VCM-StBAclm-Qt Quatsomes
2.10.1. Determination of Minimum Inhibitory Concentrations (MICs)
2.10.2. Time Killing Assays VCM-StBAclm-Qt Quatsomes
2.11. Molecular and Mechanistic Studies on VCM-StBAclm-Qt Quatsomes
2.11.1. Effect of VCM-StBAclm-Qt Quatsomes on Electrical Conductivity, DNA, and Protein Leakage
2.11.2. Bacterial Membrane Disruption
2.11.3. Fluorescence-Activated Cell Sorting (FACS) Bacterial Cell Viability
2.11.4. Reduction of MRSA Biofilm using Fluorescence Microscopy
2.12. In Vivo Antibacterial Activity
2.13. Stability Studies
2.14. Statistical Analysis of the Experiment
3. Results and Discussion
3.1. Synthesis and Characterization of StBAclm
3.2. Preparation and Characterization of VCM-StBAclm-Qt1
3.2.1. Preparation, Characterization and Morphology of VCM-StBAclm-Qt1 Quatsomes
3.2.2. Effect of Change in pH on MHD and ζ
3.2.3. Vancomycin, CHol, and StBAclm Complex and Self-Assembly of Inserted Complexes
3.3. Cytotoxicity and Hemolysis Assays
3.3.1. In Vitro Cytotoxicity Assay
3.3.2. In Vitro Hemolytic Evaluation of VCM-StBAclm-Qt1 Quatsomes
3.4. In Vitro Drug Release Behaviour
3.5. Stability Studies
3.6. In Vitro Antimicrobial Activity
3.6.1. MIC Determination
3.6.2. Fractional Inhibitory Concentration (FIC) Studies
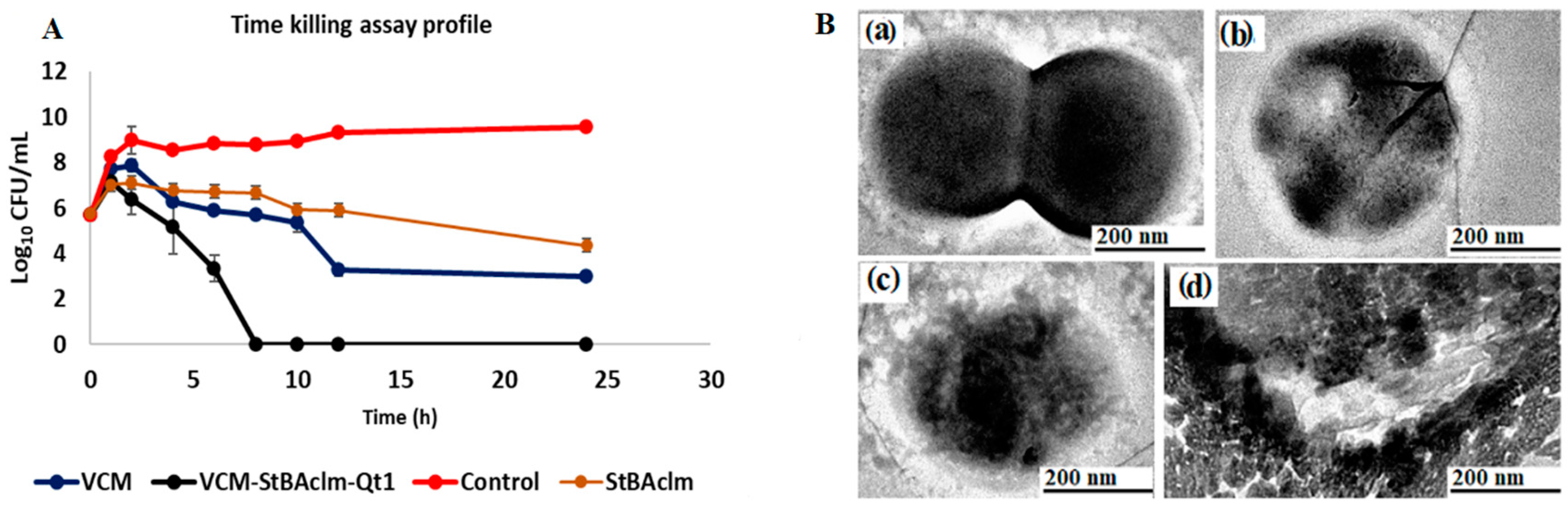
3.7. Bactericidal Time Assay of VCM-loaded StBAclm-Qt1 Quatsomes
3.8. Mechanistic Studies of VCM-loaded StBAclm-Qt1 Quatsomes
3.8.1. Bacterial Membrane Disruption
3.8.2. Fluorescence-Activated Cell Sorting (FACS) Cell Viability
3.8.3. Biofilm Eradication of VCM-Loaded StBAclm-Qt1 Quatsomes using Fluorescence Microscopy
3.9. Molecular Antibacterial Studies
3.9.1. Bacterial Cell Membrane Permeability in Terms of Relative Electric Conductivity
3.9.2. Leakage of Proteins and VCM-Loaded StBAclm-Qt1 Quatsomes Analysis
3.10. In Vivo Antibacterial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | Adenosine triphosphatase |
| A-549 | Adenocarcinomic alveolar basal epithelial cells |
| BCA | Bicinchoninic acid |
| CDC | Centers for Disease Control and Prevention |
| CTAB | Cationic hexadecyltrimethylammonium bromide |
| CFU | Colony-forming unit |
| CHol | Cholesterol |
| DCM | Dichloromethane |
| DLC | Drug loading capacity |
| DMSO | Dimethyl sulfoxide |
| DNA | Deoxyribonucleic acids |
| DSC | Differential scanning calorimetry |
| DEE | Drug encapsulation efficiency |
| FT-IR | Fourier transform infrared |
| HR-MS | High-resolution mass spectrometry |
| HEK-293 | Human embryonic kidney cell lines |
| Hep-G2 | Liver hepatocellular carcinoma cell lines |
| MCF-7 | Human breast adenocarcinoma cell lines |
| MDT | Mean dissolution time |
| MHA | Mueller–Hinton agar |
| MHD | Mean hydrodynamic diameter |
| MHB | Mueller–Hinton Broth |
| MI | Methyl iodide |
| MICs | Minimum inhibitory concentrations |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NB | Nutrient broth |
| OD | Optical density |
| PDI | Polydispersity index |
| PI | Propidium iodide |
| pDNA | Plasmid deoxyribonucleic acid |
| PBS | Phosphate saline buffer |
| RMSE | Root mean square error |
| RBC | Red blood cells |
| R2 | Correlation coefficient |
| SA | Stearylamine |
| siRNA | Small interfering ribonucleic acid |
| tBA | Tert-butyl acrylate |
| TFA | Trifluoroacetic acid |
| TIPs | Triisopropylsilane |
| UVis-Spec | UV Spectrophotometer |
| VCM | Vancomycin |
| ζ | Zeta potential |
| 1H NMR | Proton nuclear magnetic resonance |
| 13C NMR | Carbon 13 nuclear magnetic resonance |
References
- Ramanavičius, S.; Žalnėravičius, R.; Niaura, G.; Drabavičius, A.; Jagminas, A. Shell-dependent antimicrobial efficiency of cobalt ferrite nanoparticles. Nano-Struct. Nano-Objects 2018, 15, 40–47. [Google Scholar] [CrossRef]
- Jiang, X.; Li, J.; Ding, M.; Tan, H.; Ling, Q.; Zhong, Y.; Fu, Q. Synthesis and degradation of nontoxic biodegradable waterborne polyurethanes elastomer with poly (ε-caprolactone) and poly (ethylene glycol) as soft segment. Eur. Polymer J. 2007, 43, 1838–1846. [Google Scholar] [CrossRef]
- Wen, Y.; Pan, S.; Luo, X.; Zhang, X.; Zhang, W.; Feng, M. A biodegradable low molecular weight polyethylenimine derivative as low toxicity and efficient gene vector. Bioconjugate Chem. 2009, 20, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Honary, S.; Ebrahimi, P.; Hadianamrei, R. Optimization of particle size and encapsulation efficiency of vancomycin nanoparticles by response surface methodology. Pharm. Dev. Technol. 2014, 19, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, G.; Tiwari, R.; Sriwastawa, B.; Bhati, L.; Pandey, S.; Pandey, P.; Bannerjee, S.K. Drug delivery systems: An updated review. Int. J. Pharm. Investig. 2012, 2, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Bamrungsap, S.; Zhao, Z.; Chen, T.; Wang, L.; Li, C.; Fu, T.; Tan, W. Nanotechnology in therapeutics: A focus on nanoparticles as a drug delivery system. Nanomedicine 2012, 7, 1253–1271. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro-and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef]
- Xiong, M.H.; Bao, Y.; Yang, X.Z.; Zhu, Y.H.; Wang, J. Delivery of antibiotics with polymeric particles. Adv. Drug Deliv. Rev. 2014, 78, 63–76. [Google Scholar] [CrossRef]
- Hassan, D.; Omolo, C.A.; Gannimani, R.; Waddad, A.Y.; Mocktar, C.; Rambharose, S.; Agrawal, N.; Govender, T. Delivery of novel vancomycin nanoplexes for combating methicillin resistant Staphylococcus aureus (MRSA) infections. Int. J. Pharm. 2019, 558, 143–156. [Google Scholar] [CrossRef]
- Omolo, C.A.; Kalhapure, R.S.; Jadhav, M.; Rambharose, S.; Mocktar, C.; Ndesendo, V.M.; Govender, T. Pegylated oleic acid: A promising amphiphilic polymer for nano-antibiotic delivery. Eur. J. Pharm. Biopharm. 2017, 112, 96–108. [Google Scholar] [CrossRef]
- Barros, S.M.; Whitaker, S.K.; Sukthankar, P.; Avila, L.A.; Gudlur, S.; Warner, M.; Beltrao, E.I.; Tomich, J.M. A review of solute encapsulating nanoparticles used as delivery systems with emphasis on branched amphipathic peptide capsules. Arch. Biochem. Biophys. 2016, 596, 22–42. [Google Scholar] [CrossRef] [PubMed]
- Fatouros, D.G.; Lamprou, D.A.; Urquhart, A.J.; Yannopoulos, S.N.; Vizirianakis, I.S.; Zhang, S.; Koutsopoulos, S. Lipid-like self-assembling peptide nanovesicles for drug delivery. ACS Appl. Mater. Interfaces 2014, 6, 8184–8189. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Tasies, L.; Moreno-Calvo, E.; Cano-Sarabia, M.; Aguilella-Arzo, M.; Angelova, A.; Lesieur, S.; Ricart, S.; Faraudo, J.; Ventosa, N.; Veciana, J. Quatsomes: Vesicles formed by self-assembly of sterols and quaternary ammonium surfactants. Langmuir 2013, 29, 6519–6528. [Google Scholar] [CrossRef] [PubMed]
- Ferrer Tasies, L.P. Cholesterol and Compressed CO2 A Smart Molecular Building Block and Advantageous Solvent to Prepare Stable Self-Assembled Colloidal Nanostructures. Ph.D. Thesis, Universitat Autònoma de Barcelona, Barcelona, Spain, 2016. [Google Scholar]
- Caboi, F.; Monduzzi, M. Didodecyldimethylammonium bromide vesicles and lamellar liquid crystals. A multinuclear NMR and optical microscopy study. Langmuir 1996, 12, 3548–3556. [Google Scholar] [CrossRef]
- Antonietti, M.; Förster, S. Vesicles and liposomes: A self-assembly principle beyond lipids. Adv. Mater. 2003, 15, 1323–1333. [Google Scholar] [CrossRef]
- Elkin, T.; Copp, S.M.; Hamblin, R.L.; Martinez, J.S.; Montaño, G.A.; Rocha, R.C. Synthesis of terpyridine-terminated amphiphilic block copolymers and their self-assembly into metallo-polymer nanovesicles. Materials 2019, 12, 601. [Google Scholar] [CrossRef]
- Yadavalli, S.S.; Xiao, Q.; Sherman, S.E.; Hasley, W.D.; Klein, M.L.; Goulian, M.; Percec, V. Bioactive cell-like hybrids from dendrimersomes with a human cell membrane and its components. Proc. Natl. Acad. Sci. USA 2019, 116, 744–752. [Google Scholar] [CrossRef]
- Lebedeva, I.O.; Zhulina, E.B.; Borisov, O.V. Self-assembly of linear-dendritic and double dendritic block copolymers: From dendromicelles to dendrimersomes. Macromolecules 2019, 52, 3655–3667. [Google Scholar] [CrossRef]
- Tomich, J.M.; Wessel, E.; Choi, J.; Avila, L.A. Nonviral Gene Therapy: Peptiplexes. In Nucleic Acid Nanotheranostics; Elsevier: Amsterdam, The Netherlands, 2019; pp. 247–276. [Google Scholar]
- Lin, Y.L.; Chang, H.Y.; Sheng, Y.J.; Tsao, H.K. Structural and mechanical properties of polymersomes formed by rod-coil diblock copolymers. Soft Matter 2013, 9, 4802–4814. [Google Scholar] [CrossRef]
- Thomas, N.; Dong, D.; Richter, K.; Ramezanpour, M.; Vreugde, S.; Thierry, B.; Wormald, P.J.; Prestidge, C.A. Quatsomes for the treatment of Staphylococcus aureus biofilm. J. Mater. Chem. B 2015, 3, 2770–2777. [Google Scholar] [CrossRef]
- Ventosa, N.; Cabrera, I.; Veciana, J.; Santana, H.; Martinez, E.; Berlanga, J. Vesicles comprising epidermal growth factor and compositions that contain them. Cuban Patent Appl. CU 2012, 112, 2012. [Google Scholar]
- Gumí-Audenis, B.; Illa-Tuset, S.; Grimaldi, N.; Pasquina-Lemonche, L.; Ferrer-Tasies, L.; Sanz, F.; Veciana, J.; Ratera, I.; Faraudo, J.; Ventosa, N. Insights into the structure and nanomechanics of a quatsome membrane by force spectroscopy measurements and molecular simulations. Nanoscale 2018, 10, 23001–23011. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Zhang, S.; Wang, B.; Cui, S.; Yan, J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release 2006, 114, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Isomaa, B.; Reuter, J.; Djupsund, B. The subacute and chronic toxicity of cetyltrimethylammonium bromide (CTAB), a cationic surfactant, in the rat. Arch. Toxicol. 1976, 35, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, J.; Li, Q.; Shen, Y.; Ge, Z.; Zhang, W.; Chen, S. Enhanced water-solubility, antibacterial activity and biocompatibility upon introducing sulfobetaine and quaternary ammonium to chitosan. Carbohydr. Polym. 2016, 143, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Nałęcz-Jawecki, G.; Grabińska-Sota, E.; Narkiewicz, P. The toxicity of cationic surfactants in four bioassays. Ecotoxicol. Environ. Saf. 2003, 54, 87–91. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, J.; Cheng, D.; Wang, Y.; Shuai, X. A pH-sensitive micelle for codelivery of siRNA and doxorubicin to hepatoma cells. Polymer 2014, 55, 3217–3226. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, H.; Li, Y.; Chen, W.; Li, H.; Peng, K.; Zhang, Z.; Sun, X. Targeting NF-kB signaling with polymeric hybrid micelles that co-deliver siRNA and dexamethasone for arthritis therapy. Biomaterials 2017, 122, 10–22. [Google Scholar] [CrossRef]
- Liu, N.; Han, J.; Zhang, X.; Yang, Y.; Liu, Y.; Wang, Y.; Wu, G. pH-responsive zwitterionic polypeptide as a platform for anti-tumor drug delivery. Colloids Surf. B Biointerfaces 2016, 145, 401–409. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera-A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Shao, Y.; Molnar, L.F.; Jung, Y.; Kussmann, J.; Ochsenfeld, C.; Brown, S.T.; Gilbert, A.T.; Slipchenko, L.V.; Levchenko, S.V.; O’Neill, D.P. Advances in methods and algorithms in a modern quantum chemistry program package. Phys. Chem. Chem. Phys. 2006, 8, 3172–3191. [Google Scholar] [CrossRef] [PubMed]
- Ryckaert, J.P.; Ciccotti, G.; Berendsen, H.J. Numerical integration of the cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef]
- Case, D.A.; Cheatham, T.E., III; Darden, T.; Gohlke, H.; Luo, R.; Merz, K.M., Jr.; Onufriev, A.; Simmerling, C.; Wang, B.; Woods, R.J. The Amber biomolecular simulation programs. J. Comput. Chem. 2005, 26, 1668–1688. [Google Scholar] [CrossRef] [PubMed]
- Roe, D.R.; Cheatham, T.E., III. PTRAJ and CPPTRAJ: Software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef]
- Seifert, E. Originpro 9.1: Scientific data analysis and graphing software-Software review. J. Chem. Inf. Model. 2014, 54, 1552. [Google Scholar] [CrossRef]
- Raha, K.; Merz, K.M., Jr. Calculating binding free energy in protein-ligand interaction. Ann. Rep. Comput. Chem. 2005, 1, 113–130. [Google Scholar]
- Ylilauri, M.; Pentikäinen, O.T. MMGBSA as a tool to understand the binding affinities of filamin-peptide interactions. J. Chem. Inform. Model. 2013, 53, 2626–2633. [Google Scholar] [CrossRef]
- Hou, T.; Wang, J.; Li, Y.; Wang, W. Assessing the performance of the MM/PBSA and MM/GBSA methods. 1. The accuracy of binding free energy calculations based on molecular dynamics simulations. J. Chem. Inform. Model. 2010, 51, 69–82. [Google Scholar] [CrossRef]
- Xie, R.-L.; Jang, Y.-J.; Xing, L.; Zhang, B.-F.; Wang, F.-Z.; Cui, P.-F.; Cho, M.-H.; Jiang, H.-L. A novel potential biocompatible hyperbranched polyspermine for efficient lung cancer gene therapy. Int. J. Pharm. 2015, 478, 19–30. [Google Scholar] [CrossRef]
- Maji, R.; Omolo, C.A.; Agrawal, N.; Maduray, K.; Hassan, D.; Mokhtar, C.; Mackhraj, I.; Govender, T. pH-Responsive Lipid–Dendrimer Hybrid Nanoparticles: An Approach To Target and Eliminate Intracellular Pathogens. Mol. Pharm. 2019, 16, 4594–4609. [Google Scholar] [CrossRef]
- Kalhapure, R.S.; Sikwal, D.R.; Rambharose, S.; Mocktar, C.; Singh, S.; Bester, L.; Oh, J.K.; Renukuntla, J.; Govender, T. Enhancing targeted antibiotic therapy via pH responsive solid lipid nanoparticles from an acid cleavable lipid. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2067–2077. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, S.J.; Kalhapure, R.S.; Rambharose, S.; Mocktar, C.; Vepuri, S.B.; Soliman, M.; Govender, T. Ultra-small lipid-dendrimer hybrid nanoparticles as a promising strategy for antibiotic delivery: In vitro and in silico studies. Int. J. Pharm. 2016, 504, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Yan, H.; Jia, X.; Zhang, Z. Preparation and in vivo/in vitro evaluation of formononetin phospholipid/vitamin E TPGS micelles. J. Drug Target. 2016, 24, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, J.H.; Turnidge, J.D. Susceptibility test methods. In Manual of Clinical Microbiology: Dilution and disk diffusion methods, 11th ed.; American Society of Microbiology: Washington, DC, USA, 2015; pp. 1253–1273. [Google Scholar]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. In Approved Standard, 9th ed.; CLSI: Wayne, PA, USA, 2012; Volume 32, p. 63. [Google Scholar]
- Seedat, N.; Kalhapure, R.S.; Mocktar, C.; Vepuri, S.; Jadhav, M.; Soliman, M.; Govender, T. Co-encapsulation of multi-lipids and polymers enhances the performance of vancomycin in lipid–polymer hybrid nanoparticles: In vitro and in silico studies. Mater. Sci. Eng. C 2016, 61, 616–630. [Google Scholar] [CrossRef]
- Omolo, C.A.; Kalhapure, R.S.; Agrawal, N.; Jadhav, M.; Rambharose, S.; Mocktar, C.; Govender, T. A hybrid of mPEG-b-PCL and G1-PEA dendrimer for enhancing delivery of antibiotics. J. Control. Release 2018, 290, 112–128. [Google Scholar] [CrossRef]
- Hassan, D.; Omolo, C.A.; Fasiku, V.O.; Mocktar, C.; Govender, T. Novel chitosan-based pH-responsive lipid-polymer hybrid nanovesicles (OLA-LPHVs) for delivery of vancomycin against methicillin-resistant Staphylococcus aureus infections. Int. J. Biol. Macromol. 2020, 147, 385–398. [Google Scholar] [CrossRef]
- Chauhan, N.; Tyagi, A.K.; Kumar, P.; Malik, A. Antibacterial potential of Jatropha curcas synthesized silver nanoparticles against food borne pathogens. Front. Microbiol. 2016, 7, 1748. [Google Scholar] [CrossRef]
- Omolo, C.A.; Kalhapure, R.S.; Agrawal, N.; Rambharose, S.; Mocktar, C.; Govender, T. Formulation and molecular dynamics simulations of a fusidic acid nanosuspension for simultaneously enhancing solubility and antibacterial activity. Mol. Pharm. 2018, 15, 3512–3526. [Google Scholar] [CrossRef]
- O’Brien-Simpson, N.M.; Pantarat, N.; Attard, T.J.; Walsh, K.A.; Reynolds, E.C. A Rapid and quantitative flow cytometry method for the analysis of membrane disruptive antimicrobial activity. PLoS ONE 2016, 11, e0151694. [Google Scholar] [CrossRef]
- Rüger, M.; Bensch, G.; Tüngler, R.; Reichl, U. A flow cytometric method for viability assessment of Staphylococcus aureus and Burkholderia cepacia in mixed culture. Cytom. A 2012, 81, 1055–1066. [Google Scholar] [CrossRef]
- Bexfield, A.; Bond, A.E.; Roberts, E.C.; Dudley, E.; Nigam, Y.; Thomas, S.; Newton, R.P.; Ratcliffe, N.A. The antibacterial activity against MRSA strains and other bacteria of a < 500Da fraction from maggot excretions/secretions of Lucilia sericata (Diptera: Calliphoridae). Microbes Infect. 2008, 10, 325–333. [Google Scholar] [PubMed]
- Shrestha, N.K.; Scalera, N.M.; Wilson, D.A.; Procop, G.W. Rapid differentiation of methicillin-resistant and methicillin-susceptible Staphylococcus aureus by flow cytometry after brief antibiotic exposure. J. Clin. Microbiol. 2011, 49, 2116–2120. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arndt-Jovin, D.J.; Jovin, T.M. Fluorescence labeling and microscopy of DNA. Methods Cell Biol. 1989, 30, 417–448. [Google Scholar] [PubMed]
- Renggli, S.; Keck, W.; Jenal, U.; Ritz, D. Role of autofluorescence in flow cytometric analysis of Escherichia coli treated with bactericidal antibiotics. J. Bacteriol. 2013, 195, 4067–4073. [Google Scholar] [CrossRef] [PubMed]
- Fittipaldi, M.; Nocker, A.; Codony, F. Progress in understanding preferential detection of live cells using viability dyes in combination with DNA amplification. J. Microbiol. Methods 2012, 91, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Berlutti, F.; Frioni, A.; Natalizi, T.; Pantanella, F.; Valenti, P. Influence of sub-inhibitory antibiotics and flow condition on Staphylococcus aureus ATCC 6538 biofilm development and biofilm growth rate: Bio timer assay as a study model. J. Antibiot. 2014, 67, 763. [Google Scholar] [CrossRef] [PubMed]
- Kugelberg, E.; Norström, T.; Petersen, T.K.; Duvold, T.; Andersson, D.I.; Hughes, D. Establishment of a superficial skin infection model in mice by using Staphylococcus aureus and Streptococcus pyogenes. Anti. Agents Chemoth. 2005, 49, 3435–3441. [Google Scholar] [CrossRef]
- Kalhapure, R.S.; Jadhav, M.; Rambharose, S.; Mocktar, C.; Singh, S.; Renukuntla, J.; Govender, T. pH-responsive chitosan nanoparticles from a novel twin-chain anionic amphiphile for controlled and targeted delivery of vancomycin. Colloids Surf. B Biointerfaces 2017, 158, 650–657. [Google Scholar] [CrossRef]
- Helgason, T.; Awad, T.; Kristbergsson, K.; McClements, D.J.; Weiss, J. Effect of surfactant surface coverage on formation of solid lipid nanoparticles (SLN). J. Colloid Interface Sci. 2009, 334, 75–81. [Google Scholar] [CrossRef]
- Unagolla, J.M.; Jayasuriya, A.C. Drug transport mechanisms and in vitro release kinetics of vancomycin encapsulated chitosan-alginate polyelectrolyte microparticles as a controlled drug delivery system. Eur. J. Pharm. Sci. 2018, 114, 199–209. [Google Scholar] [CrossRef]
- Cerchiara, T.; Abruzzo, A.; Palomino, R.A.Ñ.; Vitali, B.; De Rose, R.; Chidichimo, G.; Ceseracciu, L.; Athanassiou, A.; Saladini, B.; Dalena, F. Spanish Broom (Spartium junceum L.) fibers impregnated with vancomycin-loaded chitosan nanoparticles as new antibacterial wound dressing: Preparation, characterization and antibacterial activity. Eur. J. Pharm. Sci. 2017, 99, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, S.J.; Kalhapure, R.S.; Jadhav, M.; Rambharose, S.; Mocktar, C.; Govender, T. Transforming linoleic acid into a nanoemulsion for enhanced activity against methicillin susceptible and resistant Staphylococcus aureus. RSC Adv. 2015, 5, 90482–90492. [Google Scholar] [CrossRef]
- Kaur, C.D.; Nahar, M.; Jain, N.K. Lymphatic targeting of zidovudine using surface-engineered liposomes. J. Drug Target. 2008, 16, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Aslantürk, Ö.S. In Vitro Cytotoxicity and Cell Viability Assays: Principles, Advantages, and Disadvantages; InTech: London, UK, 2018; Volume 2. [Google Scholar]
- Ogunjimi, A.T.; Melo, S.M.; Vargas-Rechia, C.G.; Emery, F.S.; Lopez, R.F. Hydrophilic polymeric nanoparticles prepared from Delonix galactomannan with low cytotoxicity for ocular drug delivery. Carbohydr. Polym. 2017, 157, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Gharaati Far, N.; Tohidkia, M.R.; Dehnad, A.; Omidi, Y. Efficiency and cytotoxicity analysis of cationic lipids-mediated gene transfection into AGS gastric cancer cells. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1001–1008. [Google Scholar] [CrossRef]
- King, A.; Chakrabarty, S.; Zhang, W.; Zeng, X.; Ohman, D.E.; Wood, L.F.; Abraham, S.; Rao, R.; Wynne, K.J. High antimicrobial effectiveness with low hemolytic and cytotoxic activity for PEG/quaternary copolyoxetanes. Biomacromolecules 2014, 15, 456–467. [Google Scholar] [CrossRef]
- Kim, Y.; Binauld, S.; Stenzel, M.H. Zwitterionic guanidine-based oligomers mimicking cell-penetrating peptides as a nontoxic alternative to cationic polymers to enhance the cellular uptake of micelles. Biomacromolecules 2012, 13, 3418–3426. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Chen, J.; Landers, J.; Baker, J.R., Jr. Zwitterionic Surfactant as a Promising Non-Cytotoxic Carrier for Nanoemulsion-Based Vaccine Development. ChemistrySelect 2019, 4, 9027–9032. [Google Scholar] [CrossRef]
- Mao, C.; Xie, X.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K.; Pan, H.; Chu, P.K.; Wu, S. The controlled drug release by pH-sensitive molecularly imprinted nanospheres for enhanced antibacterial activity. Mater. Sci. Eng. C 2017, 77, 84–91. [Google Scholar] [CrossRef]
- Ohno, S.; Tsuda, Y.; Nakai, K.; Fujii, S.; Nakamura, Y.; Yusa, S.-I. pH-responsive liquid marbles prepared using fluorinated fatty acid. Chem. Lett. 2016, 45, 547–549. [Google Scholar] [CrossRef]
- Stubbings, W.; Leow, P.; Yong, G.C.; Goh, F.; Körber-Irrgang, B.; Kresken, M.; Endermann, R.; Labischinski, H. In vitro spectrum of activity of finafloxacin, a novel, pH-activated fluoroquinolone, under standard and acidic conditions. Antimicrob. Agents Chemother. 2011, 55, 4394–4397. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, S.; Tulkens, P.M.; Van Bambeke, F. Contrasting effects of acidic pH on the extracellular and intracellular activities of the anti-gram-positive fluoroquinolones moxifloxacin and delafloxacin against Staphylococcus aureus. Antimicrob. Agents. Chemother. 2011, 55, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, X.; Sun, X.; Zhao, M.; Yu, C.; Lee, R.J.; Sun, F.; Zhou, Y.; Li, Y.; Teng, L. Dual-functional lipid polymeric hybrid pH-responsive nanoparticles decorated with cell penetrating peptide and folate for therapy against rheumatoid arthritis. Eur. J. Pharm. Biopharm. 2018, 130, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Akimitsu, N.; Hamamoto, H.; Inoue, R.; Shoji, M.; Akamine, A.; Takemori, K.; Hamasaki, N.; Sekimizu, K. Increase in resistance of methicillin-resistant Staphylococcus aureus to beta-lactams caused by mutations conferring resistance to benzalkonium chloride, a disinfectant widely used in hospitals. Antimicrob. Agents Chemother. 1999, 43, 3042–3043. [Google Scholar] [CrossRef]
- Borsos, S.; Blondeau, J. Antimicrobial efficacy of gatifloxacin with and without benzalkonium chloride compared with moxifloxacin against common ocular pathogens. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1892. [Google Scholar]
- Couto, I.; Costa, S.S.; Viveiros, M.; Martins, M.; Amaral, L. Efflux-mediated response of Staphylococcus aureus exposed to ethidium bromide. J. Antimicrob. Chemother. 2008, 62, 504–513. [Google Scholar] [CrossRef]
- Kügler, R.; Bouloussa, O.; Rondelez, F. Evidence of a charge-density threshold for optimum efficiency of biocidal cationic surfaces. Microbiology 2005, 151, 1341–1348. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M.; de Melo Carrasco, L.D. Cationic antimicrobial polymers and their assemblies. Int. J. Mol. Sci. 2013, 14, 9906–9946. [Google Scholar] [CrossRef]
- Sande, L.; Sanchez, M.; Montes, J.; Wolf, A.J.; Morgan, M.A.; Omri, A.; Liu, G.Y. Liposomal encapsulation of vancomycin improves killing of methicillin-resistant Staphylococcus aureus in a murine infection model. J. Antimicrob. Chemother. 2012, 67, 2191–2194. [Google Scholar] [CrossRef]
- Bhise, K.; Sau, S.; Kebriaei, R.; Rice, S.A.; Stamper, K.C.; Alsaab, H.O.; Rybak, M.J.; Iyer, A.K. Combination of Vancomycin and Cefazolin Lipid Nanoparticles for Overcoming Antibiotic Resistance of MRSA. Materials 2018, 11, 1245. [Google Scholar] [CrossRef]
- Furi, L.; Ciusa, M.L.; Knight, D.; Di Lorenzo, V.; Tocci, N.; Cirasola, D.; Aragones, L.; Coelho, J.R.; Freitas, A.T.; Marchi, E. Evaluation of reduced susceptibility to quaternary ammonium compounds and bisbiguanides in clinical isolates and laboratory-generated mutants of Staphylococcus aureus. Antimicrob. Agents Chemother. 2013, 57, 3488–3497. [Google Scholar] [CrossRef] [PubMed]
- Christine, K.; Hesje, S.D.B.; Joseph, M. Blondeau. Benzalkonium Chloride Enhances Antibacterial Activity of Gatifloxacin and Reduces its Propensity to Select for Fluoroquinolone-Resistant Strains. J. Ocul. Pharmacol. Ther. 2009, 25, 329–334. [Google Scholar] [CrossRef]
- Gheorghe, I.; Popa, M.; Măruţescu, L.G. Molecular features of virulence and resistance mechanisms in nosocomial and community-acquired Staphylococcus aureus. In Staphylococcus Aureus; IntechOpen: London, UK, 2018. [Google Scholar]
- de Souza Monteiro, A.; Neto, W.R.N.; Mendes, A.R.S.; dos Santos Pinto, B.L.; da Silva, L.C.N.; Ferreira, G.F. Effects of alterations in Staphylococcus aureus cell membrane and cell wall in antimicrobial resistance. In The Rise of Virulence and Antibiotic Resistance in Staphylococcus Aureus; IntechOpen: London, UK, 2017. [Google Scholar]
- Kehrenberg, C.; Schwarz, S.; Jacobsen, L.; Hansen, L.H.; Vester, B. A new mechanism for chloramphenicol, florfenicol and clindamycin resistance: Methylation of 23S ribosomal RNA at A2503. Mol. Microb. 2005, 57, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.F.; Hamed, M.I.; Panitch, A.; Seleem, M.N. Targeting methicillin-resistant Staphylococcus aureus with short salt-resistant synthetic peptides. Antimicrob. Agents Chemother. 2014, 58, 4113–4122. [Google Scholar] [CrossRef]
- Dong, D.; Thomas, N.; Ramezanpour, M.; Psaltis, A.J.; Huang, S.; Zhao, Y.; Thierry, B.; Wormald, P.-J.; Prestidge, C.A.; Vreugde, S.; et al. Inhibition of Staphylococcus aureus and Pseudomonas aeruginosa biofilms by quatsomes in low concentrations. Exp. Biol. Med. 2020, 245, 34–41. [Google Scholar] [CrossRef]
- Omolo, C.A.; Megrab, N.A.; Kalhapure, R.S.; Agrawal, N.; Jadhav, M.; Mocktar, C.; Rambharose, S.; Maduray, K.; Nkambule, B.; Govender, T. Liposomes with pH responsive ‘on and off’switches for targeted and intracellular delivery of antibiotics. J. Liposome Res. 2019, 1–19. [Google Scholar] [CrossRef]
- Kakihana, Y.; Cheng, L.; Fang, L.-F.; Wang, S.-Y.; Jeon, S.; Saeki, D.; Rajabzadeh, S.; Matsuyama, H. Preparation of positively charged PVDF membranes with improved antibacterial activity by blending modification: Effect of change in membrane surface material properties. Colloids Surf. A Physicochem. Eng. Asp. 2017, 533, 133–139. [Google Scholar] [CrossRef]
- Alkhalifa, S.; Jennings, M.; Granata, D.; Klein, M.; Wuest, W.M.; Minbiole, K.; Carnevale, V. Analysis of the Destabilization of Bacterial Membranes by Quaternary Ammonium Compounds: A Combined Experimental and Computational Study. ChemBioChem 2019, 21, 1510–1516. [Google Scholar] [CrossRef]
- Yasir, M.; Dutta, D.; Willcox, M.D. Mode of action of the antimicrobial peptide Mel4 is independent of Staphylococcus aureus cell membrane permeability. PLoS ONE 2019, 14, e0215703. [Google Scholar] [CrossRef]
- Li, J.; Koh, J.-J.; Liu, S.; Lakshminarayanan, R.; Verma, C.S.; Beuerman, R.W. Membrane active antimicrobial peptides: Translating mechanistic insights to design. Front. Neurosci. 2017, 11, 73. [Google Scholar] [CrossRef]
- Rosenberg, M.; Azevedo, N.F.; Ivask, A. Propidium iodide staining underestimates viability of adherent bacterial cells. Sci. Rep. 2019, 9, 6483. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhong, J.; Wei, C.; Lin, C.-W.; Ding, T. Current perspectives on viable but non-culturable state in foodborne pathogens. Front. Microbiol. 2017, 8, 580. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K.; Okada, A.; Hayashi, Y.; Ichikawa, H.; Nishimura, A.; Shibata, N.; Sugioka, N. Enhanced oral bioavailability of vancomycin in rats treated with long-term parenteral nutrition. SpringerPlus 2015, 4, 442. [Google Scholar] [CrossRef] [PubMed]
- Garrett, T.R.; Bhakoo, M.; Zhang, Z. Bacterial adhesion and biofilms on surfaces. Prog. Nat. Sci. 2008, 18, 1049–1056. [Google Scholar] [CrossRef]
- Bogino, P.; Oliva, M.; Sorroche, F.; Giordano, W. The role of bacterial biofilms and surface components in plant-bacterial associations. Int. J. Mol. Sci. 2013, 14, 15838–15859. [Google Scholar] [CrossRef] [PubMed]
- Parasion, S.; Kwiatek, M.; Gryko, R.; Mizak, L.; Malm, A. Bacteriophages as an alternative strategy for fighting biofilm development. Pol. J. Microbiol. 2014, 63, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, P.; Ehrlich, G.D.; Sedghizadeh, P.P.; Hall Stoodley, L.; Baratz, M.E.; Altman, D.T.; Sotereanos, N.G.; Costerton, J.W.; DeMeo, P. Orthopaedic biofilm infections. Curr. Orthop. Pr. 2011, 22, 558. [Google Scholar] [CrossRef]
- Alhag, M.; Renvert, S.; Polyzois, I.; Claffey, N. Re-osseointegration on rough implant surfaces previously coated with bacterial biofilm: An experimental study in the dog. Clin. Oral Implant. Res. 2008, 19, 182–187. [Google Scholar] [CrossRef]
- Li, Z.H.; Cai, M.; Liu, Y.S.; Sun, P.L.; Luo, S.L. Antibacterial activity and mechanisms of essential oil from Citrus medica L. var. sarcodactylis. Molecules 2019, 24, 1577. [Google Scholar] [CrossRef]
- Van Holle, A.; Machado, M.D.; Soares, E.V. Flocculation in ale brewing strains of Saccharomyces cerevisiae: Re-evaluation of the role of cell surface charge and hydrophobicity. Appl. Microbiol. Biotechnol. 2012, 93, 1221–1229. [Google Scholar] [CrossRef]
- Cristani, M.; D’Arrigo, M.; Mandalari, G.; Castelli, F.; Sarpietro, M.G.; Micieli, D.; Venuti, V.; Bisignano, G.; Saija, A.; Trombetta, D. Interaction of four monoterpenes contained in essential oils with model membranes: Implications for their antibacterial activity. J. Agric. Food Chem. 2007, 55, 6300–6308. [Google Scholar] [CrossRef] [PubMed]
- Rukholm, G.; Mugabe, C.; Azghani, A.O.; Omri, A. Antibacterial activity of liposomal gentamicin against Pseudomonas aeruginosa: A time-kill study. Int. J. Antimicrob. Agents 2006, 27, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.H.; Lee, L.W.; Sheu, S.Y.; Lin, P.Y. Study on the stevioside analogues of steviolbioside, steviol, and isosteviol 19-alkyl amide dimers: Synthesis and cytotoxic and antibacterial activity. Chem. Pharm. Bull. 2004, 52, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, M.; Tarning, J.; Aye Cho, T.; Anal, A. Antibacterial activities and possible modes of action of Acacia nilotica (L.) Del. against multidrug-resistant Escherichia coli and Salmonella. Molecules 2017, 22, 47. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Peng, H.; Wang, X.; Shao, F.; Yuan, Z.; Han, H. Graphene oxide exhibits broad-spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation. Nanoscale 2014, 6, 1879–1889. [Google Scholar] [CrossRef]
- Finger, S.; Wiegand, C.; Buschmann, H.J.; Hipler, U.C. Antimicrobial properties of cyclodextrin-antiseptics-complexes determined by microplate laser nephelometry and ATP bioluminescence assay. Int. J. Pharm. 2012, 436, 851–856. [Google Scholar] [CrossRef]
- Gajewicz, A.; Schaeublin, N.; Rasulev, B.; Hussain, S.; Leszczynska, D.; Puzyn, T.; Leszczynski, J. Towards understanding mechanisms governing cytotoxicity of metal oxides nanoparticles: Hints from nano-QSAR studies. J. Nanotoxicol. 2015, 9, 313–325. [Google Scholar] [CrossRef]
- Seleem, M.N.; Jain, N.; Pothayee, N.; Ranjan, A.; Riffle, J.; Sriranganathan, N. Targeting Brucella melitensis with polymeric nanoparticles containing streptomycin and doxycycline. FEMS Microbiol. 2009, 294, 24–31. [Google Scholar] [CrossRef]
- Ranjan, A.; Pothayee, N.; Seleem, M.; Jain, N.; Sriranganathan, N.; Riffle, J.; Kasimanickam, R. Drug delivery using novel nanoplexes against a Salmonella mouse infection model. J. Nanoparticle Res. 2010, 12, 905–914. [Google Scholar] [CrossRef]








| CHol: StBAclm (mg/mL) | pH | MHD (nm) | PDI | ζ (mV) | DEE% | DLC% |
|---|---|---|---|---|---|---|
| VCM loaded StAclm-Qt1 | 7.4 | 122.9 ± 3.78 | 0.169 ± 0.02 | −5.74 ± 2.57 | ||
| 6.0 | 130.7 ± 5.13 | 0.201 ± 0.04 | +9.89 ± 0.68 | 52.22 ± 8.4 | 13.20 ± 1.17% | |
| 5.5 | 145.7 ± 5.08 | 0.216 ± 0.04 | +16.0 ± 1.59 | |||
| StBAclm-Qt blank | 7.4 | 139.5 ± 4.506 | 0.064 ± 0.024 | −9.750 ± 3.020 | ||
| 6.0 | 157.4 ± 16.58 | 0.066 ± 0.015 | −6.662 ± 0.439 | |||
| 5.5 | 155.3 ± 2.85 | 0.175 ± 0.01 | +6.01 ± 0.987 |
| Energy Components (kcal/mol) | |||||
|---|---|---|---|---|---|
| Complex | ΔEvdW | ΔEelec | ΔGgas | ΔGsolv | ΔGbind |
| VCM | −26.44 ± 0.35 | 32.98 ± 0.79 | 6.53 ± 0.76 | −26.81 ± 0.76 | −20.27 ± 0.31 |
| CHol | −15.66 ± 0.30 | −0.91 ± 0.19 | −16.58 ± 0.34 | 5.02 ± 0.17 | −11.55 ±0.28 |
| StBAclm | −15.06 ± 0.50 | 33.01 ± 0.83 | 17.94 ±0.99 | −30.28 ± 0.83 | −12.33 ± 0.42 |
| Room Temperature (25 ± 1 °C) | Room Temperature (4 ± 1 °C) | |||||||
|---|---|---|---|---|---|---|---|---|
| Time (days) | MHD | PDI | ζ (mV) | Content % | MHD | PDI | ζ (mV) | Content % |
| 0 | 141.9 ± 3.78 | 0.285 ± 0.04 | −5.74 ± 2.57 | 100 ± 7.66 | 141.9 ± 3.78 | 0.285 ± 0.04 | −5.74 ± 2.57 | 100 ± 7.66 |
| 30 | 145.2 ± 5.12 | 0.265 ± 1.21 | −6.12 ± 1.23 | 98.12 ± 9.9 | 144.3 ± 2.44 | 0.242 ± 0.01 | −7.29 ± 1.45 | 99.69 ± 8.27 |
| 60 | 143.4 ± 6.77 | 0.275 ± 2.41 | −5.86 ± 2.41 | 98.91 ± 12.3 | 140.2 ± 3.21 | 0.251 ± 0.03 | −6.12 ± 1.25 | 98.87 ± 8.27 |
| 90 | 144.1 ± 6.92 | 0.264 ± 1.03 | −5.88 ± 3.10 | 99.59 ± 11.9 | 142.3 ± 2.22 | 0.263 ± 1.02 | −6.12 ± 1.25 | 98.12 ± 5.67 |
| In Vitro Antibacterial Activity at pH 6.0 | In Vitro Antibacterial Activity at pH 7.4 | |||||
|---|---|---|---|---|---|---|
| Time (h) | 24 | 48 | 72 | 24 | 48 | 72 |
| MRSA (MIC µg/mL) | MRSA (MIC µg/mL) | |||||
| Bare VCM | 31.25 | 31.25 | 31.25 | 31.25 | 31.25 | 31.25 |
| StBAclm blank quatsome | 125 | 125 | 125 | 250 | 250 | 250 |
| StBAclm surfactant | 125 | 125 | 125 | 250 | 250 | 250 |
| VCM loaded StBAclm-Qt1 | 0.97 | 0.97 | 0.97 | 3.90 | 3.90 | 3.90 |
| (MRSA) pH 6.0 | ||||
| Duration | FIC agent A | FIC agent B | Σ FIC (agent A + agent B) | Interpretation |
| 24 h | 0.031 | 0.0077 | 0.0387 | Synergy |
| 48 h | 0.031 | 0.0077 | 0.0387 | Synergy |
| 72 h | 0.031 | 0.0077 | 0.0387 | Synergy |
| (MRSA) pH 7.4 | ||||
| Duration | FIC agent A | FIC agent B | Σ FIC (agent A + agent B) | Interpretation |
| 24 h | 0.125 | 0.015 | 0.140 | Synergy |
| 48 h | 0.125 | 0.015 | 0.140 | Synergy |
| 72 h | 0.125 | 0.015 | 0.140 | Synergy |
| Parameters | Control | Bare VCM | VCM-StBAcm-Qt1 Quatsomes |
|---|---|---|---|
| Electrical conductivity (mS cm−1) | 0.321 ± 0.01 | 0.357 ± 0.02 | 0.487 ± 0.01 |
| DNA concentration (μg·mL−1) | 17.0 ± 0.490 | 4.3 ± 0.08 | 2.08 ± 0.040 |
| Protein concentration (μg·mL−1) | 158.58 ± 8.54 | 98.12 ± 4.88 | 75.94 ± 4.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, D.; Omolo, C.A.; Fasiku, V.O.; Elrashedy, A.A.; Mocktar, C.; Nkambule, B.; Soliman, M.E.S.; Govender, T. Formulation of pH-Responsive Quatsomes from Quaternary Bicephalic Surfactants and Cholesterol for Enhanced Delivery of Vancomycin against Methicillin Resistant Staphylococcus aureus. Pharmaceutics 2020, 12, 1093. https://doi.org/10.3390/pharmaceutics12111093
Hassan D, Omolo CA, Fasiku VO, Elrashedy AA, Mocktar C, Nkambule B, Soliman MES, Govender T. Formulation of pH-Responsive Quatsomes from Quaternary Bicephalic Surfactants and Cholesterol for Enhanced Delivery of Vancomycin against Methicillin Resistant Staphylococcus aureus. Pharmaceutics. 2020; 12(11):1093. https://doi.org/10.3390/pharmaceutics12111093
Chicago/Turabian StyleHassan, Daniel, Calvin A. Omolo, Victoria Oluwaseun Fasiku, Ahmed A Elrashedy, Chunderika Mocktar, Bongani Nkambule, Mahmoud E. S. Soliman, and Thirumala Govender. 2020. "Formulation of pH-Responsive Quatsomes from Quaternary Bicephalic Surfactants and Cholesterol for Enhanced Delivery of Vancomycin against Methicillin Resistant Staphylococcus aureus" Pharmaceutics 12, no. 11: 1093. https://doi.org/10.3390/pharmaceutics12111093
APA StyleHassan, D., Omolo, C. A., Fasiku, V. O., Elrashedy, A. A., Mocktar, C., Nkambule, B., Soliman, M. E. S., & Govender, T. (2020). Formulation of pH-Responsive Quatsomes from Quaternary Bicephalic Surfactants and Cholesterol for Enhanced Delivery of Vancomycin against Methicillin Resistant Staphylococcus aureus. Pharmaceutics, 12(11), 1093. https://doi.org/10.3390/pharmaceutics12111093