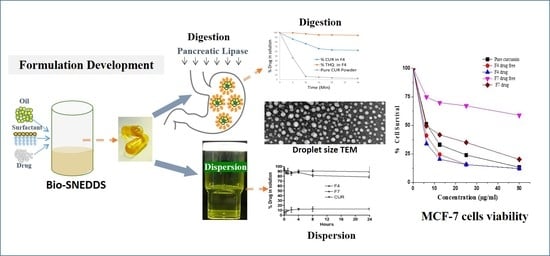
Development, Characterization Optimization, and Assessment of Curcumin-Loaded Bioactive Self-Nanoemulsifying Formulations and Their Inhibitory Effects on Human Breast Cancer MCF-7 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagent
2.2. Methods
2.2.1. Estimation of Thymoquinone Content (Phytochemical) in Black Seed Oil (BSO)
2.2.2. Gas Chromatography Mass Spectrometry (GC–MS) Analysis of BSO
2.2.3. Curcumin and Thymoquinone Bio-SNEDDS Formulation Development
2.2.4. Assessment of the Formulation Efficiency
2.2.5. Droplet Size and Polydispersity Index (PDI) Measurement
2.2.6. CUR and THQ Equilibrium Solubility
2.2.7. Transmission Electron Microscopy (TEM)
2.2.8. Physical Stability Assessment of CUR Bio-SNEDDS
2.2.9. Determination of Antimicrobial Activity
Disk Diffusion Method
Estimation of Antioxidant Activity
β-Carotene–Linoleic Acid Assay
In Vitro Dynamic Dispersion Studies
In Vitro Lipolysis Experiments
Cell Viability (MTT Cytotoxic) Assay
control × 100
Statistical Analysis
3. Results and Discussion
3.1. Chemical Constituents of BSO
3.2. Visual Assessment, Dispersion Time, Droplet Size, and PDI Analysis
3.3. Effect of Surfactant (HCO30) on Particle Size
3.4. Effect of Surfactant on the Solubility of CUR
3.5. Equilibrium Solubility Studies
3.6. Physical Stability Assessment of CUR Bio-SNEDDS
3.7. Transmission Electron Microscopy (TEM)
3.8. In Vitro Dynamic Dispersion Studies
3.9. In Vitro Lipolysis Studies
3.10. Antimicrobial Activity
3.10.1. Disk Diffusion Assays
3.10.2. Antioxidant Activity
3.10.3. Bio-SNEDDS Enhances Curcumin-Induced Inhibition of MCF-7 Cell Growth
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gadsby, R. New treatments for type 2 diabetes—The DPP4 inhibitors. Prim. Care Diabetes 2007, 1, 209–211. [Google Scholar] [CrossRef]
- Malamatari, M.; Somavarapu, S.; Kachrimanis, K.; Buckton, G.; Taylor, K.M. Preparation of respirable nanoparticle agglomerates of the low melting and ductile drug ibuprofen: Impact of formulation parameters. Powder Technol. 2017, 308, 123–134. [Google Scholar] [CrossRef]
- Noh, J.H.; Kim, S.-K.; Cho, Y.-J.; Nam, H.-U.; Kim, I.-J.; Jeong, I.-K.; Choi, M.-G.; Yoo, H.-J.; Ahn, Y.-H.; Bae, H.-Y.; et al. Current status of diabetes management in elderly Koreans with diabetes. Diabetes Res. Clin. Pr. 2007, 77, S71–S75. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Alexandridis, P. Temperature-Dependent Adsorption of Pluronic F127 Block Copolymers onto Carbon Black Particles Dispersed in Aqueous Media. J. Phys. Chem. B 2002, 106, 10834–10844. [Google Scholar] [CrossRef]
- Guttoff, M.; Saberi, A.H.; McClements, D.J. Formation of vitamin D nanoemulsion-based delivery systems by spontaneous emulsification: Factors affecting particle size and stability. Food Chem. 2015, 171, 117–122. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Lu, Z.; Wu, H.; Lv, F. Study on the antibiotic activity of microcapsule curcumin against foodborne pathogens. Int. J. Food Microbiol. 2009, 136, 71–74. [Google Scholar] [CrossRef]
- Lim, G.P.; Chu, T.; Yang, F.; Beech, W.; Frautschy, S.A.; Cole, G.M. The Curry Spice Curcumin Reduces Oxidative Damage and Amyloid Pathology in an Alzheimer Transgenic Mouse. J. Neurosci. 2001, 21, 8370–8377. [Google Scholar] [CrossRef]
- Wilken, R.; Veena, M.S.; Wang, M.B.; Srivatsan, E.S. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol. Cancer 2011, 10, 12. [Google Scholar] [CrossRef] [Green Version]
- Kazi, M.; Shahba, A.A.; Alrashoud, S.; AlWadei, M.; Sherif, A.Y.; Alanazi, F.K. Bioactive Self-Nanoemulsifying Drug Delivery Systems (Bio-SNEDDS) for Combined Oral Delivery of Curcumin and Piperine. Molecules 2020, 25, 1703. [Google Scholar] [CrossRef] [Green Version]
- Baetta, R.; Corsini, A. Pharmacology of Dipeptidyl Peptidase-4 Inhibitors. Drugs 2011, 71, 1441–1467. [Google Scholar] [CrossRef]
- Kalam, M.A.; Raish, M.; Ahmed, A.; Alkharfy, K.M.; Mohsin, K.; Alshamsan, A.; Al-Jenoobi, F.I.; Al-Mohizea, A.M.; Shakeel, F. Oral bioavailability enhancement and hepatoprotective effects of thymoquinone by self-nanoemulsifying drug delivery system. Mater. Sci. Eng. C 2017, 76, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Woo, C.C.; Kumar, A.P.; Sethi, G.; Tan, K.H.B. Thymoquinone: Potential cure for inflammatory disorders and cancer. Biochem. Pharmacol. 2012, 83, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.-G.; Lee, S.-Y.; Huang, Z.; Lim, D.Y.; Chen, H.; Jung, S.K.; Bode, A.M.; Lee, K.W.; Dong, Z. Curcumin Suppresses Proliferation of Colon Cancer Cells by Targeting CDK2. Cancer Prev. Res. 2014, 7, 466–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zu, J.-N.; Li, J.; Chen, C.; Xi, C.-Y.; Yan, J. Curcumin promotes the spinal cord repair via inhibition of glial scar formation and inflammation. Neurosci. Lett. 2014, 560, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Akhondian, J.; Kianifar, H.R.; Raoofziaee, M.; Moayedpour, A.; Toosi, M.B.; Khajedaluee, M. The effect of thymoquinone on intractable pediatric seizures (pilot study). Epilepsy Res. 2011, 93, 39–43. [Google Scholar] [CrossRef]
- Decalf, J.; Tarbell, K.V.; Casrouge, A.; Price, J.D.; Linder, G.; Mottez, E.; Sultanik, P.; Mallet, V.; Pol, S.; Duffy, D.; et al. Inhibition of DPP 4 activity in humans establishes its in vivo role in CXCL 10 post-translational modification: Prospective placebo-controlled clinical studies. EMBO Mol. Med. 2016, 8, 679–683. [Google Scholar] [CrossRef]
- Alwadei, M.; Kazi, M.; Alanazi, F.K. Novel oral dosage regimen based on self-nanoemulsifying drug delivery systems for codelivery of phytochemicals-curcumin and thymoquinone. Saudi Pharm. J. 2019, 27, 866–876. [Google Scholar] [CrossRef]
- Kazi, M.; Alhajri, A.; AlShehri, S.M.; Elzayat, E.M.; Al Meanazel, O.T.; Shakeel, F.; Noman, O.M.; Altamimi, M.A.; Alanazi, F.K. Enhancing Oral Bioavailability of Apigenin Using a Bioactive Self-Nanoemulsifying Drug Delivery System (Bio-SNEDDS): In Vitro, In Vivo and Stability Evaluations. Pharmaceutics 2020, 12, 749. [Google Scholar] [CrossRef]
- Kazi, M.; Shariare, M.; Al-Bgomi, M.; Hussain, M.; Alanazi, F. Simultaneous determination of Curcumin (Cur) and Thymoquinone (THQ) in lipid based self-nanoemulsifying systems and its application to the commercial product using UHPLC-UV-Vis spectrophotometer. Curr. Pharm. Anal. 2018, 14, 277–285. [Google Scholar] [CrossRef]
- Kommuru, T.; Gurley, B.; Khan, M.; Reddy, I. Self-emulsifying drug delivery systems (SEDDS) of coenzyme Q10: Formulation development and bioavailability assessment. Int. J. Pharm. 2001, 212, 233–246. [Google Scholar] [CrossRef]
- Craig, D. An investigation into the mechanisms of self-emulsification using particle size analysis and low frequency dielectric spectroscopy. Int. J. Pharm. 1995, 114, 103–110. [Google Scholar] [CrossRef]
- Gursoy, R.N.; Benita, S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed. Pharmacother. 2004, 58, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Aref, H.L. Variability in antimicrobial activity of latex from two varieties of Ficus carica. Afr. J. Microbiol. Res. 2011, 5, 1361–1367. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Mohsin, K. Design of lipid-based formulations for oral administration of poorly water-soluble drug fenofibrate: Effects of digestion. AAPS PharmSciTech 2012, 13, 637–646. [Google Scholar] [CrossRef] [Green Version]
- Pouton, C.W. Formulation of self-emulsifying drug delivery systems. Adv. Drug Deliv. Rev. 1997, 25, 47–58. [Google Scholar] [CrossRef]
- Kang, B.K.; Lee, J.S.; Chon, S.K.; Jeong, S.Y.; Yuk, S.H.; Khang, G.; Lee, H.B.; Cho, S.H. Development of self-microemulsifying drug delivery systems (SMEDDS) for oral bioavailability enhancement of simvastatin in beagle dogs. Int. J. Pharm. 2004, 274, 65–73. [Google Scholar] [CrossRef]
- Zidan, A.S.; Rahman, Z.; Sayeed, V.; Raw, A.; Yu, L.; Khan, M.A. Crystallinity evaluation of tacrolimus solid dispersions by chemometric analysis. Int. J. Pharm. 2012, 423, 341–350. [Google Scholar] [CrossRef]
- Mohsin, K.; Long, M.A.; Pouton, C.W. Design of Lipid-Based Formulations for Oral Administration of Poorly Water-Soluble Drugs: Precipitation of Drug after Dispersion of Formulations in Aqueous Solution. J. Pharm. Sci. 2009, 98, 3582–3595. [Google Scholar] [CrossRef]
- Porter, C.J.H.; Trevaskis, N.L.; Charman, W.N. Lipids and lipid-based formulations: Optimizing the oral delivery of lipophilic drugs. Nat. Rev. Drug Discov. 2007, 6, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Hintzen, F.; Perera, G.; Hauptstein, S.; Müller, C.; Laffleur, F.; Bernkop-Schnürch, A. In vivo evaluation of an oral self-microemulsifying drug delivery system (SMEDDS) for leuprorelin. Int. J. Pharm. 2014, 472, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, Y.; Zhang, L.; Wang, Q.; Zhang, D. Stability of nanosuspensions in drug delivery. J. Control. Release 2013, 172, 1126–1141. [Google Scholar] [CrossRef]
- Kazi, M.; Alanazi, F.K.; Hussain, M.D. In vitro Methods for In vitro-In vivo Correlation (IVIVC) for Poorly Water Soluble Drugs: Lipid Based Formulation Perspective. Curr. Drug Deliv. 2018, 15, 918–929. [Google Scholar] [CrossRef] [PubMed]
- Devraj, R.; Williams, H.D.; Warren, D.B.; Mohsin, K.; Porter, C.J.; Pouton, C.W. In vitro assessment of drug-free and fenofibrate-containing lipid formulations using dispersion and digestion testing gives detailed insights into the likely fate of formulations in the intestine. Eur. J. Pharm. Sci. 2013, 49, 748–760. [Google Scholar] [CrossRef]
- Fatouros, D.G.; Nielsen, F.; Douroumis, D.; Hadjileontiadis, L.J.; Müllertz, A. In vitro–in vivo correlations of self-emulsifying drug delivery systems combining the dynamic lipolysis model and neuro-fuzzy networks. Eur. J. Pharm. Biopharm. 2008, 69, 887–898. [Google Scholar] [CrossRef] [Green Version]
- Dai, W.-G.; Dong, L.C.; Shi, X.; Nguyen, J.; Evans, J.; Xu, Y.; Creasey, A.A. Evaluation of drug precipitation of solubility-enhancing liquid formulations using milligram quantities of a new molecular entity (NME). J. Pharm. Sci. 2007, 96, 2957–2969. [Google Scholar] [CrossRef]
- Higashino, H.; Hasegawa, T.; Yamamoto, M.; Matsui, R.; Masaoka, Y.; Kataoka, M.; Sakuma, S.; Yamashita, S. In Vitro–in Vivo Correlation of the Effect of Supersaturation on the Intestinal Absorption of BCS Class 2 Drugs. Mol. Pharm. 2014, 11, 746–754. [Google Scholar] [CrossRef]
- Cuiné, J.F.; Charman, W.N.; Pouton, C.W.; Edwards, G.A.; Porter, C.J. Increasing the Proportional Content of Surfactant (Cremophor EL) Relative to Lipid in Self-emulsifying Lipid-based Formulations of Danazol Reduces Oral Bioavailability in Beagle Dogs. Pharm. Res. 2007, 24, 748–757. [Google Scholar] [CrossRef]
- Zhou, Q.-M.; Wang, X.-F.; Liu, X.-J.; Zhang, H.; Lu, Y.-Y.; Su, S.-B. Curcumin enhanced antiproliferative effect of mitomycin C in human breast cancer MCF-7 cells in vitro and in vivo. Acta Pharmacol. Sin. 2011, 32, 1402–1410. [Google Scholar] [CrossRef] [Green Version]
- Jiang, M.; Huang, O.; Zhang, X.; Xie, Z.; Shen, A.; Liu, H.; Geng, M.; Shen, K. Curcumin induces cell death and restores tamoxifen sensitivity in the antiestrogen-resistant breast cancer cell lines MCF-7/LCC2 and MCF-7/LCC9. Molecules 2013, 18, 701–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]










| No | Formulation Compositions | Appearance | Spontaneity | Particle Size (nm) | PDI |
|---|---|---|---|---|---|
| F1 | BSO | Turbid | nondispersed | 2668.50 ± 32.11 | 1.00 |
| F2 | BSO:I998 (7:3) | Turbid | <1 min | 1934.54 ± 15.02 | 1.00 |
| F3 | BSO:I988 (7:3)/HCO30 (1/1) | Bluish | <1 min | 75.22 ± 8.34 | 0.694 |
| F4 | BSO:I988 (7:3)/KolliphorEL (1/1) | Transparent | <1 min | 28.53 ± 0.18 | 0.129 |
| F5 | BSO:I988 (1:1)/HCO30 (1/1) | Bluish | <1 min | 81.09 ± 15.12 | 0.447 |
| F6 | BSO/HCO30 (1/1) | Bluish | <1 min | 102.41 ± 17.89 | 0.651 |
| F7 | HCO30 | Transparent | 1–5 min | 19.81 ± 0.39 | 0.186 |
| No. | Formulation Ratios (% w/w) | Solubility of CUR (mg/g) | Solubility of THQ (mg/g) |
|---|---|---|---|
| F1 | BSO | 0.977 ± 0.013 | 3.014 ± 0.018 |
| F2 | BSO:I998 (7:3) | 2.066 ± 0.006 | 2.094 ± 0.010 |
| F3 | BSO:I988 (7:3)/HCO30 (1/1) | 16.328 ± 0.049 | 1.266 ± 0.005 |
| F4 | BSO:I988 (7:3)/KolliphorEL (1/1) | 20.695 ± 0.052 | 1.207 ± 0.005 |
| F5 | BSO:I988 (1:1)/HCO30 (1/1) | 15.276 ± 0.064 | 0.873 ± 0.005 |
| F6 | BSO/HCO30 (1/1) | 14.554 ± 0.036 | 1.273 ± 0.011 |
| F7 | HCO30 | 45.300± 0.049 | NP |
| Formulation | 0 Months | 3 Months | ||||
|---|---|---|---|---|---|---|
| Drug % | Z-Ave (d.nm) | ZP. (mV) | Drug % | Z-Ave (d.nm) | ZP. (mV) | |
| F4 | 100% | 28.53 ± 0.18 | −22.17 ± 2.90 | 97.36 ± 3.45 | 28.31 ± 0.87 | −21.59 ± 1.89 |
| F7 | 100% | 19.81 ± 0.39 | −10.39 ± 0.66 | 93.88 ± 1.62 | 18.99 ± 0.43 | −12.32 ± 1.22 |
| Appearance | ||||||
| F4 | Transparent | Transparent | ||||
| F7 | Transparent | Transparent | ||||
| Sample | (%) Antioxidant Activity against Conc. (1000 µg/mL) | Radical Scavenging Activity (%DPPH against Conc.) | ||||
|---|---|---|---|---|---|---|
| 10 (µg/mL) | 50 (µg/mL) | 100 (µg/mL) | 500 (µg/mL) | 1000 (µg/mL) | ||
| F4D | 57.2 ± 2.8 | 12.7 ± 4.1 | 20.3 ± 4.3 | 34.3 ± 3.9 | 56.3 ± 4.1 | 68.7 ± 3.2 |
| F4 | 19.3 ± 1.4 | - | - | - | - | 21.3 ± 2.4 |
| F7D | 48.3 ± 3.2 | 6.21 ± 4.2 | 12.2 ± 3.1 | 22.3 ± 2.4 | 38.3 ± 4.6 | 51.6 ± 3.7 |
| F7 | 2.6 ± 2.8 | - | - | - | - | 3.7 ± 2.2 |
| Ascorbic acid | NT | 80.7 ± 2.5 | 85.1 ± 1.3 | 85 ± 3.2 | 88.7 ± 2.7 | 90.7 ± 4.4 |
| Rutin | 89.3 | NT | NT | NT | NT | NT |
| Sample | MCF-7 IC50 (µg/mL) |
|---|---|
| Pure CUR control | 6.67 ± 0.5 |
| Drug-loaded F4 Bio-SNEDDS | 4.76 ± 0.3 |
| Drug-free F4 Bio-SNEDDS | 5.2 ± 0.4 |
| Drug-loaded F7 SNEDDS | 5.94 ± 0.5 |
| Drug-free F7 SNEDDS | >50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazi, M.; A. Nasr, F.; Noman, O.; Alharbi, A.; Alqahtani, M.S.; Alanazi, F.K. Development, Characterization Optimization, and Assessment of Curcumin-Loaded Bioactive Self-Nanoemulsifying Formulations and Their Inhibitory Effects on Human Breast Cancer MCF-7 Cells. Pharmaceutics 2020, 12, 1107. https://doi.org/10.3390/pharmaceutics12111107
Kazi M, A. Nasr F, Noman O, Alharbi A, Alqahtani MS, Alanazi FK. Development, Characterization Optimization, and Assessment of Curcumin-Loaded Bioactive Self-Nanoemulsifying Formulations and Their Inhibitory Effects on Human Breast Cancer MCF-7 Cells. Pharmaceutics. 2020; 12(11):1107. https://doi.org/10.3390/pharmaceutics12111107
Chicago/Turabian StyleKazi, Mohsin, Fahd A. Nasr, Omar Noman, Abdulrahman Alharbi, Mohammed S. Alqahtani, and Fars K. Alanazi. 2020. "Development, Characterization Optimization, and Assessment of Curcumin-Loaded Bioactive Self-Nanoemulsifying Formulations and Their Inhibitory Effects on Human Breast Cancer MCF-7 Cells" Pharmaceutics 12, no. 11: 1107. https://doi.org/10.3390/pharmaceutics12111107
APA StyleKazi, M., A. Nasr, F., Noman, O., Alharbi, A., Alqahtani, M. S., & Alanazi, F. K. (2020). Development, Characterization Optimization, and Assessment of Curcumin-Loaded Bioactive Self-Nanoemulsifying Formulations and Their Inhibitory Effects on Human Breast Cancer MCF-7 Cells. Pharmaceutics, 12(11), 1107. https://doi.org/10.3390/pharmaceutics12111107