PEG Graft Polymer Carriers of Antioxidants: In Vitro Evaluation for Transdermal Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Permeation Tests in Franz Diffusion Cells
2.4. Cell Culture
2.5. MTT Cytotoxicity Assay
2.6. Apoptosis and Cell Cycle Analysis by Flow Cytometry
2.7. Cell Senescence Test
3. Results
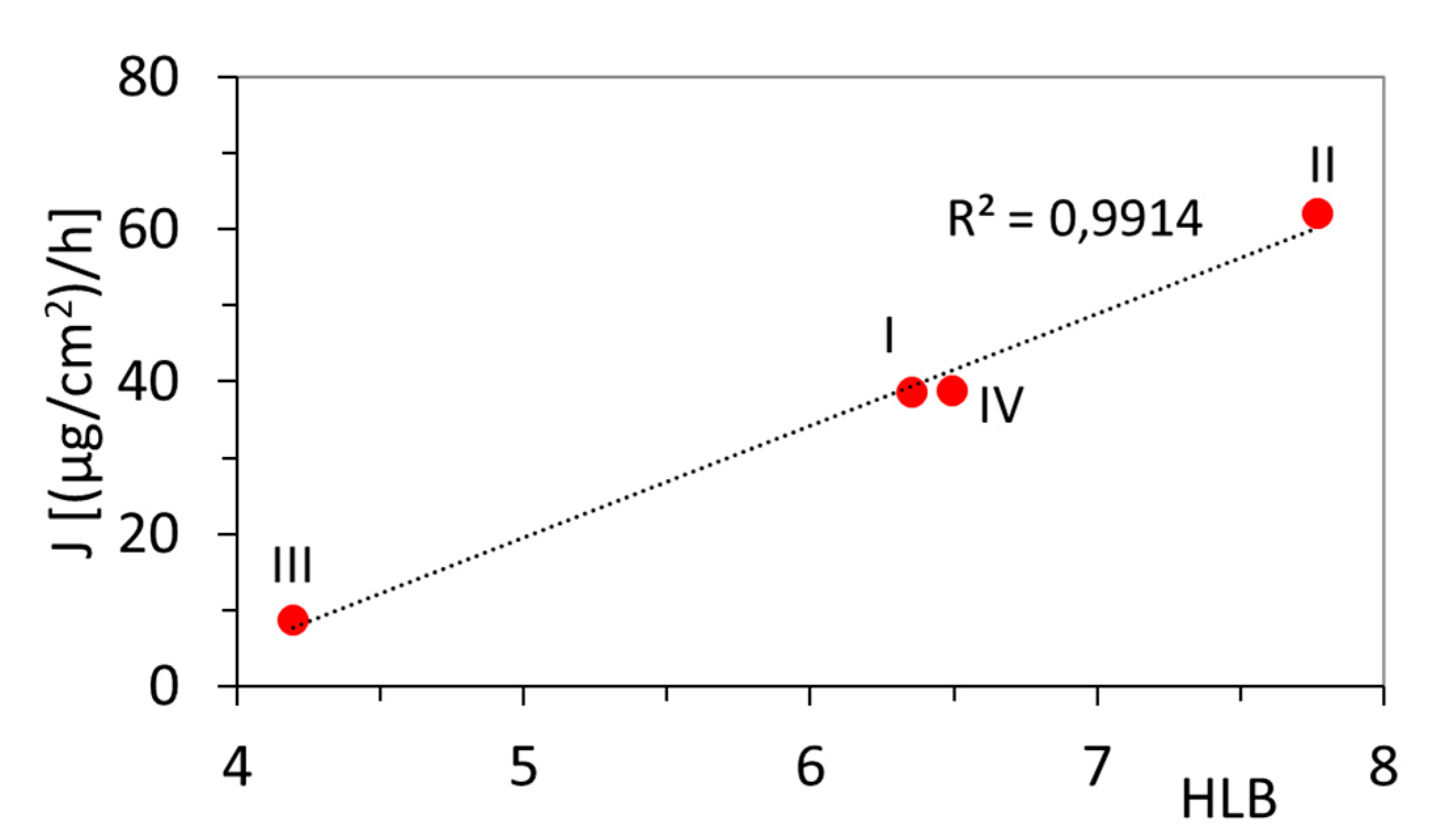
3.1. Permeation Tests
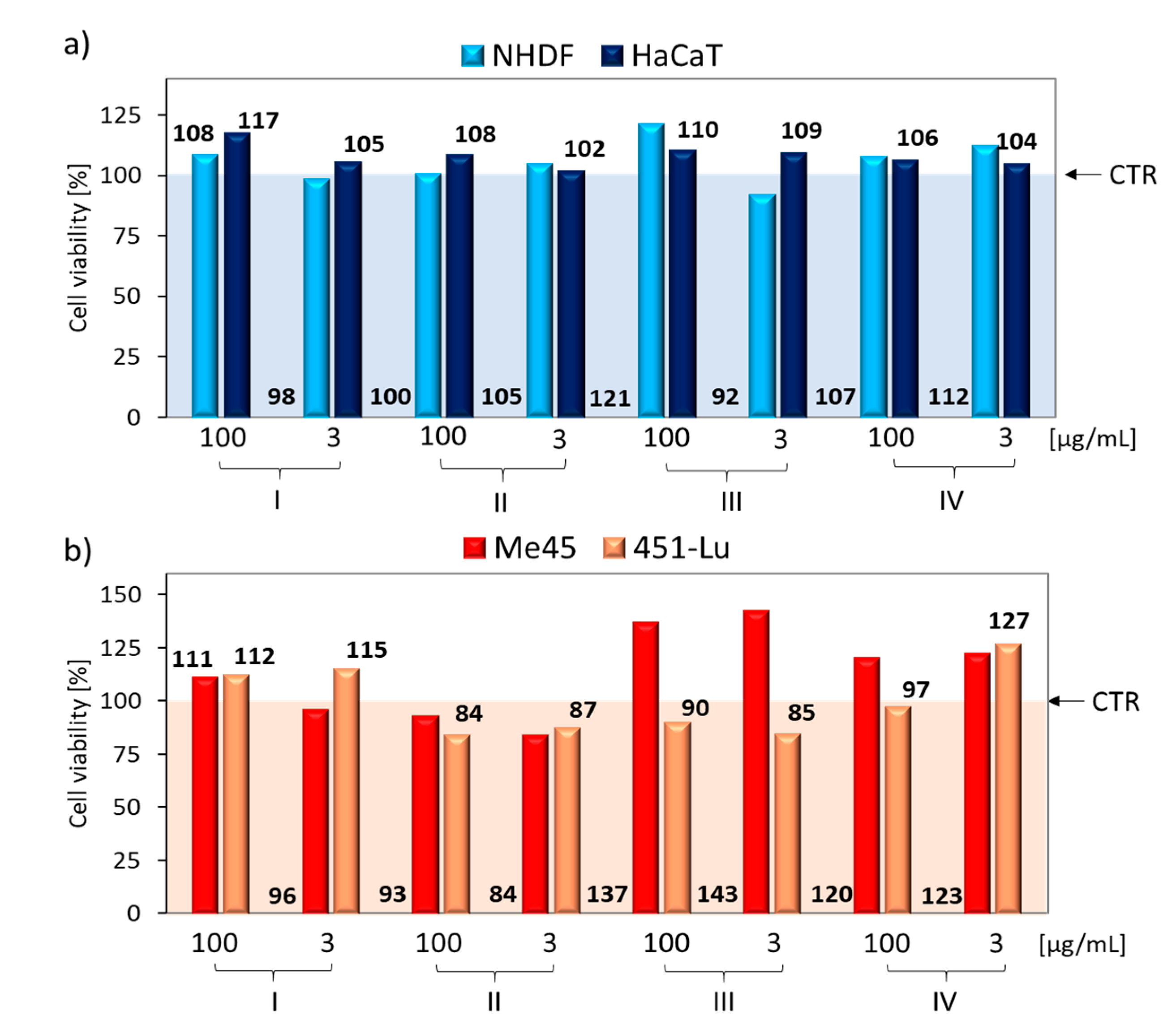
3.2. Cytotoxicity
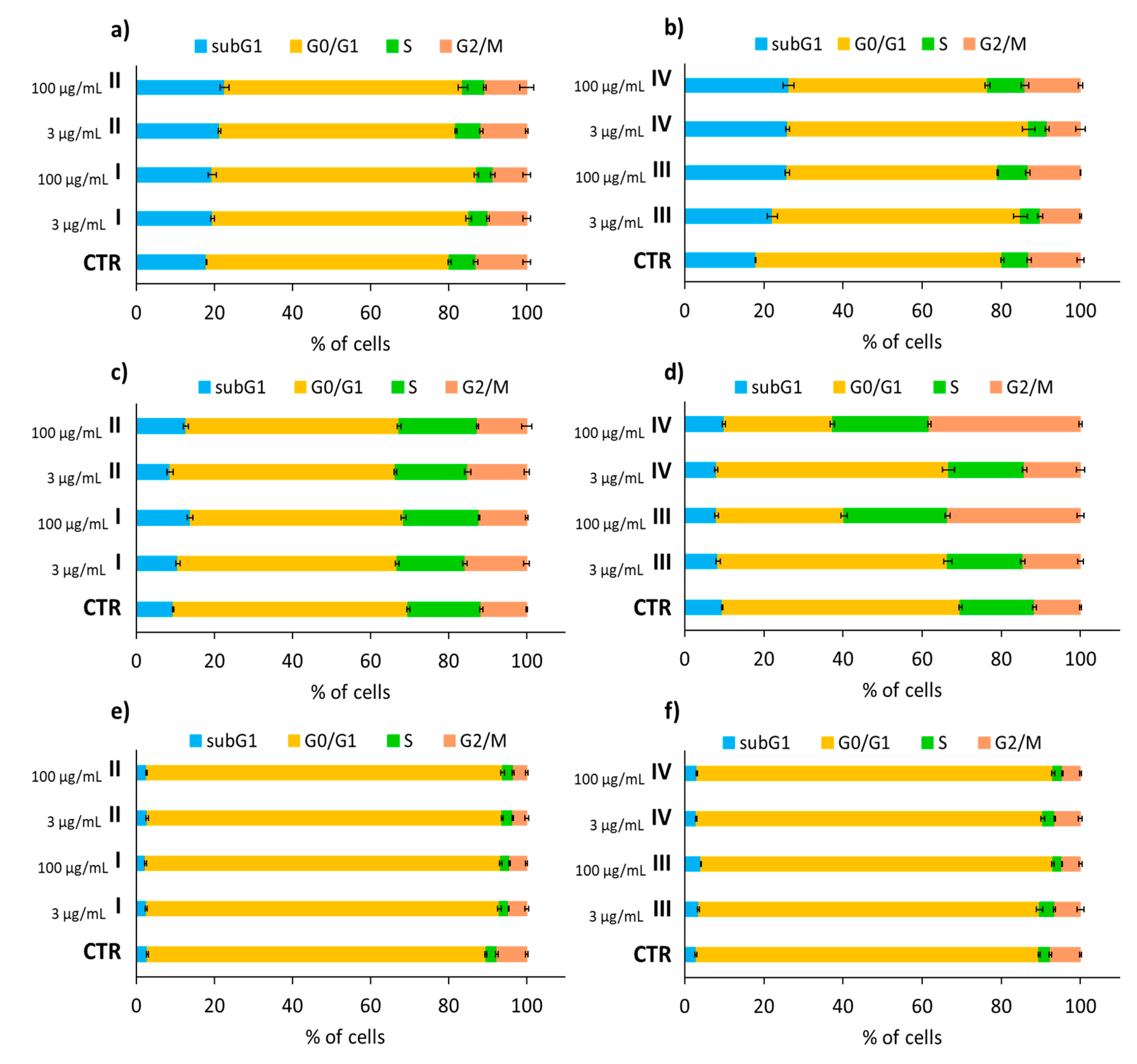
3.3. Cell Cycle Analysis
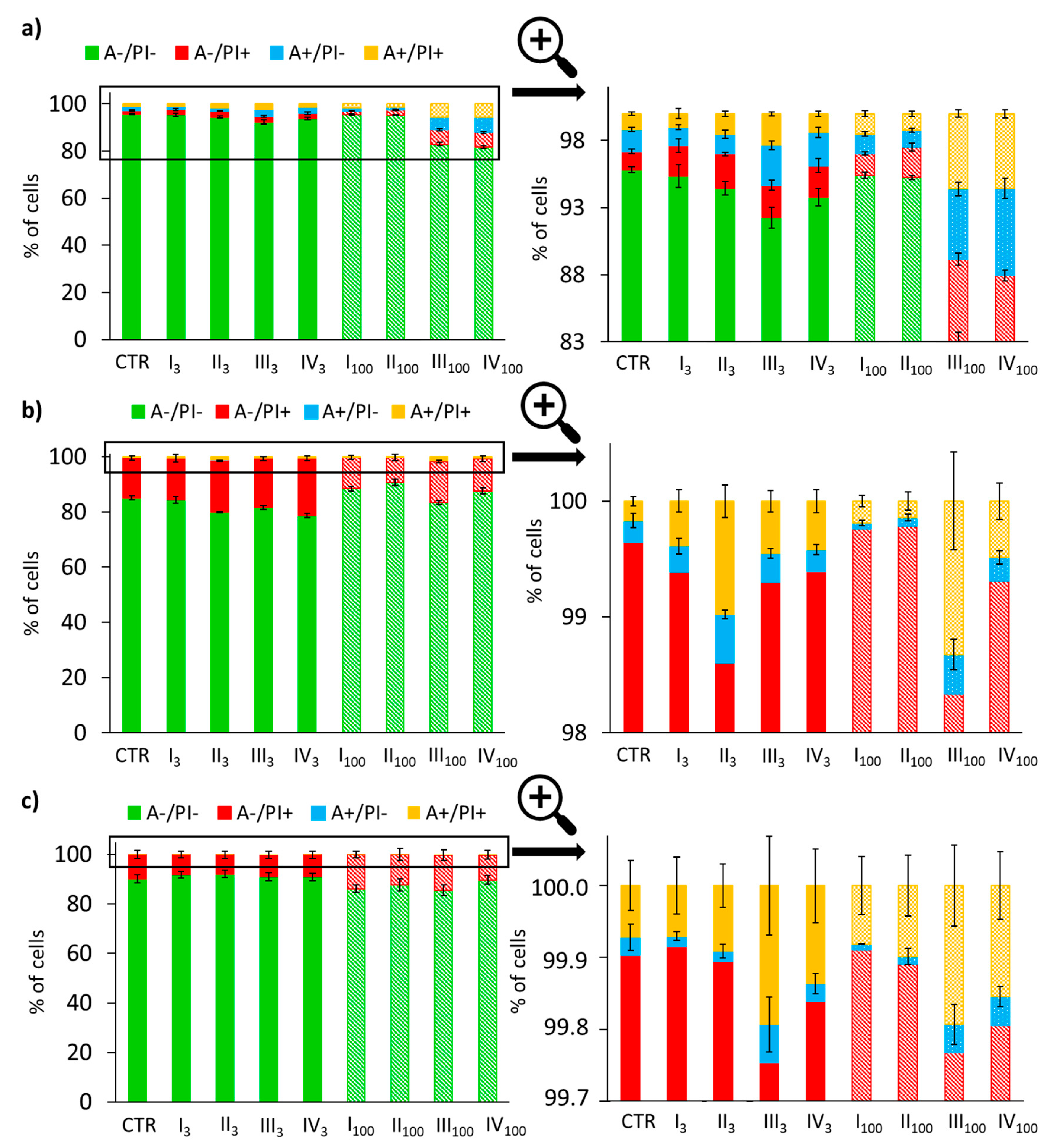
3.4. Analysis of Apoptotic and Necrotic Changes
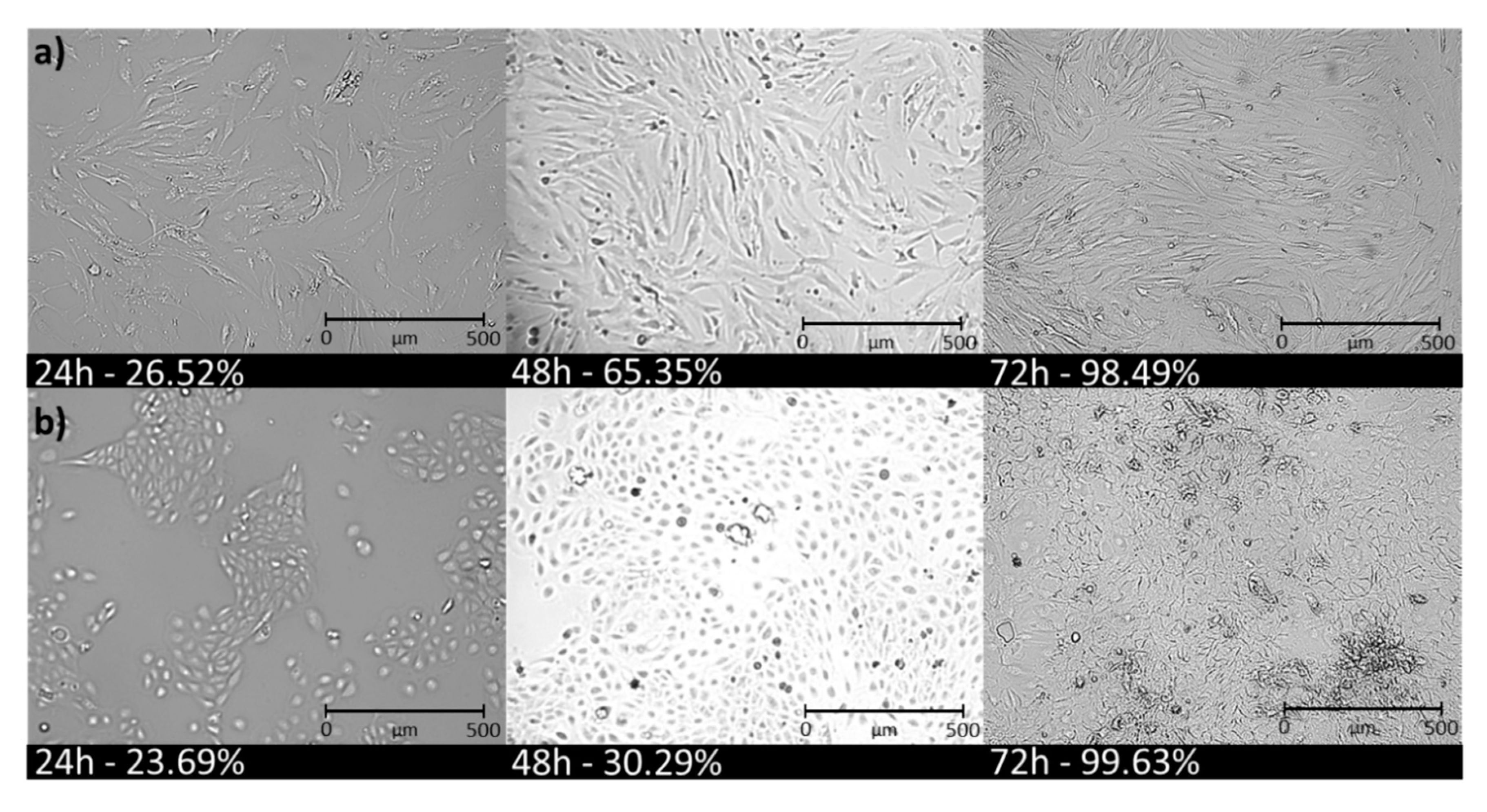
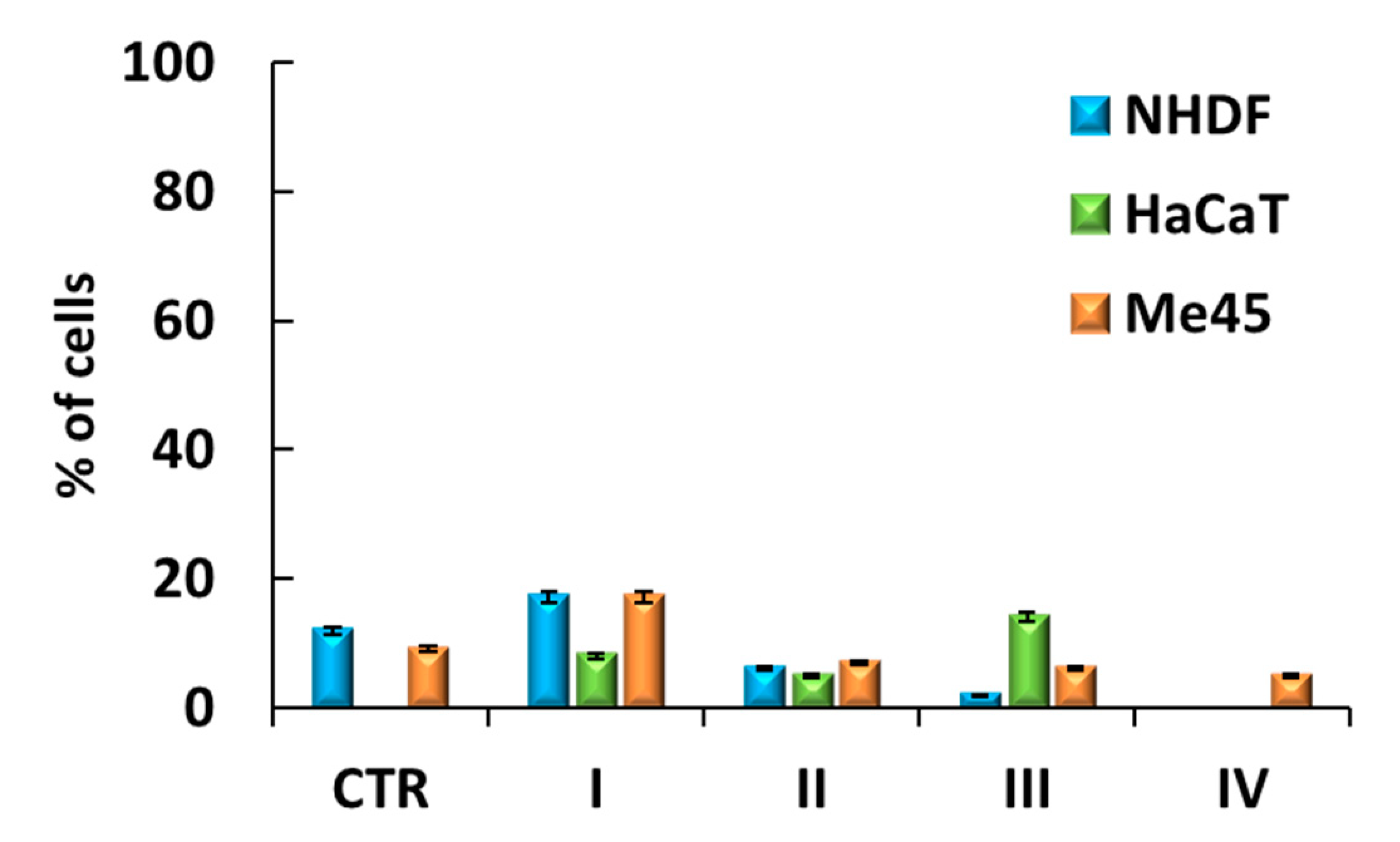
3.5. Cell Senescence Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ammala, A. Biodegradable polymers as encapsulation materials for cosmetics and personal care markets. Int. J. Cosmet. Sci. 2013, 35, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, R.; Duncan, R. Polymeric carriers: Preclinical safety and the regulatory implications for design and development of polymer therapeutics. Adv. Drug Deliv. Rev. 2009, 61, 1220–1231. [Google Scholar] [CrossRef] [PubMed]
- Říhová, B. Biocompatibility of biomaterials: Hemocompatibility, immunocompatiblity and biocompatibility of solid polymeric materials and soluble targetable polymeric carriers. Adv. Drug Deliv. Rev. 1996, 12, 157–176. [Google Scholar] [CrossRef]
- Iurciuc-Tincu, C.-E.; Stamate Cretan, M.; Purcar, V.; Popa, M.; Daraba, O.M.; Atanase, L.I.; Ochiuz, L. Drug delivery system based on pH-sensitive biocompatible poly(2-vinyl pyridine)-b-poly(ethylene oxide) nanomicelles loaded with curcumin and 5-fluorouracil. Polymers 2020, 12, 1450. [Google Scholar] [CrossRef]
- Luo, G.-F.; Chen, W.-H.; Zhang, X.-Z. Poly(N-isopropylacrylamide)-based thermally responsive micelles. ACS Macro Lett. 2020, 9, 872–881. [Google Scholar] [CrossRef]
- Marturano, V.; Cerruti, P.; Giamberini, M.; Tylkowski, B.; Ambrogi, V. Light-responsive polymer micro- and nano-capsules. Polymers 2017, 9, 8. [Google Scholar] [CrossRef]
- Seo, J.-E.; Kim, S.; Kim, B.-H. In vitro skin absorption tests of three types of parabens using a Franz diffusion cell. J. Expo. Sci. Env. Epid. 2017, 27, 320–325. [Google Scholar] [CrossRef]
- Salamanca, C.H.; Barrera-Ocampo, A.; Lasso, J.C.; Camacho, N.; Yarce, C.J. Franz diffusion cell approach for pre-formulation characterisation of ketoprofen semi-solid dosage forms. Pharmaceutics 2018, 10, 148. [Google Scholar] [CrossRef] [Green Version]
- Ilka, R.; Mohseni, M.; Kianirad, M.; Naseripour, M.; Ashtari, K.; Mehravi, B. Nanogel-based natural polymers as smart carriers for the controlled delivery of Timolol Maleate through the cornea for glaucoma. Int. J. Biol. Macromol. 2018, 109, 955–962. [Google Scholar] [CrossRef]
- Abnoos, M.; Mohseni, M.; Mousavi, S.A.J.; Ashtari, K.; Ilka, R.; Mehravi, B. Chitosan-alginate nano-carrier for transdermal delivery of pirfenidone in idiopathic pulmonary fibrosis. Int. J. Biol. Macromol. 2018, 118, 1319–1325. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Chi, H.; Wang, S. An alternative choice of lidocaine-loaded liposomes: Lidocaine-loaded lipid–polymer hybrid nanoparticles for local anesthetic therapy. Drug Deliv. 2016, 23, 1254–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niles, A.L.; Riss, T.L. Multiplexed viability, cytotoxicity, and caspase activity assays. Methods Mol. Biol. 2015, 1219, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Crowley, L.C.; Marfell, B.J.; Scott, A.P.; Waterhouse, N.J. Quantitation of apoptosis and necrosis by Annexin V binding, propidium iodide uptake, and flow cytometry. Cold Spring Harb. Protoc. 2016, 11, 953–957. [Google Scholar] [CrossRef] [PubMed]
- Nunez, R. DNA Measurement and cell cycle analysis by flow cytometry. Curr. Issues Mol. Biol. 2001, 3, 67–70. [Google Scholar] [PubMed]
- Bielas, R.; Mielańczyk, A.; Skonieczna, M.; Mielańczyk, Ł.; Neugebauer, D. Choline supported poly(ionic liquid) graft copolymers as novel delivery systems of anionic pharmaceuticals for anti-inflammatory and anti-coagulant therapy. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bielas, R.; Siewniak, A.; Skonieczna, M.; Adamiec, M.; Mielańczyk, Ł.; Neugebauer, D. Choline based polymethacrylate matrix with pharmaceutical cations as co-delivery system for antibacterial and anti-inflammatory combined therapy. J. Mol. Liq. 2019, 285, 114–122. [Google Scholar] [CrossRef]
- Mielańczyk, A.; Skonieczna, M.; Neugebauer, D. Cellular response to star-shaped polyacids. Solution behavior and conjugation advantages. Toxicol. Lett. 2017, 274, 42–50. [Google Scholar] [CrossRef]
- Mielańczyk, A.; Skonieczna, M.; Mielańczyk, Ł.; Neugebauer, D. In vitro evaluation of doxorubicin conjugates based on sugar core nonlinear polymethacrylates toward anticancer drug delivery. Bioconjug. Chem. 2016, 27, 893–904. [Google Scholar] [CrossRef]
- Mielańczyk, A.; Skonieczna, M.; Bernaczek, K.; Neugebauer, D. Fluorescein nanocarriers based on cationic star copolymers with acetal linked sugar cores. Synthesis and biochemical characterization. RSC Adv. 2014, 4, 31904–31913. [Google Scholar] [CrossRef]
- Mielańczyk, A.; Mrowiec, K.; Kupczak, M.; Mielańczyk, Ł.; Scieglinska, D.; Gogler-Piglowska, A.; Michalski, M.; Gabriel, A.; Neugebauer, D.; Skonieczna, M. Synthesis and in vitro cytotoxicity evaluation of star-shaped polymethacrylic conjugates with methotrexate or acitretin as potential antipsoriatic prodrugs. Eur. J. Pharmacol. 2020, 866, 172804. [Google Scholar] [CrossRef]
- Boskabadi, M.; Saeedi, M.; Akbari, J.; Morteza-Semnani, K.; Hashemi, S.M.H.; Babaei, A. Topical gel of vitamin A solid lipid nanoparticles: A hopeful promise as a dermal delivery system. Colloids Surf. B Biointerfaces 2019, 171, 150–157. [Google Scholar] [CrossRef]
- Lu, L.; Du, Y.; Ismail, M.; Ling, L.; Yao, C.; Fu, Z.; Li, X. Liposomes assembled from dimeric retinoic acid phospholipid with improved pharmacokinetic properties. Eur. J. Pharm. Sci. 2018, 112, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Odrobińska, J.; Niesyto, K.; Erfurt, K.; Siewniak, A.; Mielańczyk, A.; Neugebauer, D. Retinol-containing graft copolymers for delivery of skin-curing agents. Pharmaceutics 2019, 11, 378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odrobińska, J.; Neugebauer, D. Micellar carriers based on amphiphilic PEG/PCL graft copolymers for delivery of active substances. Polymers 2020, 12, 2876. [Google Scholar] [CrossRef]
- Odrobińska, J.; Neugebauer, D. PEG Grafted polymethacrylates bearing antioxidants as a new class of polymer conjugates for application in cosmetology. Materials 2020, 13, 3455. [Google Scholar] [CrossRef]
- Odrobińska, J.; Mielańczyk, Ł.; Neugebauer, D. 4-n-Butylresorcinol-based linear and graft polymethacrylates for arbutin and vitamins delivery by micellar systems. Polymers 2020, 12, 330. [Google Scholar] [CrossRef] [Green Version]
- Odrobińska, J.; Neugebauer, D. Retinol derivative as bioinitiator in the synthesis of hydroxyl-functionalized polymethacrylates for micellar delivery systems. EXPRESS Polym. Lett. 2019, 13, 806–817. [Google Scholar] [CrossRef]
- Borys, P.; Rybak, A.; Grzywna, Z.J. On the analysis of concentration-dependent diffusion using transient sorption and permeation measurements by the D1–D8 system. Ind. Eng. Chem. Res. 2013, 52, 8887–8896. [Google Scholar] [CrossRef]
- Ashrafi, P.; Sun, Y.; Davey, N.; Wilkinson, S.C.; Moss, G.P. The influence of diffusion cell type and experimental temperature on machine learning models of skin permeability. J. Pharm. Pharmacol. 2020, 72, 197–208. [Google Scholar] [CrossRef]
- Sahle, F.F.; Balzus, B.; Gerecke, C.; Kleuser, B.; Bodmeier, R. Formulation and in vitro evaluation of polymeric enteric nanoparticles as dermal carriers with pH-dependent targeting potential. Eur. J. Pharm. Sci. 2016, 92, 98–109. [Google Scholar] [CrossRef]
- Kosakowska, K.A.; Casey, B.K.; Kurtz, S.L.; Lawson, L.B.; Grayson, S.M. Evaluation of amphiphilic star/linear–dendritic polymer reverse micelles for transdermal drug delivery: Directing carrier properties by tailoring core versus peripheral branching. Biomacromolecules 2018, 19, 3163–3176. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Zhao, S.; Wang, H.; Liu, Y.; Zhang, Y.; Sun, G. Self-assembly of retinoid nanoparticles for melanoma therapy. Int. J. Nanomed. 2019, 14, 7963–7973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emami, J.; Rezazadeh, M.; Mashayekhi, M.; Rostami, M.; Jahanian-Najafabadi, A. A novel mixed polymeric micelle for co-delivery of paclitaxel and retinoic acid and overcoming multidrug resistance: Synthesis, characterization, cytotoxicity, and pharmacokinetic evaluation. Drug Dev. Ind. Pharm. 2018, 44, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.-L.; Yu, W.; Xu, F.; Zhang, L.-L. Novel thermo-responsive self-assembly micelles from a double brush-shaped PNIPAM-g-(PA-b-PEG-b-PA)-g-PNIPAM block copolymer with PNIPAM polymers as side chains. J. Polym. Sci. Polym. Chem. 2012, 50, 2053–2067. [Google Scholar] [CrossRef]
- Zhao, Y.; He, G.; Guo, W.; Bao, L.; Yi, M.; Gong, Y.; Zhang, S. Self-assembled micelles prepared from amphiphilic copolymers bearing cell outer membrane phosphorylcholine zwitterions for a potential anti-phagocytic clearance carrier. Polym. Chem. 2016, 7, 5698–5708. [Google Scholar] [CrossRef]
- Twarużek, M.; Zastempowska, E.; Soszczyńska, E.; Ałtyn, I. The use of in vitro assays for the assessment of cytotoxicity on the example of MTT test. Folia Biol. Oecologica 2018, 14, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Ciapetti, G.; Cenni, E.; Pratelli, L.; Pizzoferrato, A. In vitro evaluation of cell/biomaterial interaction by MTT assay. Biomaterials 1993, 14, 359–364. [Google Scholar] [CrossRef]
- Sugimoto, K.; Nishimura, T.; Nomura, K.; Sugimoto, K.; Kuriki, T. Inhibitory effects of α-arbutin on melanin synthesis in cultured human melanoma cells and a three-dimensional human skin model. Biol. Pharm. Bull. 2004, 27, 510–514. [Google Scholar] [CrossRef] [Green Version]
- Parisi, O.I.; Aiello, D.; Casula, M.F.; Puoci, F.; Malivindi, R.; Scrivano, L.; Testa, F. Mesoporous nanocrystalline TiO2 loaded with ferulic acid for sunscreen and photo-protection: Safety and efficacy assessment. RSC Adv. 2016, 6, 83767–83775. [Google Scholar] [CrossRef]
- Maksymiak, M.; Debowska, R.; Jelonek, K.; Kowalczuk, M.; Adamus, G. Structural characterization of biocompatible lipoic acid–oligo-(3-hydroxybutyrate) conjugates by electrospray ionization massspectrometry. Rapid Commun. Mass Spectrom. 2013, 27, 773–783. [Google Scholar] [CrossRef]
- Cheng, S.-L.; Liu, R.H.; Sheu, J.-N.; Chen, S.-T.; Sinchaikul, S.; Tsay, G.J. Toxicogenomics of A375 human malignant melanoma cells treated with arbutin. J. Biomed. Sci. 2007, 14, 87–105. [Google Scholar] [CrossRef]
- Huang, M.-T.; Smart, R.C.; Wong, C.-Q.; Conney, A.H. Inhibitory effect of curcumin, chlorogenic acid, caffeic acid, and ferulic acid on tumor promotion in mouse skin by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1988, 48, 5941–5946. [Google Scholar]

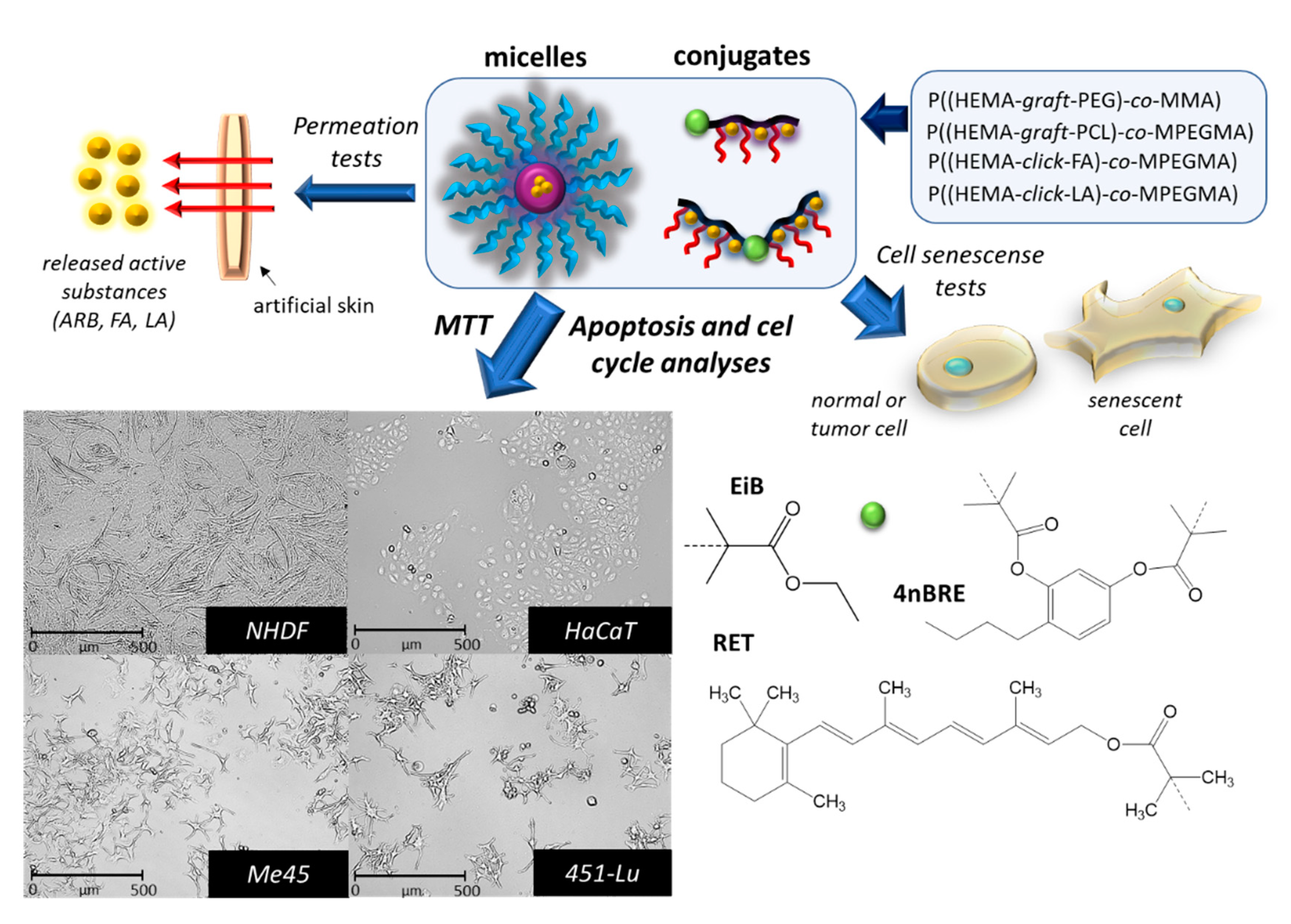

| No. | Copolymer | I | AS | DPn | DPPEG | Fhphil. (wt %) | Ref. |
|---|---|---|---|---|---|---|---|
| I | P((HEMA-graft-PEG)-co-MMA) | RETBr | ARB | 89 | 5 | 32 | [23] |
| II | P((HEMA-graft-PCL9000)-co-MPEGMA) | RETBr | ARB | 173 | 80 | 39 | [24] |
| III | P((HEMA-click-FA)-co-MPEGMA | 4nBREBr2 | FA | 198 | 34 | 21 | [25] |
| IV | P((HEMA-click-LA)-co-MPEGMA | EiBBr | LA | 163 | 45 | 33 |
| No. | DLC (%) | DC (%) | Rmax (%)/T (h) | Dh ± SD (nm) | CMC (mg/mL) |
|---|---|---|---|---|---|
| I | 99 | - | 94/1.5 | 420 ± 77 | 0.0836 |
| II | 77 | - | 99/1.5 | 280 ± 19 | 0.0080 |
| III | - | 32 | 49/4.0 | 204 ± 15 | - |
| IV | - | 38 | 96/0.5 | 82 ± 20 | - |
| No. | DLCapr (%) | DCapr (%) | FCRmax (%) | NR (%) | Tmax (h) | HLB | ||
|---|---|---|---|---|---|---|---|---|
| I | 31 | - | 69 | 31 | 3 | 6 | 6.35 | 38.66 |
| II | 40 | - | 48 | 52 | 1 | 6 | 7.76 | 62.12 |
| III | - | 11 | 73 | 27 | 4 | 6 | 4.19 | 8.89 |
| IV | - | 31 | 59 | 41 | 1 | 6 | 6.49 | 38.98 |
| No. | NHDF (%) | HaCat (%) | ||||
|---|---|---|---|---|---|---|
| conc. [µg/mL] | 0 | 100 | 3 | 0 | 100 | 3 |
| CTR | 63.38 | - | - | 77.69 | - | - |
| I | 72.06 | 74.35 | 98.40 | 95.76 | ||
| II | 68.65 | 71.40 | 94.30 | 92.30 | ||
| III | 99.42 | 100.00 | 90.25 | 94.39 | ||
| IV | 64.54 | 65.23 | 91.77 | 98.04 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odrobińska, J.; Skonieczna, M.; Neugebauer, D. PEG Graft Polymer Carriers of Antioxidants: In Vitro Evaluation for Transdermal Delivery. Pharmaceutics 2020, 12, 1178. https://doi.org/10.3390/pharmaceutics12121178
Odrobińska J, Skonieczna M, Neugebauer D. PEG Graft Polymer Carriers of Antioxidants: In Vitro Evaluation for Transdermal Delivery. Pharmaceutics. 2020; 12(12):1178. https://doi.org/10.3390/pharmaceutics12121178
Chicago/Turabian StyleOdrobińska, Justyna, Magdalena Skonieczna, and Dorota Neugebauer. 2020. "PEG Graft Polymer Carriers of Antioxidants: In Vitro Evaluation for Transdermal Delivery" Pharmaceutics 12, no. 12: 1178. https://doi.org/10.3390/pharmaceutics12121178
APA StyleOdrobińska, J., Skonieczna, M., & Neugebauer, D. (2020). PEG Graft Polymer Carriers of Antioxidants: In Vitro Evaluation for Transdermal Delivery. Pharmaceutics, 12(12), 1178. https://doi.org/10.3390/pharmaceutics12121178







