Pharmacokinetics, Pharmacodynamics and Drug–Drug Interactions of New Anti-Migraine Drugs—Lasmiditan, Gepants, and Calcitonin-Gene-Related Peptide (CGRP) Receptor Monoclonal Antibodies
Abstract
1. Introduction
2. Lasmiditan
2.1. Pharmacokinetics and Pharmacodynamics
2.2. Interactions with Serotonergic Drugs
2.3. Interactions with P-gp and BCRP Substrates
2.4. Interactions with Heart Rate Lowering Drugs
2.5. Interactions with Central Nervous System (CNS) Depressants
2.6. Potential Effect of Lasmiditan on CYP450 Enzymes
2.7. Effect of Other Drugs on Lasmiditan’s Pharmacokinetics
3. CGRP Receptor Antagonists—Ubrogepant and Rimegepant
3.1. Ubrogepant
3.1.1. Drug–Drug Interactions
CYP3A4 Inhibitors
CYP3A4 Inducers
BCRP- and/or P-gp-Only Inhibitors
3.1.2. Moderate Food Interaction—Grapefruit Juice
3.1.3. Other
3.1.4. Disease Interactions
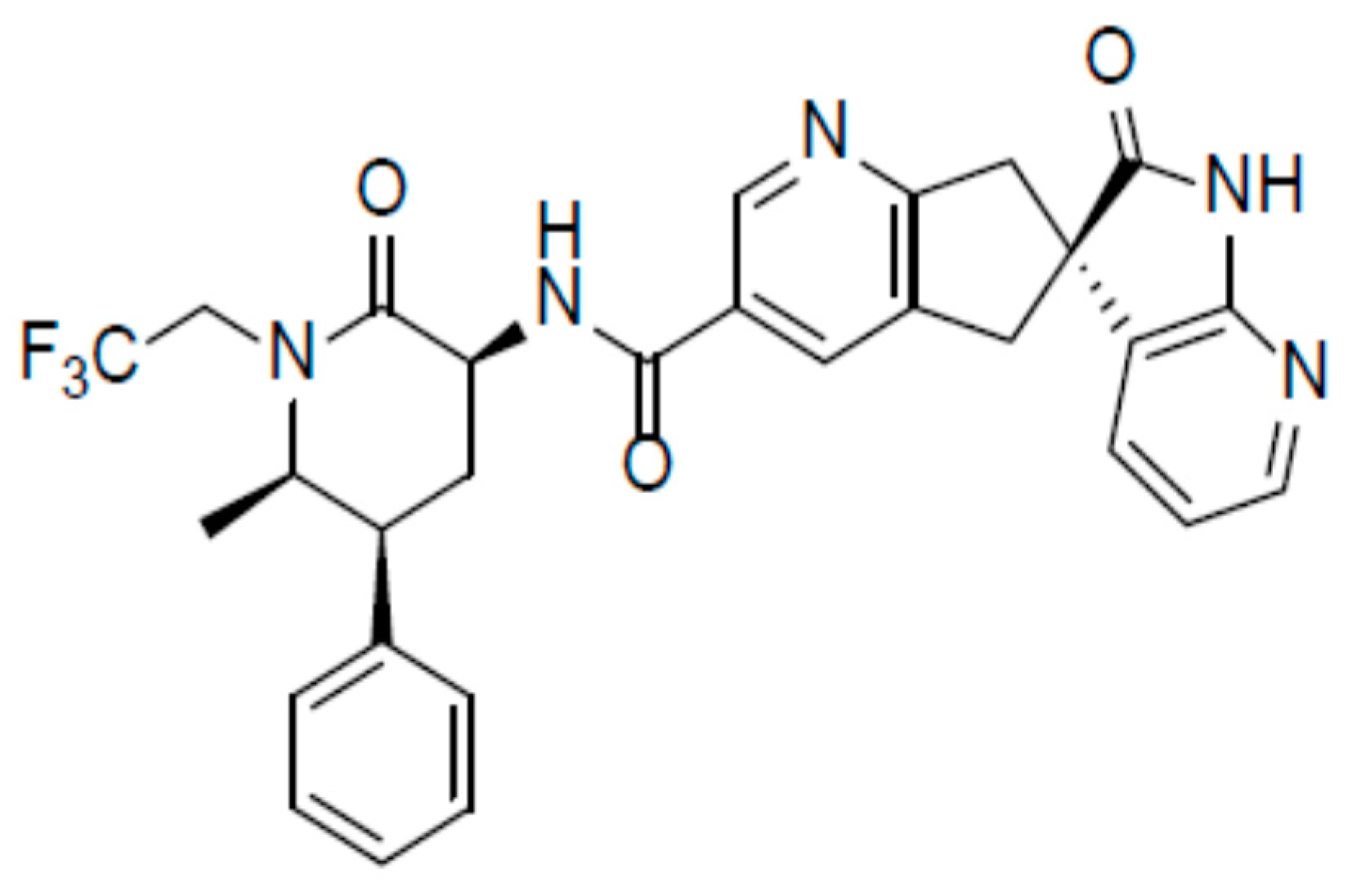
3.2. Rimegepant
3.2.1. Drug–Drug Interactions
CYP3A4 Inhibitors
CYP3A4 Inducers
CYP2C9 Inhibitors
Membrane Transporters
Other—Rimegepant as a Perpetrator
3.2.2. Disease Interactions
3.2.3. Moderate Food Interaction—Grapefruit Juice
4. Anti-CGRP Monoclonal Antibodies (mAbs)
4.1. Pharmacokinetics and Pharmacodynamics of mAbs and the Risk of Interactions
4.2. Erenumab
4.3. Fremanezumab
4.4. Galcanezumab
4.5. Eptinezumab
5. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- Burch, R.C.; Buse, D.C.; Lipton, R.B. Migraine: Epidemiology, Burden, and Comorbidity. Neurol Clin. 2019, 37, 631–649. [Google Scholar] [CrossRef]
- Pomes, L.M.; Guglielmetti, M.; Bertamino, E.; Simmaco, M.; Borro, M. Optimising migraine treatment: From drug-drug interactions to personalized medicine. J. Headache Pain. 2019, 20, 56. [Google Scholar] [CrossRef] [PubMed]
- Szkutnik-Fiedler, D. Current pharmacotherapy of migraine. Headache and COVID-19. Farm Wsp. 2020, 13, 76–83. (In Polish) [Google Scholar]
- Greb, E. Headache May Predict Clinical Evolution of COVID-19. Available online: https://www.medscape.com/viewarticle/932637#vp_2 (accessed on 25 June 2020).
- American Headache Society (AHS) Annual Meeting 2020: Presented. 13 June 2020. Available online: https://americanheadachesociety.org/events/virtual-annual-scientific-meeting/ (accessed on 30 June 2020).
- Bolay, H.; Gül, A.; Baykan, B. COVID-19 is a Real Headache! Headache 2020, 60, 1415–1421. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-Cov-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Kocot-Kępska, M.; Wordliczek, J.; Woroń, J.; Boczar, K.; Przewłocka, B.; Malec-Milewska, M.; Kübler, A.; Dobrogowski, J. Polish Association for the Study of Pain Position Statement on managing pain in the face of COVID-19 due to SARS-CoV-2 infection. Ból 2020, 21, 11–16. (In Polish) [Google Scholar] [CrossRef]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef]
- Tassorelli, C.; Diener, H.C.; Dodick, D.W.; Silberstein, S.D.; Lipton, R.B.; Ashina, M.; Becker, W.J.; Ferrari, M.D.; Goadsby, P.J.; Pozo-Rosich, P.; et al. International Headache Society Clinical Trials Standing Committee. Guidelines of the International Headache Society for controlled trials of preventive treatment of chronic migraine in adults. Cephalalgia 2018, 38, 815–832. [Google Scholar] [CrossRef]
- Diener, H.C.; Tassorelli, C.; Dodick, D.W.; Silberstein, S.D.; Lipton, R.B.; Ashina, M.; Becker, W.J.; Ferrari, M.D.; Goadsby, P.J.; Pozo-Rosich, P.; et al. International Headache Society Clinical Trials Standing Committee. Guidelines of the International Headache Society for controlled trials of acute treatment of migraine attacks in adults, 4th edn. Cephalalgia 2019, 39, 687–710. [Google Scholar] [CrossRef]
- American Headache Society. The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache 2019, 59, 1–18. [Google Scholar] [CrossRef]
- Brola, W.; Sobolewski, P. New strategies for migraine treatment and prevention. Aktualn Neurol. 2019, 19, 132–140. (In Polish) [Google Scholar] [CrossRef]
- Lamb, Y.N. Lasmiditan: First Approval. Drugs 2019, 79, 1989–1996. [Google Scholar] [CrossRef]
- Negro, A.; Martelletti, P. Gepants for the treatment of migraine. Expert Opin Investig Drugs 2019, 28, 555–567. [Google Scholar] [CrossRef]
- Scuteri, D.; Adornetto, A.; Rombolà, L.; Naturale, M.D.; Morrone, L.A.; Bagetta, G.; Paolo Tonin, P.; Corasaniti, M.T. New Trends in Migraine Pharmacology: Targeting Calcitonin Gene–Related Peptide (CGRP) With Monoclonal Antibodies. Front. Pharmacol. 2019, 10, 363. [Google Scholar] [CrossRef]
- Raffaelli, B.; Neeb, L.; Reuter, U. Monoclonal antibodies for the prevention of migraine. Expert Opin. Biol. Ther. 2019, 19, 1307–1317. [Google Scholar] [CrossRef]
- MaassenVanDenBrink, A.; de Vries, T.; Danser, A.H.J. Headache medication and the COVID-19 pandemic. J. Headache Pain 2020, 21, 38. [Google Scholar] [CrossRef]
- Choi, Y.H. Interpretation of Drug Interaction Using Systemic and Local Tissue Exposure Changes. Pharmaceutics 2020, 12, 417. [Google Scholar] [CrossRef]
- Ansari, H.; Ziad, S. Drug-Drug Interactions in Headache Medicine. Headache 2016, 56, 1241–1248. [Google Scholar] [CrossRef]
- Clemow, D.B.; Johnson, K.W.; Hochstetler, H.M.; Ossipov, M.H.; Hake, A.M.; Blumenfeld, A.M. Lasmiditan mechanism of action—Review of a selective 5-HT(1F) agonist. J. Headache Pain 2020, 21, 71. [Google Scholar] [CrossRef]
- Hou, M.; Xing, H.; Li, C.; Wang, X.; Deng, D.; Li, J.; Zhang, P.; Chen, J. Short-term efficacy and safety of lasmiditan, a novel 5-HT1F receptor agonist, for the cute treatment of migraine: A systematic review and meta-analysis. J. Headache Pain 2020, 21, 66. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, Y.; Gao, B.; Wang, Z.; Chen, Z.; Wang, Z. Lasmiditan for Acute Treatment of Migraine in Adults: A Systematic Review and Meta-analysis of Randomized Controlled Trials. CNS Drugs 2020, 34, 1015–1024. [Google Scholar] [CrossRef]
- Doty, E.G.; Krege, J.H.; Pohl, G.; Case, M.; Dowsett, S.A.; Tepper, S.J. Pain Freedom at 2 to 8 Hours With Lasmiditan: A Comparison With Rimegepant and Ubrogepant. Headache 2020. [Google Scholar] [CrossRef]
- Curto, M.; Cipolla, F.; Cisale, G.Y.; Capi, M.; Spuntarelli, V.; Guglielmetti, M.; Martelletti, P.; Lionetto, L. Profiling lasmiditan as a treatment option for migraine. Expert. Opin. Pharmacother. 2020, 21, 147–153. [Google Scholar] [CrossRef]
- Macone, A.E.; Perloff, M.D. Lasmiditan: Its Development and Potential Use. Clin. Pharmacol. Drug Dev. 2020, 9, 292–296. [Google Scholar] [CrossRef]
- Tsai, M.; Case, M.; Ardayfio, P.; Hochstetler, H.; Wilbraham, D. Effects of Lasmiditan on Cardiovascular Parameters and Pharmacokinetics in Healthy Subjects Receiving Oral Doses of Propranolol. Clin. Pharmacol. Drug Dev. 2020, 9, 629–638. [Google Scholar] [CrossRef]
- REYVOW (Lasmiditan) Tablets, Eli Lilly and Company. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211280s000lbl.pdf (accessed on 30 June 2020).
- Vila-Pueyo, M. Targeted 5-HT1F Therapies for Migraine. Neurotherapeutics 2018, 15, 291–303. [Google Scholar] [CrossRef]
- Absorption, Metabolism and Excretion of [14C]-Lasmiditan—Single Oral Dose Administration. Available online: https://clinicaltrials.gov/ct2/show/results/NCT03040362?term=lasmiditan&draw=3&rank=3 (accessed on 14 November 2020).
- Komori, M.; Mimura, H.; Tsai, M.; Ozeki, A.; Takaichi, G.; Wilbraham, D. Safety, Tolerability, and Pharmacokinetics of Lasmiditan in Healthy Japanese and Caucasian Subjects. Rinsho Jpn. J. Clin. Pharmacol. Ther. 2020, 51, 119–127. [Google Scholar] [CrossRef]
- A Study of Lasmiditan in Healthy Elderly Participants. Available online: https://clinicaltrials.gov/ct2/show/NCT03406260 (accessed on 14 November 2020).
- Study of Oral Lasmiditan in Participants with Normal and Impaired Renal Function. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03009162?term=lasmiditan&draw=2&rank=16 (accessed on 14 November 2020).
- Pharmacokinetic Single Dose Study of Oral Lasmiditan in Participants with Normal and Impaired Hepatic Function. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03040479?term=lasmiditan&draw=2&rank=28 (accessed on 14 November 2020).
- Lupi, C.; Benemei, S.; Guerzoni, S.; Pellesi, L.; Negro, A. Pharmacokinetics and pharmacodynamics of new acute treatments for migraine. Expert. Opin. Drug Metab. Toxicol. 2019, 15, 189–198. [Google Scholar] [CrossRef]
- Kuca, B.; Silberstein, S.D.; Wietecha, L.; Berg, P.H.; Dozier, G.; Lipton, R.B. COL MIG-301 Study Group. Lasmiditan is an effective acute treatment for migraine: A phase 3 randomized study. Neurology 2018, 91, e2222–e2232. [Google Scholar] [CrossRef]
- Schim, J.; Ailani, J.; Loo, L.; Krege, J.H.; Baygani, S.; Hundemer, H.; Port, M. Efficacy and safety of lasmiditan in patients on concomitant migraine preventive medications: Findings from samurai and spartan phase 3 trials. Headache 2019, 59, 109–110. [Google Scholar] [CrossRef]
- Krege, J.H.; Rizzoli, P.B.; Liffick, E.; Doty, E.G.; Dowsett, S.A.; Wang, J.; Buchanan, A.S. Safety findings from Phase 3 lasmiditan studies for acute treatment of migraine: Results from SAMURAI and SPARTAN. Cephalalgia 2019, 39, 957–966. [Google Scholar] [CrossRef]
- Loo, L.S.; Ailani, J.; Schim, J.; Baygani, S.; Hundemer, H.P.; Port, M.; Krege, J.H. Efficacy and safety of lasmiditan in patients using concomitant migraine preventive medications: Findings from SAMURAI and SPARTAN, two randomized phase 3 trials. J. Headache Pain 2019, 20, 84. [Google Scholar] [CrossRef]
- Berg, P.H.; Wilbraham, D.; Tsai, M. Effects of lasmiditan when coadministered with sumatriptan: Results of a randomized, double-blind, crossover study in healthy subjects. Headache 2019, 59, 115. [Google Scholar] [CrossRef]
- Wilbraham, D.; Luffer-Atlas, D.; Tsai, M.; Ruff, D. Multiple-ascending dose, safety, tolerability, pharmacokinetic, and drug-drug interaction study with lasmiditan. Headache 2020, 60, 95–96. Available online: https://headachejournal.onlinelibrary.wiley.com/doi/full/10.1111/head.13854 (accessed on 14 November 2020).
- Lasmiditan (LY573144). Clinical Pharmacology and Biopharmaceutics Review(s). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/211280Orig1s000ClinPharmR.pdf (accessed on 14 November 2020).
- Clemow, D.B.; Baygani, S.K.; Hauck, P.M.; Hultman, C.B. Lasmiditan in patients with common migraine comorbidities: A post hoc efficacy and safety analysis of two phase 3 randomized clinical trials. Curr. Med. Res. Opin. 2020, 36, 1791–1806. [Google Scholar] [CrossRef]
- Francescangeli, J.; Karamchandani, K.; Powell, M.; Bonavia, A. The Serotonin Syndrome: From Molecular Mechanisms to Clinical Practice. Int. J. Mol. Sci. 2019, 20, 2288. [Google Scholar] [CrossRef]
- Lasmiditan. Available online: https://go.drugbank.com/drugs/DB11732 (accessed on 5 September 2020).
- Lasmiditan Drug Interactions. Available online: https://www.drugs.com/druginteractions/lasmiditan.html (accessed on 10 September 2020).
- In Vitro Drug Interaction Studies—Cytochrome P450 Enzyme- and Transporter-Mediated Drug Interactions. Guidance for Industry. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER). January 2020, Clinical Pharmacology. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/vitro-drug-interaction-studies-cytochrome-p450-enzyme-and-transporter-mediated-drug-interactions (accessed on 10 September 2020).
- Moreno-Ajona, D.; Pérez-Rodríguez, A.; Goadsby, P.J. Gepants, calcitonin-gene-related peptide receptor antagonists: What could be their role in migraine treatment? Curr. Opin. Neurol. 2020, 33, 309–315. [Google Scholar] [CrossRef]
- Edvinsson, L. Role of CGRP in Migraine. Handb. Exp. Pharmacol. 2019, 255, 121–130. [Google Scholar] [CrossRef]
- Raffaelli, B.; Reuter, U. The Biology of Monoclonal Antibodies: Focus on Calcitonin Gene-Related Peptide for Prophylactic Migraine Therapy. Neurotherapeutics 2018, 15, 324–335. [Google Scholar] [CrossRef]
- Stępień, A. Modern migraine treatment. Ból 2019, 20, 39–44. (In Polish) [Google Scholar] [CrossRef]
- UBRELVY (Ubrogepant) Tablets, Allergan Inc. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211765s000lbl.pdf (accessed on 30 June 2020).
- Scott, L.J. Ubrogepant: First Approval. Drugs 2020, 80, 323–328. [Google Scholar] [CrossRef]
- Efficacy, Safety, and Tolerability Study of Oral Ubrogepant in the Acute Treatment of Migraine (ACHIEVE I). Available online: https://clinicaltrials.gov/ct2/show/NCT02828020 (accessed on 7 July 2020).
- Yang, Y.; Chen, M.; Sun, Y.; Gao, B.; Chen, Z.; Wang, Z. Safety and Efficacy of Ubrogepant for the Acute Treatment of Episodic Migraine: A Meta-Analysis of Randomized Clinical Trials. CNS Drugs 2020, 34, 463–471. [Google Scholar] [CrossRef]
- Dhir, A. Ubrogepant to treat migraine. Drugs Today 2020, 56, 459–467. [Google Scholar] [CrossRef]
- Blumenfeld, A.M.; Edvinsson, L.; Jakate, A.; Banerjee, P. Pharmacology and Pharmacokinetics of Ubrogepant: A Potent, Selective Calcitonin Gene-Related Peptide Receptor Antagonist for the Acute Treatment of Migraine. J. Fam. Pract. 2020, 69 (Suppl. 1), S8–S12. [Google Scholar]
- Ailani, J.; Lipton, R.B.; Hutchinson, S.; Knievel, K.; Lu, K.; Butler, M.; Yu, S.Y.; Finnegan, M.; Severt, L.; Trugman, J.M. Long-Term Safety Evaluation of Ubrogepant for the Acute Treatment of Migraine: Phase 3, Randomized, 52-Week Extension Trial. Headache 2020, 60, 141–152. [Google Scholar] [CrossRef]
- Ailani, J. Clinical Efficacy and Safety of Ubrogepant for the Acute Treatment of Migraine. J. Fam. Pract. 2020, 69 (Suppl. 1), S13–S22. [Google Scholar]
- Jakate, A.; Boinpally, R.; Butler, M.; Lu, K.; McGeeney, D.; Periclou, A. Evaluation of the Pharmacokinetic Interaction of Ubrogepant Coadministered With Sumatriptan and of the Safety of Ubrogepant With Triptans. Headache 2020, 60, 1340–1350. [Google Scholar] [CrossRef]
- Lipton, R.B.; Dodick, D.W.; Ailani, J.; Lu, K.; Finnegan, M.; Szegedi, A.; Trugman, J.M. Effect of Ubrogepant vs Placebo on Pain and the Most Bothersome Associated Symptom in the Acute Treatment of Migraine: The ACHIEVE II Randomized Clinical Trial. JAMA 2019, 322, 1887–1898. [Google Scholar] [CrossRef]
- Jakate, A.; Boinpally, R.; Butler, M.; Lu, K.; Womack, K.; McGeeney, D.; Periclou, A. Evaluation of the pharmacokinetic interaction and safety of ubrogepant coadministered with acetaminophen or nonsteroidal anti-inflammatory drugs: A randomized trial. Cephalalgia Reports. 2020, 3, 2515816320921186. [Google Scholar] [CrossRef]
- Ubrogepant. Clinical Pharmacology Review(s). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/211765Orig1s000ClinPharmR.pdf (accessed on 14 November 2020).
- Ankrom, W.; Bondiskey, P.; Li, C.C.; Palcza, J.; Liu, W.; Dockendorf, M.F.; Matthews, C.; Panebianco, D.; Reynders, T.; Wagner, J.A.; et al. Ubrogepant Is Not Associated With Clinically Meaningful Elevations of Alanine Aminotransferase in Healthy Adult Males. Clin. Transl. Sci. 2020, 13, 462–472. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Tepper, S.J.; Watkins, P.B.; Ayele, G.; Miceli, R.; Butler, M.; Severt, L.; Finnegan, M.; Szegedi, A.; Trugman, J.M.; et al. Safety and tolerability of ubrogepant following intermittent, high-frequency dosing: Randomized, placebo-controlled trial in healthy adults. Cephalalgia 2019, 39, 1753–1761. [Google Scholar] [CrossRef]
- Ubrogepant. Available online: https://go.drugbank.com/drugs/DB15328 (accessed on 5 September 2020).
- Ubrogepant Drug Interactions. Available online: https://www.drugs.com/druginteractions/ubrogepant.html (accessed on 10 September 2020).
- Jakate, A.; Boinpally, R.; Butler, M.; Lu, K.; McGeeney, D.; Periclou, A. Single Therapeutic and Supratherapeutic Doses of Ubrogepant Do Not Affect Cardiac Repolarization in Healthy Adults: Results From a Randomized Trial. Clin. Pharmacol. Ther. 2020, 107, 1014–1022. [Google Scholar] [CrossRef]
- Scott, L.J. Rimegepant: First Approval. Drugs 2020, 80, 741–746. [Google Scholar] [CrossRef]
- NURTEC ODT (Rimegepant) Orally Disintegrating Tablets, Biohaven Pharmaceuticals Inc. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212728s000lbl.pdf (accessed on 10 September 2020).
- Lipton, R.B.; Croop, R.; Stock, E.G.; Stock, D.A.; Morris, B.A.; Frost, M.; Dubowchik, G.M.; Conway, C.M.; Coric, V.; Goadsby, P.J. Rimegepant, an Oral Calcitonin Gene-Related Peptide Receptor Antagonist, for Migraine. N. Engl. J. Med. 2019, 381, 142–149. [Google Scholar] [CrossRef]
- Edvinsson, L. Rimegepant oral disintegrating tablet for migraine. Lancet 2019, 394, 711–712. [Google Scholar] [CrossRef]
- DeFalco, A.P.; Lazim, R.; Cope, N.E. Rimegepant Orally Disintegrating Tablet for Acute Migraine Treatment: A Review. Ann. Pharmacother. 2020, 1060028020954800. [Google Scholar] [CrossRef]
- Rimegepant Drug Interactions. Available online: https://www.drugs.com/druginteractions/rimegepant.html (accessed on 10 September 2020).
- Rimegepant. Available online: https://go.drugbank.com/drugs/DB12457 (accessed on 10 September 2020).
- Gao, B.; Yang, Y.; Wang, Z.; Sun, Y.; Chen, Z.; Zhu, Y.; Wang, Z. Efficacy and Safety of Rimegepant for the Acute Treatment of Migraine: Evidence From Randomized Controlled Trials. Front. Pharmacol. 2020, 10, 1577. [Google Scholar] [CrossRef]
- Croop, R.; Goadsby, P.J.; Stock, D.A.; Conway, C.M.; Forshaw, M.; Stock, E.G.; Coric, V.; Lipton, R.B. Efficacy, safety, and tolerability of rimegepant orally disintegrating tablet for the acute treatment of migraine: A randomised, phase 3, double-blind, placebo-controlled trial. Lancet 2019, 394, 737–745. [Google Scholar] [CrossRef]
- Hutchinson, S.; Schim, J.; Lipton, R.; Thiry, A.; Morris, B.; Coric, V.; Croop, R. Safety of rimegepant 75 mg in adults with migraine: No effects of age, sex, or race in 3 phase 3 trials. Cephalalgia 2019, 39, 196. [Google Scholar] [CrossRef]
- Berman, G.; Croop, R.; Kudrow, D.; Halverson, P.; Lovegren, M.; Thiry, A.C.; Conway, C.M.; Coric, V.; Lipton, R.B. Safety of Rimegepant, an Oral CGRP Receptor Antagonist, Plus CGRP Monoclonal Antibodies for Migraine. Headache 2020, 60, 1734–1742. [Google Scholar] [CrossRef]
- Rimegepant. Clinical Microbiology/Virology Review(s). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/212728Orig1s000ClinPharmR.pdf (accessed on 14 November 2020).
- Hutchinson, S.; Schim, J.; Lipton, R.B.; Croop, R.; Jensen, C.M.; Thiry, A.C.; Stock, E.G.; Conway, C.M.; Lovegren, M.; Coric, V.; et al. Oral rimegepant 75 mg is safe and well tolerated in adults with migraine and cardiovascular risk factors: Results of a multicenter, long-term, open-label safety study. Headache 2020, 60, 115. Available online: https://headachejournal.onlinelibrary.wiley.com/doi/full/10.1111/head.13854 (accessed on 14 November 2020).
- Croop, R.; Stringfellow, J.; Hanna, M.; Jensen, C.M.; Ivans, A.; Coric, V. Oral rimegepant produces no significant effect on blood pressure when administered concomitantly with sc sumatriptan. Headache 2020, 60, 116. Available online: https://headachejournal.onlinelibrary.wiley.com/doi/full/10.1111/head.13854 (accessed on 14 November 2020).
- Summary of Product Characteristics for AIMOVIG (erenumab) 70 mg and 140 mg, Solution for Injection in Pre-Filled Syringe, Solution for Injection in Pre-Filled Pen. Available online: https://www.ema.europa.eu/en/documents/product-information/aimovig-epar-product-information_en.pdf (accessed on 7 July 2020).
- Summary of Product Characteristics for AJOVY (fremanezumab) 225 mg Solution for Injection in Pre-Filled Syringe and Solution for Injection in Pre-Filled Pen. Available online: https://www.ema.europa.eu/en/documents/product-information/ajovy-epar-product-information_en.pdf (accessed on 10 September 2020).
- Summary of Product Characteristics for EMGALITY (galcanezumab) 120 mg Solution for Injection in Pre-Filled Pen. Available online: https://www.ema.europa.eu/en/documents/product-information/emgality-epar-product-information_en.pdf (accessed on 10 September 2020).
- Lundbeck Seattle BioPharmaceuticals, Inc. VYEPTI™ (Eptinezumab) Injections, for Intravenous Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761119s000lbl.pdf (accessed on 8 August 2020).
- González-Hernández, A.; Marichal-Cancino, B.A.; García-Boll, E.; Villalón, C.M. The locus of action of CGRPergic monoclonal antibodies against migraine: Peripheral over central mechanisms. CNS Neurol. Disord. Drug Targets 2020. [Google Scholar] [CrossRef]
- A Study to Evaluate the Efficacy and Safety of Erenumab (AMG 334) in Chronic Migraine Prevention. Available online: https://clinicaltrials.gov/ct2/show/NCT02066415 (accessed on 7 July 2020).
- Zhu, C.; Guan, J.; Xiao, H.; Luo, W.; Tong, R. Erenumab safety and efficacy in migraine: A systematic review and meta-analysis of randomized clinical trials. Medicine 2019, 98, e18483. [Google Scholar] [CrossRef]
- Andreou, A.P.; Fuccaro, M.; Lambru, G. The role of erenumab in the treatment of migraine. Ther. Adv. Neurol. Disord. 2020, 13, 1756286420927119. [Google Scholar] [CrossRef]
- Efficacy and Safety of Subcutaneous Administration of Fremanezumab (TEV-48125) for the Preventive Treatment of Migraine (HALO). Available online: https://clinicaltrials.gov/ct2/show/NCT02638103 (accessed on 7 July 2020).
- Urits, I.; Clark, G.; An, D.; Wesp, B.; Zhou, R.; Amgalan, A.; Berger, A.A.; Kassem, H.; Ngo, A.L.; Kaye, A.D.; et al. An Evidence-Based Review of Fremanezumab for the Treatment of Migraine. Pain Ther. 2020, 9, 195–215. [Google Scholar] [CrossRef]
- Gao, B.; Sun, N.; Yang, Y.; Sun, Y.; Chen, M.; Chen, Z.; Wang, Z. Safety and Efficacy of Fremanezumab for the Prevention of Migraine: A Meta-Analysis From Randomized Controlled Trials. Front. Neurol. 2020, 11, 435. [Google Scholar] [CrossRef]
- Brandes, J.L.; Kudrow, D.; Yeung, P.P.; Sakai, F.; Aycardi, E.; Blankenbiller, T.; Grozinski-Wolff, M.; Yang, R.; Ma, Y. Effects of fremanezumab on the use of acute headache medication and associated symptoms of migraine in patients with episodic migraine. Cephalalgia 2020, 40, 470–477. [Google Scholar] [CrossRef]
- Ferrari, M.D.; Diener, H.C.; Ning, X.; Galic, M.; Cohen, J.M.; Yang, R.; Mueller, M.; Ahn, A.H.; Schwartz, Y.C.; Grozinski-Wolff, M.; et al. Fremanezumab versus placebo for migraine prevention in patients with documented failure to up to four migraine preventive medication classes (FOCUS): A randomised, double-blind, placebo-controlled, phase 3b trial. Lancet 2019, 394, 1030–1040. [Google Scholar] [CrossRef]
- Fiedler-Kelly, J.; Passarell, J.; Ludwig, E.; Levi, M.; Cohen-Barak, O. Effect of Fremanezumab Monthly and Quarterly Doses on Efficacy Responses. Headache 2020, 60, 1376–1391. [Google Scholar] [CrossRef]
- Lionetto, L.; Cipolla, F.; Guglielmetti, M.; Martelletti, P. Fremanezumab for the prevention of chronic and episodic migraine. Drugs Today 2019, 55, 265–276. [Google Scholar] [CrossRef]
- Gklinos, P.; Mitsikostas, D.D. Galcanezumab in Migraine Prevention: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Ther. Adv. Neurol. Disord. 2020, 13, 1756286420918088. [Google Scholar] [CrossRef]
- A Study of Galcanezumab in Participants with Episodic Cluster Headache. Available online: https://clinicaltrials.gov/ct2/show/NCT02397473 (accessed on 7 July 2020).
- Ren, Z.; Zhang, H.; Wang, R.; Yuan, Q.; Pan, L.; Chen, C. The treatment efficacy of galcanezumab for migraine: A meta-analysis of randomized controlled trials. Clin Neurol. Neurosurg. 2019, 186, 105428. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, X.; Li, Q. Efficacy and safety of eptinezumab for preventive treatment of migraine: A systematic review and meta-analysis. J. Neurol. 2020. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.; Gao, B.; Xuan, H.; Zhu, Y.; Chen, Z.; Wang, Z. Different doses of galcanezumab versus placebo in patients with migraine and cluster headache: A meta-analysis of randomized controlled trials. J. Headache Pain 2020, 21, 14. [Google Scholar] [CrossRef]
- Ruff, D.D.; Ford, J.H.; Tockhorn-Heidenreich, A.; Sexson, M.; Govindan, S.; Pearlman, E.M.; Wang, S.J.; Khan, A.; Aurora, S.K. Efficacy of galcanezumab in patients with chronic migraine and a history of preventive treatment failure. Cephalalgia 2019, 39, 931–944. [Google Scholar] [CrossRef]
- Martin, V.; Samaan, K.H.; Aurora, S.; Pearlman, E.M.; Zhou, C.; Li, X.; Pallay, R. Efficacy and Safety of Galcanezumab for the Preventive Treatment of Migraine: A Narrative Review. Adv. Ther. 2020, 37, 2034–2049. [Google Scholar] [CrossRef]
- Scott, L.J. Galcanezumab: A Review in the Prevention of Migraine and Treatment of Episodic Cluster Headache. Drugs 2020, 80, 893–904. [Google Scholar] [CrossRef]
- Oakes, T.M.; Kovacs, R.; Rosen, N.; Doty, E.; Kemmer, P.; Aurora, S.K.; Camporeale, A. Evaluation of Cardiovascular Outcomes in Adult Patients With Episodic or Chronic Migraine Treated With Galcanezumab: Data From Three Phase 3, Randomized, Double-Blind, Placebo-Controlled EVOLVE-1, EVOLVE-2, and REGAIN Studies. Headache 2020, 60, 110–123. [Google Scholar] [CrossRef]
- Mulleners, W.M.; Kim, B.K.; Láinez, M.J.A.; Lanteri-Minet, M.; Pozo-Rosich, P.; Wang, S.; Tockhorn-Heidenreich, A.; Aurora, S.K.; Nichols, R.M.; Yunes-Medina, L.; et al. Safety and efficacy of galcanezumab in patients for whom previous migraine preventive medication from two to four categories had failed (CONQUER): A multicentre, randomised, double-blind, placebo-controlled, phase 3b trial. Lancet Neurol. 2020, 19, 814–825. [Google Scholar] [CrossRef]
- Evaluate Efficacy & Safety of Eptinezumab Administered Intravenously in Subjects Experiencing Acute Attack of Migraine (RELIEF). Available online: https://clinicaltrials.gov/ct2/show/NCT04152083 (accessed on 7 July 2020).
- Scuteri, D.; Corasaniti, M.T.; Tonin, P.; Bagetta, G. Eptinezumab for the treatment of migraine. Drugs Today 2019, 55, 695–703. [Google Scholar] [CrossRef]
- Lipton, R.B.; Goadsby, P.J.; Smith, J.; Schaeffler, B.A.; Biondi, D.M.; Hirman, J.; Pederson, S.; Allan, B.; Cady, R. Efficacy and safety of eptinezumab in patients with chronic migraine: PROMISE-2. Neurology 2020, 94, e1365–e1377. [Google Scholar] [CrossRef]
- Okoniewska, K.; Marczak, M.; Grabowski, T.; Okoniewski, J.; Ozimek, M. Differences at particular stages of pharmacokinetics between classic small molecule drugs and biological drugs, on the example of therapeutic monoclonal antibodies. In Innowacje w Medycynie i Farmakoterapii; PFO Vetos-Farma Sp. z o.o: Bielawa, Poland, March 2016; pp. 104–120. Available online: https://www.researchgate.net/publication/311953339 (accessed on 7 July 2020). (In Polish)
- Fiedler-Kelly, J.B.; Cohen-Barak, O.; Morris, D.N.; Ludwig, E.; Rasamoelisolo, M.; Shen, H.; Levi, M. Population pharmacokinetic modelling and simulation of fremanezumab in healthy subjects and patients with migraine. Br. J. Clin. Pharmacol. 2019, 85, 2721–2733. [Google Scholar] [CrossRef]
- Kielbasa, W.; Quinlan, T. Population Pharmacokinetics of Galcanezumab, an Anti-CGRP Antibody, Following Subcutaneous Dosing to Healthy Individuals and Patients With Migraine. J. Clin. Pharmacol. 2020, 60, 229–239. [Google Scholar] [CrossRef]
- Lamb, Y.N. Galcanezumab: First Global Approval. Drugs 2018, 78, 1769–1775. [Google Scholar] [CrossRef]
- Stauffer, V.L.; Turner, I.; Kemmer, P.; Kielbasa, W.; Day, K.; Port, M.; Quinlan, T.; Camporeale, A. Effect of age on pharmacokinetics, efficacy, and safety of galcanezumab treatment in adult patients with migraine: Results from six phase 2 and phase 3 randomized clinical trials. J. Headache Pain 2020, 21, 79. [Google Scholar] [CrossRef]
- Dhillon, S. Eptinezumab: First Approval. Drugs 2020, 80, 733–739. [Google Scholar] [CrossRef]
- Baker, B.; Schaeffler, B.; Beliveau, M.; Rubets, I.; Pederson, S.; Trinh, M.; Smith, J.; Latham, J. Population pharmacokinetic and exposure-response analysis of eptinezumab in the treatment of episodic and chronic migraine. Pharmacol. Res. Perspect. 2020, 8, e00567. [Google Scholar] [CrossRef]
- Castelli, M.S.; McGonigle, P.; Hornby, P.J. The pharmacology and therapeutic applications of monoclonal antibodies. Pharmacol. Res. Perspect. 2019, 7, e00535. [Google Scholar] [CrossRef]
- Vaisman-Mentesh, A.; Gutierrez-Gonzalez, M.; DeKosky, B.J.; Wine, Y. The Molecular Mechanisms That Underlie the Immune Biology of Anti-drug Antibody Formation Following Treatment With Monoclonal Antibodies. Front. Immunol. 2020, 11, 1951. [Google Scholar] [CrossRef]
- Martinez, J.M.; Hindiyeh, N.; Anglin, G.; Kalidas, K.; Hodsdon, M.E.; Kielbasa, W.; Moser, B.A.; Pearlman, E.M.; Garces, S. Assessment of immunogenicity from galcanezumab phase 3 trials in patients with episodic or chronic migraine. Cephalalgia 2020, 40, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Tovey, M.G.; Lallemand, C. Immunogenicity and other problems associated with the use of biopharmaceuticals. Ther. Adv. Drug Saf. 2011, 2, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Griswold, K.E.; Bailey-Kellogg, C. Design and engineering of deimmunized biotherapeutics. Curr. Opin. Struct. Biol. 2016, 39, 79–88. [Google Scholar] [CrossRef]
- Cohen-Barak, O.; Weiss, S.; Rasamoelisolo, M.; Faulhaber, N.; Yeung, P.P.; Loupe, P.S.; Yoon, E.; Gandhi, M.D.; Spiegelstein, O.; Aycardi, E. A phase 1 study to assess the pharmacokinetics, safety, and tolerability of fremanezumab doses (225 mg, 675 mg and 900 mg) in Japanese and Caucasian healthy subjects. Cephalalgia 2018, 38, 1960–1971. [Google Scholar] [CrossRef]
- Skljarevski, V.; Matharu, M.; Millen, B.A.; Ossipov, M.H.; Kim, B.K.; Yang, J.Y. Efficacy and safety of galcanezumab for the prevention of episodic migraine: Results of the EVOLVE-2 Phase 3 randomized controlled clinical trial. Cephalalgia 2018, 38, 1442–1454. [Google Scholar] [CrossRef]
- Dodick, D.W.; Ashina, M.; Brandes, J.L.; Kudrow, D.; Lanteri-Minet, M.; Osipova, V.; Palmer, K.; Picard, H.; Mikol, D.D.; Lenz, R.A. ARISE: A Phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia 2018, 38, 1026–1037. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Reuter, U.; Hallström, Y.; Broessner, G.; Bonner, J.H.; Zhang, F.; Sapra, S.; Picard, H.; Mikol, D.D.; Lenz, R.A. A Controlled Trial of Erenumab for Episodic Migraine. N. Engl. J. Med. 2017, 377, 2123–2132. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szkutnik-Fiedler, D. Pharmacokinetics, Pharmacodynamics and Drug–Drug Interactions of New Anti-Migraine Drugs—Lasmiditan, Gepants, and Calcitonin-Gene-Related Peptide (CGRP) Receptor Monoclonal Antibodies. Pharmaceutics 2020, 12, 1180. https://doi.org/10.3390/pharmaceutics12121180
Szkutnik-Fiedler D. Pharmacokinetics, Pharmacodynamics and Drug–Drug Interactions of New Anti-Migraine Drugs—Lasmiditan, Gepants, and Calcitonin-Gene-Related Peptide (CGRP) Receptor Monoclonal Antibodies. Pharmaceutics. 2020; 12(12):1180. https://doi.org/10.3390/pharmaceutics12121180
Chicago/Turabian StyleSzkutnik-Fiedler, Danuta. 2020. "Pharmacokinetics, Pharmacodynamics and Drug–Drug Interactions of New Anti-Migraine Drugs—Lasmiditan, Gepants, and Calcitonin-Gene-Related Peptide (CGRP) Receptor Monoclonal Antibodies" Pharmaceutics 12, no. 12: 1180. https://doi.org/10.3390/pharmaceutics12121180
APA StyleSzkutnik-Fiedler, D. (2020). Pharmacokinetics, Pharmacodynamics and Drug–Drug Interactions of New Anti-Migraine Drugs—Lasmiditan, Gepants, and Calcitonin-Gene-Related Peptide (CGRP) Receptor Monoclonal Antibodies. Pharmaceutics, 12(12), 1180. https://doi.org/10.3390/pharmaceutics12121180




