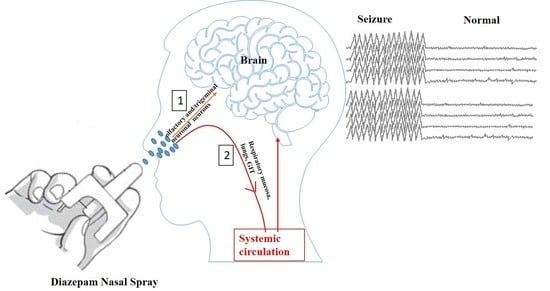
A Short Review on the Intranasal Delivery of Diazepam for Treating Acute Repetitive Seizures
Abstract
:1. Introduction
2. Treatment Options for ARS
3. Diazepam for Intranasal Administration
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cereghino, J.J. Identification and treatment of acute repetitive seizures in children and adults. Curr. Treat. Options Neurol. 2007, 9, 249–255. [Google Scholar] [CrossRef]
- Cereghino, J.J.; Cloyd, J.C.; Kuzniecky, R.I.; The North American Diastat Study Group. Rectal diazepam gel for treatment of acute repetitive seizures in adults. Arch. Neurol. 2002, 59, 1915–1920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buelow, J.M.; Shafer, P.; Shinnar, R.; Austin, J.; Dewar, S.; Long, L.; O’Hara, K.; Santilli, N. Perspectives on seizure clusters: Gaps in lexicon, awareness, and treatment. Epilepsy Behav. 2016, 57, 16–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Yu, W.; Lü, Y. The causes of new-onset epilepsy and seizures in the elderly. Neuropsychiatr. Dis. Treat. 2016, 12, 1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jafarpour, S.; Hirsch, L.J.; Gaínza-Lein, M.; Kellinghaus, C.; Detyniecki, K. Seizure cluster: Definition, prevalence, consequences, and management. Seizure 2019, 68, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Zack, M.M.; Kobau, R. National and State Estimates of the Numbers of Adults and Children with Active Epilepsy—United States, 2015. Mmwr. Morb. Mortal. Wkly. Rep. 2017, 66, 821–825. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Epilepsy. Available online: https://www.who.int/health-topics/epilepsy#tab=tab_1 (accessed on 27 November 2020).
- Haut, S.R. Seizure clustering. Epilepsy Behav. 2006, 8, 50–55. [Google Scholar] [CrossRef]
- Komaragiri, A.; Detyniecki, K.; Hirsch, L.J. Seizure clusters: A common, understudied and undertreated phenomenon in refractory epilepsy. Epilepsy Behav. 2016, 59, 83–86. [Google Scholar] [CrossRef]
- Chen, B.; Choi, H.; Hirsch, L.J.; Katz, A.; Legge, A.; Wong, R.A.; Jiang, A.; Kato, K.; Buchsbaum, R.; Detyniecki, K. Prevalence and risk factors of seizure clusters in adult patients with epilepsy. Epilepsy Res. 2017, 133, 98–102. [Google Scholar] [CrossRef]
- McKee, H.R.; Abou-Khalil, B. Outpatient pharmacotherapy and modes of administration for acute repetitive and prolonged seizures. CNS Drugs 2015, 29, 55–70. [Google Scholar] [CrossRef]
- Spencer, D. Hope for new treatments for acute repetitive seizures. Epilepsy Curr. 2014, 14, 147–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciccone, O.; Mathews, M.; Birbeck, G.L. Management of acute seizures in children: A review with special consideration of care in resource-limited settings. Afr. J. Emerg. Med. 2017, 7, S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, L.J., Jr. Molecular mechanisms of antiseizure drug activity at GABAA receptors. Seizure 2013, 22, 589–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, P.J.; Duncan, J.S. Cognitive decline in severe intractable epilepsy. Epilepsia 2005, 46, 1780–1787. [Google Scholar] [CrossRef] [PubMed]
- Kapur, J.; Macdonald, R.L. Rapid Seizure-Induced Reduction of Benzodiazepine and Zn2+ Sensitivity of Hippocampal Dentate Granule Cell GABAA Receptors. J. Neurosci. 1997, 17, 7532–7540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenblatt, D.; Arendt, R.; Abernethy, D.R.; Giles, H.; Sellers, E.; Shader, R. In vitro quantitation of benzodiazepine lipophilicity: Relation to in vivo distribution. BJA Br. J. Anaesth. 1983, 55, 985–989. [Google Scholar] [CrossRef] [Green Version]
- Adeyemo, M.A.; Idowu, S.O. Correlation of lipophilicity descriptors with pharmacokinetic parameters of selected benzodiazepines. Afr. J. Biomed. Res. 2016, 19, 213–218. [Google Scholar]
- Agarwal, S.K.; Cloyd, J.C. Development of benzodiazepines for out-of-hospital management of seizure emergencies. Neurol. Clin. Pr. 2015, 5, 80–85. [Google Scholar] [CrossRef] [Green Version]
- Manno, E.M. Status epilepticus: Current treatment strategies. Neurohospitalist 2011, 1, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Rogawski, M.A.; Heller, A.H. Diazepam buccal film for the treatment of acute seizures. Epilepsy Behav. 2019, 101, 106537. [Google Scholar] [CrossRef] [Green Version]
- Ivaturi, V.D. Intranasal and Rectal Diazepam for Rescue Therapy: Assessment of Pharmacokinetics and Tolerability; Retrieved from the University of Minnesota Digital Conservancy: Twin Cities, MN, USA, 2020; Available online: http://hdl.handle.net/11299/100044 (accessed on 29 November 2020).
- Rey, E.; Tréluyer, J.-M.; Pons, G. Pharmacokinetic optimisation of benzodiazepine therapy for acute seizures. Clin. Pharmacokinet. 1999, 36, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Maglalang, P.D.; Rautiola, D.; Siegel, R.A.; Fine, J.M.; Hanson, L.R.; Coles, L.D.; Cloyd, J.C. Rescue therapies for seizure emergencies: New modes of administration. Epilepsia 2018, 59, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Detyniecki, K.; Van Ess, P.J.; Sequeira, D.J.; Wheless, J.W.; Meng, T.-C.; Pullman, W.E. Safety and efficacy of midazolam nasal spray in the outpatient treatment of patients with seizure clusters—A randomized, double-blind, placebo-controlled trial. Epilepsia 2019, 60, 1797–1808. [Google Scholar] [CrossRef]
- Anderson, M. Buccal midazolam for pediatric convulsive seizures: Efficacy, safety, and patient acceptability. Patient Prefer. Adherence 2013, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- Sarma, A.K.; Khandker, N.; Kurczewski, L.; Brophy, G.M. Medical management of epileptic seizures: Challenges and solutions. Neuropsychiatr. Dis. Treat. 2016, 12, 467. [Google Scholar]
- Malu, C.K.K.; Kahamba, D.M.; Walker, T.D.; Mukampunga, C.; Musalu, E.M.; Kokolomani, J.; Mayamba, R.M.K.; Wilmshurst, J.M.; Dubru, J.-M.; Misson, J.-P. Efficacy of sublingual lorazepam versus intrarectal diazepam for prolonged convulsions in Sub-Saharan Africa. J. Child Neurol. 2014, 29, 895–902. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, J.; Robertson, S.; Norris, E.; Appleton, R.; Whitehouse, W.P.; Phillips, B.; Martland, T.; Berry, K.; Collier, J.; Smith, S. Safety and efficacy of buccal midazolam versus rectal diazepam for emergency treatment of seizures in children: A randomised controlled trial. Lancet 2005, 366, 205–210. [Google Scholar] [CrossRef]
- Von Blomberg, A.; Kay, L.; Knake, S.; Fuest, S.; Zöllner, J.P.; Reif, P.S.; Herrmann, E.; Balaban, Ü.; Schubert-Bast, S.; Rosenow, F.; et al. Efficacy, Tolerability, and Safety of Concentrated Intranasal Midazolam Spray as Emergency Medication in Epilepsy Patients During Video-EEG Monitoring. CNS Drugs 2020, 34, 545–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhaliwal, J.S.; Saadabadi, A. Diazepam. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Agarwal, S.K.; Kriel, R.L.; Brundage, R.C.; Ivaturi, V.D.; Cloyd, J.C. A pilot study assessing the bioavailability and pharmacokinetics of diazepam after intranasal and intravenous administration in healthy volunteers. Epilepsy Res. 2013, 105, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Hogan, R.E.; Tarquinio, D.; Sperling, M.R.; Klein, P.; Miller, I.; Segal, E.B.; Rabinowicz, A.L.; Carrazana, E. Pharmacokinetics and safety of VALTOCO (NRL-1; diazepam nasal spray) in patients with epilepsy during seizure (ictal/peri-ictal) and nonseizure (interictal) conditions: A phase 1, open-label study. Epilepsia 2020, 61, 935–943. [Google Scholar] [CrossRef]
- Grower, M.F.; Russell, E.A., Jr.; Getter, L. Solubility of injectable valium in intravenous solutions. Anesth. Prog. 1978, 25, 158. [Google Scholar] [PubMed]
- Wermeling, D.P.H.; Miller, J.L.; Archer, S.M.; Manaligod, J.M.; Rudy, A.C. Bioavailability and pharmacokinetics of lorazepam after intranasal, intravenous, and intramuscular administration. J. Clin. Pharmacol. 2001, 41, 1225–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vyas, T.K.; Shahiwala, A.; Marathe, S.; Misra, A. Intranasal drug delivery for brain targeting. Curr. Drug Deliv. 2005, 2, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Cloyd, J.C.; Siegel, R.A. A review of intranasal formulations for the treatment of seizure emergencies. J. Control. Release 2016, 237, 147–159. [Google Scholar] [CrossRef]
- Tanimoto, S.; Pesco Koplowitz, L.; Lowenthal, R.E.; Koplowitz, B.; Rabinowicz, A.L.; Carrazana, E. Evaluation of Pharmacokinetics and Dose Proportionality of Diazepam After Intranasal Administration of NRL-1 to Healthy Volunteers. Clin. Pharmacol. Drug Dev. 2020, 9, 719–727. [Google Scholar] [CrossRef] [Green Version]
- Maggio, E.T.; Pillion, D.J. High efficiency intranasal drug delivery using Intravail® alkylsaccharide absorption enhancers. Drug Deliv. Transl. Res. 2013, 3, 16–25. [Google Scholar] [CrossRef]
- Ali, J.; Ali, M.; Baboota, S.; Kaur Sahni, J.; Ramassamy, C.; Dao, L. Potential of nanoparticulate drug delivery systems by intranasal administration. Curr. Pharm. Des. 2010, 16, 1644–1653. [Google Scholar] [CrossRef]
- Arora, P.; Sharma, S.; Garg, S. Permeability issues in nasal drug delivery. Drug Discov. Today 2002, 7, 967–975. [Google Scholar] [CrossRef]
- Ugwoke, M.I.; Verbeke, N.; Kinget, R. The biopharmaceutical aspects of nasal mucoadhesive drug delivery. J. Pharm. Pharmacol. 2001, 53, 3–22. [Google Scholar] [CrossRef]
- Choi, H.-G.; Jung, J.-H.; Ryu, J.-M.; Yoon, S.-J.; Oh, Y.-K.; Kim, C.-K. Development of in situ-gelling and mucoadhesive acetaminophen liquid suppository. Int. J. Pharm. 1998, 165, 33–44. [Google Scholar] [CrossRef]
- Kaur, P.; Kim, K. Pharmacokinetics and brain uptake of diazepam after intravenous and intranasal administration in rats and rabbits. Int. J. Pharm. 2008, 364, 27–35. [Google Scholar] [CrossRef]
- Ivaturi, V.D.; Riss, J.R.; Kriel, R.L.; Cloyd, J.C. Pharmacokinetics and tolerability of intranasal diazepam and midazolam in healthy adult volunteers. Acta Neurol. Scand. 2009, 120, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Schrier, L.; Zuiker, R.; Merkus, F.W.H.M.; Klaassen, E.S.; Guan, Z.; Tuk, B.; van Gerven, J.M.A.; van der Geest, R.; Groeneveld, G.J. Pharmacokinetics and pharmacodynamics of a new highly concentrated intranasal midazolam formulation for conscious sedation. Br. J. Clin. Pharmacol. 2017, 83, 721–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- French, J.A. Benzo versus benzo: And the winner is…. Epilepsy Curr. 2011, 11, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Lissauer, S.; Kenny, J.; Jefferis, O.; Wingfield, T.; Miller, A.; Chagaluka, G.; Kalilani-Phiri, L.; Molyneux, E. Buccal, intranasal or intravenous lorazepam for the treatment of acute convulsions in children in Malawi: An open randomized trial: Le lorazépam par voie orale, intranasale ou intraveineuse pour le traitement des convulsions aiguës chez l’enfant au Malawi: Étude ouverte randomisée. Afr. J. Emerg. Med. 2015, 5, 120–126. [Google Scholar]
- Gizurarson, S.; Gudbrandsson, F.K.; Jonsson, H.; Bechgaard, E. Intranasal administration of diazepam aiming at the treatment of acute seizures: Clinical trials in healthy volunteers. Biol. Pharm. Bull. 1999, 22, 425–427. [Google Scholar] [CrossRef] [Green Version]
- Lindhardt, K.; Gizurarson, S.; Stefánsson, S.B.; Òlafsson, D.R.; Bechgaard, E. Electroencephalographic effects and serum concentrations after intranasal and intravenous administration of diazepam to healthy volunteers. Br. J. Clin. Pharm. 2001, 52, 521–527. [Google Scholar] [CrossRef] [Green Version]
- Ivaturi, V.D.; Riss, J.R.; Kriel, R.L.; Siegel, R.A.; Cloyd, J.C. Bioavailability and tolerability of intranasal diazepam in healthy adult volunteers. Epilepsy Res. 2009, 84, 120–126. [Google Scholar] [CrossRef]
- Lau, S.W.J.; Slattery, J.T. Absorption of diazepam and lorazepam following intranasal administration. Int. J. Pharm. 1989, 54, 171–174. [Google Scholar] [CrossRef]
- Bechgaard, E.; Gizurarson, S.; Hjortkjær, R.K. Pharmacokinetic and Pharmacodynamic Response after Intranasal Administration of Diazepam to Rabbits. J. Pharm. Pharmacol. 1997, 49, 747–750. [Google Scholar] [CrossRef]
- Platt, S.R.; Randell, S.C.; Scott, K.C.; Chrisman, C.L.; Hill, R.C.; Gronwall, R.R. Comparison of plasma benzodiazepine concentrations following intranasal and intravenous administration of diazepam to dogs. Am. J. Vet. Res. 2000, 61, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Nandi, I.; Kim, K.H. Development of an ethyl laurate-based microemulsion for rapid-onset intranasal delivery of diazepam. Int. J. Pharm. 2002, 237, 77–85. [Google Scholar] [CrossRef]
- Lindhardt, K.; Ólafsson, D.R.; Gizurarson, S.; Bechgaard, E. Intranasal bioavailability of diazepam in sheep correlated to rabbit and man. Int. J. Pharm. 2002, 231, 67–72. [Google Scholar] [CrossRef]
- Hou, H. Development of Novel Intranasal Diazepam Formulations for the Treatment of Seizure Emergencies; University of Minnesota: Minneapolis, MN, USA, 2006. [Google Scholar]
- Jiao, C.-M.; Liu, H.-Z.; Jing, L.-Y.; Zhang, Y.; Li, S.-M. Preparation and evaluation of diazepam microemulsion for intranasal delivery. Chin. J. Pharm. 2008, 4, 1–284. [Google Scholar]
- Botner, S.; Sintov, A.C. Intranasal Delivery of Two Benzodiazepines, Midazolam and Diazepam, by a Microemulsion System. Pharmacol. Pharm. 2011, 9. [Google Scholar] [CrossRef] [Green Version]
- Musulin, S.E.; Mariani, C.L.; Papich, M.G. Diazepam pharmacokinetics after nasal drop and atomized nasal administration in dogs. J. Vet. Pharmacol. Ther. 2011, 34, 17–24. [Google Scholar] [CrossRef]
- Sheng, P.; Zhang, R.-T.; Wang, H.; Nie, H.; Fu, X.-H. The effect of l-menthol on the intranasal absorption to diazepam. Chin. J. Hosp. Pharm. 2012, 24, 172–182. [Google Scholar]
- Sheng, P.; Zhang, R.-T.; Wang, H.; Huang, Z.; Fu, X.-H. Intranasal Absorption Kinetics of Diazepam and Promoting Effect of Menthol on The Absorption of Diazepam. Eval. Anal. Drug Use Hosp. China 2013, 19, 34–40. [Google Scholar]
- Ivaturi, V.; Kriel, R.; Brundage, R.; Loewen, G.; Mansbach, H.; Cloyd, J. Bioavailability of Intranasal vs. Rectal Diazepam. Epilepsy Res. 2013, 103, 254–261. [Google Scholar] [CrossRef]
- Bream, G.; Leibowitz, M.; Wargin, B.; Abernathy, K.; Henney, H.; Ward, D. Assessment of Pharmacokinetic Linearity and Relative Bioavailability of an Intranasal Diazepam Formulation Compared with Diazepam Rectal Gel in Healthy Adult Subjects (P02. 214); AAN Enterprises: St. Paul, MN, USA, 2013. [Google Scholar]
- Sperling, M.R.; Haas, K.F.; Krauss, G.; Seif Eddeine, H.; Henney, H.R.; Rabinowicz, A.L.; Bream, G.; Squillacote, D.; Carrazana, E.J. Dosing feasibility and tolerability of intranasal diazepam in adults with epilepsy. Epilepsia 2014, 55, 1544–1550. [Google Scholar] [CrossRef]
- Henney, H.R.; Sperling, M.R.; Rabinowicz, A.L.; Bream, G.; Carrazana, E.J. Assessment of pharmacokinetics and tolerability of intranasal diazepam relative to rectal gel in healthy adults. Epilepsy Res. 2014, 108, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Winter, T.; Lis, L.; Georg, G.I.; Siegel, R.A. Rapid Delivery of Diazepam from Supersaturated Solutions Prepared Using Prodrug/Enzyme Mixtures: Toward Intranasal Treatment of Seizure Emergencies. AAPS J. 2014, 16, 577–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inokuchi, R.; Ohashi-Fukuda, N.; Nakamura, K.; Wada, T.; Gunshin, M.; Kitsuta, Y.; Nakajima, S.; Yahagi, N. Comparison of intranasal and intravenous diazepam on status epilepticus in stroke patients: A retrospective cohort study. Med. Baltim. 2015, 94, e555. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Sharma, R.K.; Sharma, N.; Gabrani, R.; Sharma, S.K.; Ali, J.; Dang, S. Nose-To-Brain Delivery of PLGA-Diazepam Nanoparticles. AAPS Pharmscitech 2015, 16, 1108–1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramreddy, S.; Janapareddi, K. Brain targeting of chitosan-based diazepam mucoadhesive microemulsions via nasal route: Formulation optimization, characterization, pharmacokinetic and pharmacodynamic evaluation. Drug Dev. Ind. Pharm. 2019, 45, 147–158. [Google Scholar] [CrossRef]
- Rautiola, D.; Maglalang, P.D.; Cheryala, N.; Nelson, K.M.; Georg, G.I.; Fine, J.M.; Svitak, A.L.; Faltesek, K.A.; Hanson, L.R.; Mishra, U.; et al. Intranasal Coadministration of a Diazepam Prodrug with a Converting Enzyme Results in Rapid Absorption of Diazepam in Rats. J. Pharmacol. Exp. Ther. 2019, 370, 796–805. [Google Scholar] [CrossRef]
- Hogan, R.; Sperling, M.; Klein, P.; Segal, E.; Carrazana, E. Pharmacokinetic Study of Valtoco™ (NRL-1; Diazepam Nasal Spray) in Patients with Epilepsy under Ictal and Inter-Ictal Conditions—Interim Report (P3.5-009); Neurology: Minneapolis, MN, USA, 2019; Volume 92, 46p. [Google Scholar]
- Hogan, R.E.; Gidal, B.E.; Koplowitz, B.; Koplowitz, L.P.; Lowenthal, R.E.; Carrazana, E. Bioavailability and safety of diazepam intranasal solution compared to oral and rectal diazepam in healthy volunteers. Epilepsia 2020, 61, 455–464. [Google Scholar] [CrossRef]
- Tarquinio, D.; Hogan, R.E.; Sperling, M.R.; Wheless, J.W.; Dlugos, D.; Miller, I.; Rabinowicz, A.L.; Carrazana, E. Safety and Tolerability of NRL-1, an Intranasal Formulation of Diazepam, in Subjects with Epilepsy in a Phase 1, Open-Label Study: Focus on Adverse Events Relevant to Clinicians and Patients (2044). Neurology 2020, 94, 2044. [Google Scholar]
- Miller, I.; Wheless, J.W.; Hogan, R.E.; Dlugos, D.; Biton, V.; Cascino, G.D.; Sperling, M.R.; Liow, K.; Vazquez, B.; Ayala, R.; et al. Safety and Tolerability of NRL-1, an Intranasal Formulation of Diazepam, in Relationship to Usage Frequency in Subjects with Epilepsy: Interim Results From a Phase 3, Open-label, Repeat Dose Study (1992). Neurology 2020, 94, 1992. [Google Scholar]
- Dean, P.; Santilli, N.; Wheless, J.W.; Vazquez, B.; Segal, E.B.; Miller, I.; Hogan, R.E.; Carrazana, E.; Rabinowicz, A.L. Low Rate of Medication Errors Supports the Ability of Patients and Caregivers to Administer NRL-1, an Intranasal Formulation of Diazepam: Interim Results from a Phase 3, Open-Label, Repeat Dose Study (1875). Neurology 2020, 94, 1875. [Google Scholar]
- Acorda. Development of a Nasal Form of Diazepam Stopped. Epilepsy Found. 2016, 214, 161–165. Available online: https://www.epilepsy.com/article/2016/5/development-nasal-form-diazepam-stopped (accessed on 29 November 2020).
- Neurelis, Inc. Neurelis Intranasal Diazepam Treatment for Epilepsy Granted Fast Track Designation by FDA; Neurelis, Inc.: San Diego, CA, USA, 2017. [Google Scholar]


| Drug | Volume of Distribution (Vd), L/kg | Clearance (L/h/Kg) | Distribution Half-Life (t1/2α) (min) | Elimination Half-Life (t1/2β) (min) | Onset of Action (min) | Duration of Action |
|---|---|---|---|---|---|---|
| Diazepam | 0.89 ± 0.18 | 0.0388 ± 0.015 | 1.9–13.3 | 32.9 ± 8.8 | 1–3 | <2 h |
| Midazolam | 0.80 ± 0.19 | 0.42 ± 0.17 | 18.6 ± 14.4 | 2.4 ± 0.8 | ~2 | 3–4 h |
| Lorazepam | 1.14 ± 0.03 | 0.063 ± 0.009 | <11 | 14.3 ± 2.5 | 1–3 | <72 h |
| Route of Administration | Onset of Action (min) | Peak Plasma Levels (min) | Bioavailability |
|---|---|---|---|
| Oral | 15–60 | 30–90 | 100% |
| IM | 15–30 | 30–60 | 60% |
| Rectal | 5–10 | 10–45 | 80–90% |
| Intranasal | <5 | >60 | 97% |
| Drug | Brand Name | Route | Excipients |
|---|---|---|---|
| Diazepam | Valium® | Intravenous | 40% propylene glycol, 10% ethyl alcohol; 5% Na benzoate and, benzoic acid as buffers, and 1.5% benzyl alcohol as a preservative [34]. |
| Diazepam | Diastat® | Rectal | Propylene glycol, ethyl alcohol (10%), hydroxypropyl methylcellulose, sodium benzoate, benzyl alcohol (1.5%), benzoic acid and water. |
| Diazepam | Valtoco® (Available doses: 5 mg, 10 mg, 15 mg) | Intranasal | Benzyl alcohol (10.5 mg per 0.1 mL), dehydrated alcohol, n-dodecyl beta-d-maltoside, and vitamin E. |
| Lorazepam | Temesta®, solution for injection | Intravenous | Macrogol 400, benzyl alcohol 21 mg/ml, propylene glycol. |
| Lorazepam | Temesta Expidet® | Orodispersible | Gelatin, mannitol |
| Midazolam | Midazolam injection, USP | Intravenous | 0.8% sodium chloride and 0.01% edetate disodium, with 1% benzyl alcohol as preservative; the pH is adjusted to 3 to 3.6 with hydrochloric acid and, if necessary, sodium hydroxide |
| Midazolam | Seizalam™ | Intramuscular | 1% benzyl alcohol as preservative, 0.01% edetate disodium, and 0.8% sodium chloride, pH is adjusted to ~3. |
| Midazolam | Nayzilam® | Intranasal | Ethanol, PEG-6 methyl ether, polyethylene glycol 400, propylene glycol and purified water. |
| Midazolam hydrochloride | Buccolam® | Oromucosal solution | Sodium chloride, water for injections, hydrochloric acid (for pH adjustment and conversion of midazolam to the hydrochloride salt), sodium hydroxide (for pH adjustment |
| Midazolam maleate | Epistatus® | Oromucosal solution | Ethanol, saccharin sodium, glycerol purified water, sodium hydroxide (for pH adjustment), liquid maltitol |
| Formulations/Routes of Administration | Study Design | Subjects | Results | Conclusions | Ref. |
|---|---|---|---|---|---|
| Intranasal diazepam (10 mg) and lorazepam (4 mg) formulated using non-ionic surfactant (Cremophor EL) | Crossover trial | Healthy adults | Diazepam: Bioavailability = 84% and 72%, Tmax = 1 h, Cmax = 175 ng/mL. Peak concentration following intranasal administration was 27% to that of IV administration. Lorazepam: Bioavailability: 35–63%, Tmax = 0.5 to 4 h, Cmax = 18.7 ± 5.9 ng/mL, Cmax: 33–94% to that following IV administration. | Intranasal administration of diazepam and lorazepam would have limited potential in the acute treatment of seizures. | [52] |
| Intranasal diazepam in a mixture of 5% glycofurol and polyethylene glycol 200 versus commercial IV diazepam (Stesolid® Dumex-Alpharma), 2 mg dose | Open crossover trail | Healthy students | Intranasal diazepam: Cmax= 39 ± 17 ng/mL, tmax = 18 ± 11 min, t1/2 = 17.8 ± 15.5, AUC0–30min = 1095 ± 412 ng.min/mL. Intravenous diazepam: t1/2 = 14.4 ± 7.0, AUC0–30min = 2972 ± 980 ng.min/mL. | Intranasal diazepam could be an alternative to IV and rectal administration for treating acute seizures | [49] |
| Intranasal diazepam in polyethylene glycol 300 (4 mg and 7 mg dose) versus Stesolid Novum® intravenous diazepam (5 mg dose) | Double-blind, randomized, crossover design | Healthy volunteers | Mean differences between before and after drug administration values of P300-N100 amplitude differences were −0.9 (6.5, 4.7), −6.4 (−10.1, −2,7), −8.6 (−11.4, −5.8) and −9.6 (−12.1, −7.1) for placebo, 4 mg intranasal, 7 mg intranasal and 5 mg diazepam preparations, respectively. 4 and 7 mg intranasal diazepam formulations showed bioavailabilities of 45% and 42%, respectively. | Based on the electroencephalographic effects and blood concentration data, PEG300 may be used to deliver effective nasal dose of diazepam for the acute treatment of epilepsy | [50] |
| Intranasal diazepam in ten vehicles of different polarity to achieve tmax ≤ 5 min | - | Rabbits | Pure glycofurol 75, tetraethyleneglycol, poly(ethylene glycol) 200 and 30% glycofurol in tetraethyleneglycol showed very rapid pharmacodynamic response (1.5–3.5 min) compared to more polar liquids | Water-free low-molecular-weight glycols might be used as an alternative to IV injection for acute situations. | [53] |
| Intranasal diazepam versus IV diazepam (0.5 mg/kg) | Crossover design | Healthy adult greyhounds | IV: Cpeak: 1316 ± 216 µg/L, Tpeak was ≤ 3 min Intranasal: Cpeak: 448 ± 41 µg/L), Tpeak: 4.5 ± 1.5 min, Bioavailability: 80 ± 9% | Plasma concentrations exceed 300 µg/L (therapeutic concentration). Intranasal diazepam may be useful for treatment of seizures in dogs in place of IV administration | [54] |
| Intranasal ethyl laurate-based microemulsion systems of diazepam (1–2 mg/kg) and comparison with IV administration (1 mg/kg). | - | Rabbits | Tween 80–23.3%, propylene glycol–23.3% ethanol–15% H2O at 2 mg/kg dose resulted in rapid-onset of action (2–3 min) of diazepam with 50% bioavailability. | Ethyl laurate-based microemulsion of diazepam may be useful in the treatment of status epilepticus. | [55] |
| Diazepam intranasal (7 mg) versus diazepam intravenous (3 mg). Results were compared with rabbit and human data | Crossover design | Sheep | Mean nasal bioavailability, tmax and Cmax were 15 ± 8%, 5 ± 3 min and 934 ± 593 ng/mL, respectively. Bioavailability of diazepam in sheep was lower than rabbit (54%, p < 0.001) and man (34%, p < 0.05). | Correlation of bioavailability (rate and the extent of absorption) was not optimal between sheep, man and rabbit. | [56] |
| Supersaturated solution of diazepam in glycofurol/water for intranasal administration | - | MDCK epithelial cells as a nasal mucosa model | Steady-state flux of diazepam was obtained across MDCK epithelial cell monolayers from supersaturated solutions, which increased proportionally with increasing degree of saturation | Supersaturated diazepam solutions may be used for intranasal delivery | [57] |
| Diazepam was intravenously (1 mg/kg) or intranasally (2 mg/kg) administered to rats and rabbits | - | New Zealand white rabbits and Sprague–Dawley rats | Rats: Tmax = 5 min in rats, AUCbrain/AUCplasma ratios after IV (3.03 ± 0.07) and intranasal (3.00 ± 0.32) administration were nearly identical. Bioavailability in rat plasma (68.4%) and brain (67.7%) Rabbits: Tmax = 10 min, AUCbrain/AUCplasma ratios after intranasal administration (3.77 ± 0.17) were slightly lower than from IV administration (4.23 ± 0.08). Bioavailability in rabbit plasma (51.6%) and brain (45.9%) | No significant nose-to-brain transport (via olfactory epithelium) of diazepam was observed. Diazepam was mostly transported acorss the blood–brain barrier after intranasal administration. | [44] |
| Intranasal diazepam microemulsion | - | Bufo gargarizans | Miglyol 812 (8.0%), Tween 80 (21.3%), PEG400 (10.7%) and water (60.0%) containing microemulsion of diazepam showed only slight nasal ciliotoxicity | Microemulsions of Miglyol 812-Tween80-PEG400-water system with diazepam could be used for intranasal administration | [58] |
| 5 mg of diazepam and midazolam via both intranasal and IV routes | Four-way, randomized crossover trial. | Healthy adult volunteers | Diazepam: Cmax = 179.2° ng/mL, Tmax = 28.8 min Midazolam: Cmax = 62.8° ng/mL and Tmax 21.6 min. Intranasal administration resulted in rapid absorption with transient discomfort. Diazepam had a longer half-life, with an extended duration of action | Diazepam and midazolam were rapidly absorbed following intranasal administration with transient discomfort. | [45] |
| Intranasal formulation of diazepam (5 mg and 10 mg) in a glycofurol–water cosolvent system was investigated | Randomized, single-blind, three-way crossover | Healthy volunteers | The estimated bioavailability was 75% with pain and tolerability scores around 2–2.3 and 4.4–4.7 following administration of 5 and 10 mg doses, respectively | Intranasal diazepam provided a reasonable bioavailability, but was not well tolerated | [51] |
| Alcohol-free microemulsion system for intranasal delivery of diazepam or midazolam (2.5% by weight) | Randomized cross-over design | Rabbits | Diazepam: Cmax = 8.40 ± 3.00, Absolute bioavailability = 33.45 ± 12.36%, tmax = 18.33 ± 23.09 min Midazolam: Cmax = 46.62 ±17.38, tmax = 9.25 ± 6.75 min, Absolute bioavailability = 35.19 ±11.83% | Midazolam and diazepam microemulsion system could achieve rapid-onset of action following intranasal administration | [59] |
| Pharmacokinetics of diazepam following IV administration versus administration as intranasal drop versus atomized nasal administration | Randomized block design | Dogs | Mean diazepam concentrations following intranasal administration reached >300 ng/mL within 5 min in both groups. Diazepam bioavailability after intranasal drop and atomized nasal administration was 42% and 41%, respectively | Intranasal administration yielded rapid anticonvulsant concentrations of diazepam in dogs | [60] |
| Effect of l-menthol on absorption of intranasal diazepam | - | Mice | The effect of diazepam via intranasal administration was strengthened in the presence of l-menthol | Intranasal diazepam with l-menthol may result in sedative-hypnotic action and control epileptic seizures | [61] |
| Effect of menthol as a penetration enhancer on the absorption intranasal diazepam | Rabbit | At 0.2%, menthol increased the absorption of diazepam [k = (0.4424 ± 0.0023)/h] with quick absorption [t1/2 =(0.32 ± 0.07)h] | 0.2% menthol helped in the intranasal absorption of diazepam through passive diffusion | [62] | |
| Tolerability and pharmacokinetics of two intranasal diazepam formulations were compared with rectal gel (Diastat®) | Double blind, 4-period, 4-way crossover study | Healthy volunteers | Mean Cmax (± SD) was 181.8 ± 84.16, 151.3 ± 108.1 and 180.7 ± 82.1 ng/mL for Nas-A 10 mg, Nas-B 10 mg and Nas-B 13.4 mg respectively; while Cmax for the rectal gel was 160.9 ± 109.4 ng/mL. Median tmax was 0.75 h for all treatments. Intranasal formulations were well tolerated and exhibited relatively rapid but variable absorption with bioavailability of 70–90% compared to diazepam rectal gel | Intranasal diazepam could be an alternative to rectal diazepam | [63] |
| Dose proportionality of 5 mg and 20 mg of intranasal diazepam formulations. Relative bioavailability of 20 mg intranasal diazepam versus 20 mg rectal gel | Phase 1, single-center, randomized, open-label, three-period crossover study | Healthy subjects | Intranasal diazepam solutions (5 and 20 mg) showed dose proportionality with median time to Cmax of 1 h. Administration of a single dose of 20 mg intranasal diazepam resulted in similar plasma concentrations of diazepam and metabolite concentration, with less variability than with 20 mg rectal gel | Diazepam nasal solution (20 mg) showed comparable bioavailability as 20 mg rectal gel | [64] |
| Diazepam nasal spray (0.2 mg/kg) | Open-label study | Tmax of diazepam was 45 min with comparable dose-normalized mean Cmax and AUC0–12 values of diazepam among patients regardless of the timing of administration in relation to seizure. | Diazepam nasal spray could be used during the convulsive phase of tonic-clonic seizures or in the postictal periods following tonic-clonic or other seizure types. | [65] | |
| Intranasal diazepam formulation versus an equivalent dose of rectal diazepam (20 mg) | Phase 1, open-label, 3-period crossover study. | Healthy adults | Mean Cmax values of diazepam nasal spray and rectal gel were found to be 378 ± 106 and 328 ± 152 ng/mL, achieved at 1.0 and 1.5 h, respectively. Both intranasal and rectal diazepam were well tolerated with mild to moderate adverse events. | Single-dose of 20 mg diazepam nasal spray is tolerable and comparable in bioavailability to that of diazepam rectal gel. | [66] |
| Supersaturated diazepam solution using a prodrug/enzyme system (Avizafone, a peptide prodrug of diazepam, delivered with—Aspergillus oryzae protease) | - | Madin-Darby canine kidney II-wild type | Prodrug-protease mixtures upon apical exposure onto MDCKII-wt monolayers showed 2–17.6-fold higher diazepam flux (S = 1.3–15.3) compared to saturated diazepam (S = 0.7). | Intranasal avizafone-protease system with diazepam may provide rapid delivery. | [67] |
| Effectiveness of intranasal diazepam as an effective alternative to IV diazepam based on the medical records | Retrospective study | Stroke patients presenting with status epilepticus. | Intranasal diazepam was administered 9 times faster compared to IV diazepam resulting in about 3-fold reduction in the time to seizure activity termination following arrival at the hospital (3 min vs 9.5 min in the IV group, p = 0.030) | Intranasal diazepam could be a safe, quick and easier alternative to intravenous administration. | [68] |
| Diazepam-loaded poly(lactic-co-glycolic acid) nanoparticles | - | Rats | Gamma scintigraphy using technetium-99m-labeled (99mTc) showed a higher uptake of diazepam from nanoparticles compared to diazepam suspension in Sprague-Dawley rats. | PLGA nanoparticles of diazepam could be used in the treatment of status epilepticus | [69] |
| Mucoadhesive microemulsions of diazepam for intranasal administration versus Calmpose (i.v) and microemulsions | - | Rats | Diazepam microemulsion composed of oleic acid (5%), surfactant mixture (50%), water (45%), and chitosan (0.5%) showed significantly high flux of 846.96 ± 34 µg/cm2/h and AUCbrain = 1206.49 ± 145.8 compared to Calmpose (i.v) and microemulsion. | Mucoadhesive microemulsions showed higher absorption compared to IV administration | [70] |
| Coadministration of a hydrophilic diazepam prodrug (avizafone) and converting enzyme, human aminopeptidase B | - | Rats | Single doses of intranasal avizafone equivalent to 0.500, 1.00, and 1.50 mg/kg of diazepam resulted in 77.8% ± 6.0%, 112% ± 10%, and 114% ± 7% bioavailability with Cmax plasma concentrations 71.5 ± 9.3, 388 ± 31, and 355 ± 187 ng/mL; and tmax of 5, 8, and 5 min for each dose level, respectively. | Rapid and complete absorption by co-administering avizafone with aminopeptidase B | [71] |
| 1 dose period (5, 10, and 20 mg) followed by a 2-dose period (2 × 10 mg) with a minimum 28-day washout | Phase 1, open-label, randomized, crossover study | Healthy adult volunteers | Plasma concentration-time profiles showed similar patterns in a dose-dependent manner. The Cmax values of diazepam were 85.6, 133.6, and 235.3 ng/mL for 5-, 10-, and 20-mg doses, respectively. Dose-normalized AUC0–∞ values were comparable between the 2 × 10-mg and single 10-mg doses. | NRL-1 could be a potential therapy for managing seizure emergencies. | [38] |
| Valtoco™ (NRL-1; diazepam nasal spray) formulated with Intravail® A3 | Open-label study | Patients with epilepsy | Pharmacokinetic parameters in ictal/peri-ictal and inter-ictal conditions were similar (tmax: 3.31 ± 2.10 vs. 2.79 ± 1.89; Cmax: 156 ± 17 vs. 179 ± 18 ng/mL; AUC: 518 ± 30 vs. 566 ± 33 hr·ng/mL, respectively) | Valtoco™ showed a good safety profile in patients with epilepsy | [72] |
| Bioavailability and tolerability of intranasal diazepam containing Intravail® vs diazepam rectal gel | Phase 1, open-label, randomized, single-dose, crossover study | Healthy adult subjects | Tmax was similar for diazepam nasal spray and rectal gel, which were slower than oral diazepam in fasted individuals | Intravail® provided therapeutic levels of diazepam comparable to rectal diazepam with no damage to the nasal mucosa | [73] |
| Tolerability of NRL-1 (Valtoco®, diazepam nasal spray formulated with Intravail A3) and adverse events in patients | Open-label, Phase 3 study | Adults and children/adolescents with epilepsy | Of the 57 subjects, 17 subjects (29.8%) reported treatment emergent adverse events (TEAEs) with no treatment discontinuation. Treatment-related TEAEs were observed in 8 subjects (14%). Dysgeusia was reported in 3 subjects (5.3%) and nasal discomfort in 2 subjects) | NRL-1 demonstrated an acceptable safety/tolerability profile | [74] |
| Long-term safety and tolerability of NRL-1 (Valtoco®, diazepam nasal spray formulated with Intravail A3). A dose of 5, 10, 15, or 20 mg was administered based on patient weight | Phase 3, open-label, study | Patients (including adults and children/adolescents | Out of 132 enrolled subjects, NRL-1 was used moderately in 65 (49.2%) and frequently in 67 (50.8%) patients. Overall, 91 patients (68.9%) had TEAEs | Repeat dosing of NRL-1 showed an acceptable safety/tolerability profile similar to diazepam administered via other routes | [75] |
| Type of dosing errors and extent as a substitution for the ability of patients/caregivers to properly administer NRL-1 | Phase 3, open-label, study | Pediatric and adult patients with epilepsy | Patients/caregivers reported 31 dosing errors in 23 patients (1.2% of the administered 2486 doses). 80.6% of these errors were associated with doses requiring spray into both nostrils and 4 patients had multiple errors. Partial dosing errors were 48.4%, improper dosing errors were 12.9%, mechanical dosing time were 9.7% and 29.0% were other/unknown errors. | Most errors occurred when dose administration is required into both nostrils | [76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boddu, S.H.S.; Kumari, S. A Short Review on the Intranasal Delivery of Diazepam for Treating Acute Repetitive Seizures. Pharmaceutics 2020, 12, 1167. https://doi.org/10.3390/pharmaceutics12121167
Boddu SHS, Kumari S. A Short Review on the Intranasal Delivery of Diazepam for Treating Acute Repetitive Seizures. Pharmaceutics. 2020; 12(12):1167. https://doi.org/10.3390/pharmaceutics12121167
Chicago/Turabian StyleBoddu, Sai H. S., and Sneha Kumari. 2020. "A Short Review on the Intranasal Delivery of Diazepam for Treating Acute Repetitive Seizures" Pharmaceutics 12, no. 12: 1167. https://doi.org/10.3390/pharmaceutics12121167
APA StyleBoddu, S. H. S., & Kumari, S. (2020). A Short Review on the Intranasal Delivery of Diazepam for Treating Acute Repetitive Seizures. Pharmaceutics, 12(12), 1167. https://doi.org/10.3390/pharmaceutics12121167