Nanoparticle-Mediated Dual Targeting: An Approach for Enhanced Baicalin Delivery to the Liver
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of BA-loaded-LA-Modified-CL-NPs
2.3. Optimization of BA-Loaded-LA-CL-NPs
2.4. Selection of the Optimized Formulation
2.5. Characterization of BA-loaded-LA-Modified-CL-NPs (F1→6)
2.5.1. Particle size and Polydispersity
2.5.2. Determination of Zeta Potential
2.5.3. Encapsulation Efficiency (EE%)
2.6. Physico-Chemical Characterization of Optimized BA-loaded-LA-Modified-CL-NPs
2.6.1. Attenuated Total Reflectance-Infrared (ATR-IR) Spectroscopy
2.6.2. In Vitro Release Studies
2.6.3. Transmission Electron Microscopy
2.7. Short-Term Stability Study
2.8. In Vivo Studies
2.8.1. Preparation of 99mTc-BA complex
2.8.2. Animals
2.8.3. Study Design and Drug Administration
2.8.4. Pharmacokinetic and Biodistribution Studies
2.9. Statistical Analysis
3. Results and Discussion
3.1. Optimization of BA-loaded-LA-Modified-CL-NPs
3.2. Attenuated Total Reflectance-Infrared ATR-IR Spectroscopy
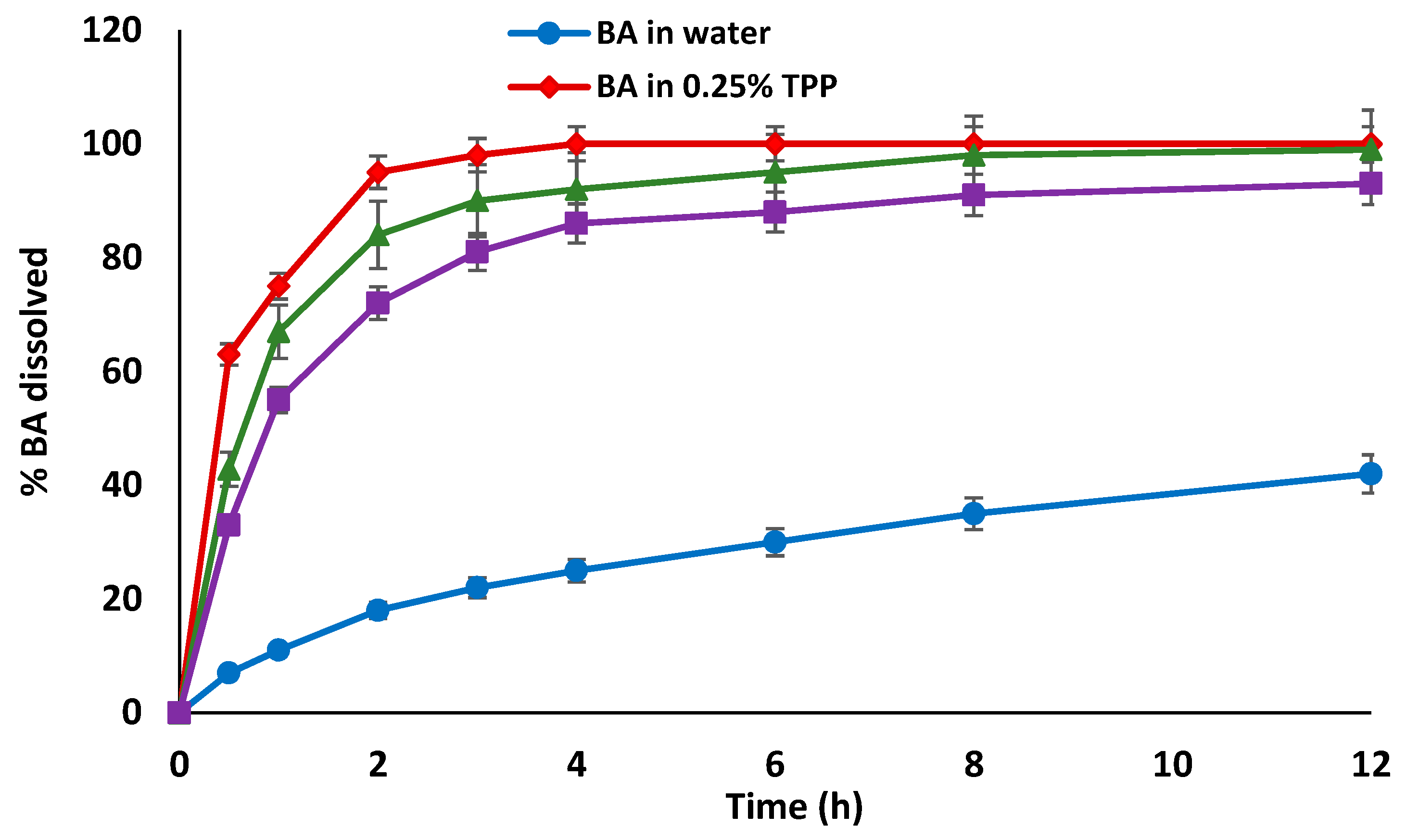
3.3. In Vitro Release Studies
3.4. Morphological Characterization
3.5. Short-term stability studies
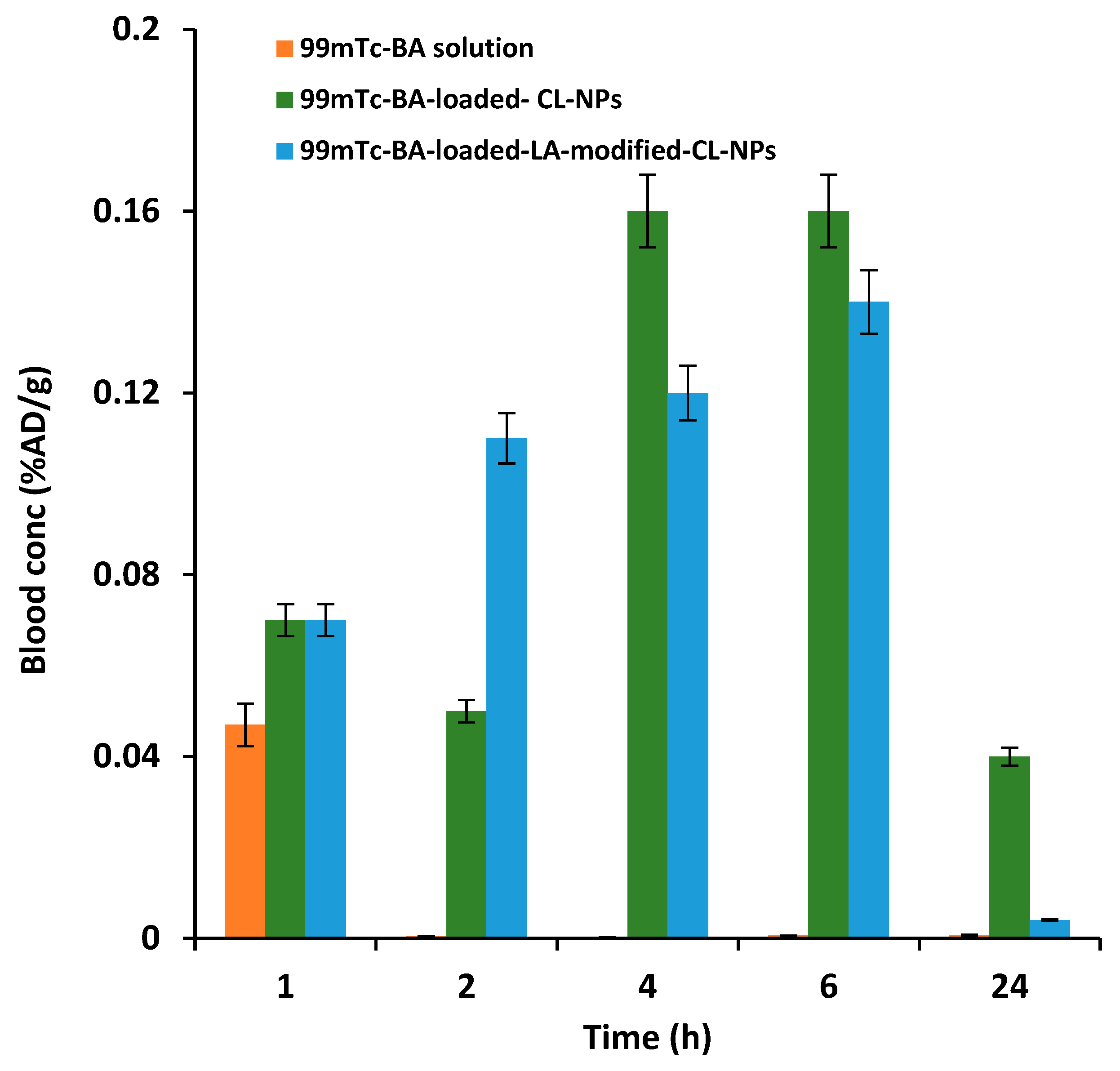
3.6. In vivo Pharmacokinetic and Biodistribution Studies
3.6.1. Radiolabelling of BA
3.6.2. Liver-Targeting Assessment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, C.; Lin, G.; Zuo, Z. Pharmacological effects and pharmacokinetics properties of Radix Scutellariae and its bioactive flavones. Biopharm. Drug Dispos. 2011, 32, 427–445. [Google Scholar] [CrossRef]
- Wu, S.; Sun, A.; Liu, R. Separation and purification of baicalin and wogonoside from the Chinese medicinal plant Scutellaria baicalensis Georgi by high-speed counter-current chromatography. J. Chromatogr. A 2005, 1066, 243–247. [Google Scholar] [CrossRef]
- Luo, J.; Dong, B.; Wang, K.; Cai, S.; Liu, T.; Cheng, X.; Lei, D.; Chen, Y.; Li, Y.; Kong, J.; et al. Baicalin inhibits biofilm formation, attenuates the quorum sensing-controlled virulence and enhances Pseudomonas aeruginosa clearance in a mouse peritoneal implant infection model. PLoS ONE 2017, 12, e0176883. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Shi, G.; Shao, J.; Wu, D.; Yan, Y.; Zhang, M.; Cui, Y.; Wang, C. In vitro antifungal activity of baicalin against Candida albicans biofilms via apoptotic induction. Microb. Pathog. 2015, 87, 21–29. [Google Scholar] [CrossRef]
- Huang, H.; Zhou, W.; Zhu, H.; Zhou, P.; Shi, X. Baicalin benefits the anti-HBV therapy via inhibiting HBV viral RNAs. Toxicol. Appl. Pharmacol. 2017, 323, 36–43. [Google Scholar] [CrossRef]
- Lee, W.; Ku, S.K.; Bae, J.S. Anti-inflammatory effects of Baicalin, Baicalein, and Wogonin in vitro and in vivo. Inflammation 2015, 38, 110–125. [Google Scholar] [CrossRef]
- Tsai, C.C.; Lin, M.T.; Wang, J.J.; Liao, J.F.; Huang, W.T. The antipyretic effects of baicalin in lipopolysaccharide-evoked fever in rabbits. Neuropharmacology 2006, 5, 709–717. [Google Scholar] [CrossRef]
- Deng, Y.F.; Aluko, R.E.; Jin, Q.; Zhang, Y.; Yuan, L.J. Inhibitory activities of baicalin against renin and angiotensin-converting enzyme. Pharm. Biol. 2012, 50, 401–406. [Google Scholar] [CrossRef]
- De Carvalho, R.S.; Duarte, F.S.; de Lima, T.C. Involvement of GABAergic non-benzodiazepine sites in the anxiolytic-like and sedative effects of the flavonoid baicalein in mice. Behav. Brain Res. 2011, 221, 75–82. [Google Scholar] [CrossRef]
- Lee, W.; Ku, S.K.; Bae, J.S. Antiplatelet, anticoagulant, and profibrinolytic activities of baicalin. Arch. Pharm. Res. 2015, 38, 893–903. [Google Scholar] [CrossRef]
- Liau, P.R.; Wu, M.S.; Lee, C.K. Inhibitory Effects of Scutellaria baicalensis Root Extract on Linoleic Acid Hydroperoxide-induced Lung Mitochondrial Lipid Peroxidation and Antioxidant Activities. Molecules 2019, 24, 2143. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.C.; Day, Y.J.; Lee, H.C.; Liou, J.T.; Chou, A.H.; Liu, F.C. Baicalin Attenuates IL-17-Mediated Acetaminophen-Induced Liver Injury in a Mouse Model. PLoS ONE 2016, 11, e0166856. [Google Scholar] [CrossRef] [Green Version]
- Gong, W.Y.; Zhao, Z.X.; Liu, B.J.; Lu, L.W.; Dong, J.C. Exploring the chemopreventive properties and perspectives of baicalin and its aglycone baicalein in solid tumors. Eur. J. Med. Chem. 2017, 126, 844–852. [Google Scholar] [CrossRef]
- Zuo, W.; Wu, H.; Zhang, K.; Lv, P.; Xu, F.; Jiang, W.; Zheng, L.; Zhao, J. Baicalin promotes the viability of Schwann cells in vitro by regulating neurotrophic factors. Exp. Ther. Med. 2017, 14, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Lv, H.; Jiang, K.; Gao, Y. Enhanced bioavailability after oral and pulmonary administration of baicalein nanocrystal. Inter. J. pharm. 2011, 420, 180–188. [Google Scholar] [CrossRef]
- Chen, H.; Gao, Y.; Wu, J.; Chen, Y.; Chen, B.; Hu, J.; Zhou, J. Exploring therapeutic potentials of baicalin and its aglycone baicalein for hematological malignancies. Cancer Lett. 2014, 354, 5–11. [Google Scholar] [CrossRef]
- Xing, J.; Chen, X.; Sun, Y.; Luan, Y.; Zhong, D. Interaction of baicalin and baicalein with antibiotics in the gastrointestinal tract. J. Pharm. Pharmacol. 2005, 57, 743–750. [Google Scholar] [CrossRef]
- Xing, J.; Chen, X.; Zhong, D. Absorption and enterohepatic circulation of baicalin in rats. Life Sci. 2005, 78, 140–146. [Google Scholar] [CrossRef]
- Li, M.; Shi, A.; Pang, H.; Xue, W.; Li, Y.; Cao, G.; Yan, B.; Dong, F.; Li, K.; Xiao, W. Safety, tolerability, and pharmacokinetics of a single ascending dose of baicalein chewable tablets in healthy subjects. J. Ethnopharmacol. 2014, 156, 210–215. [Google Scholar] [CrossRef]
- He, X.; Pei, L.; Tong, H.H.; Zheng, Y. Comparison of spray freeze drying and the solvent evaporation method for preparing solid dispersions of baicalein with Pluronic F68 to improve dissolution and oral bioavailability. AAPS Pharmscitech. 2011, 12, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.; Xia, X.; Yang, Y.; Ye, J.; Dong, W.; Ma, P.; Jin, Y.; Liu, Y. Co-encapsulation of paclitaxel and baicalein in nanoemulsions to overcome multidrug resistance via oxidative stress augmentation and P-glycoprotein inhibition. Inter. J. Pharm. 2016, 513, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xi, M.; Duan, X.; Wang, Y.; Kong, F. Delivery of baicalein and paclitaxel using self-assembled nanoparticles: Synergistic antitumor effect in vitro and in vivo. Inter. J. Nanomed. 2015, 10, 3737. [Google Scholar]
- Wissing, S.; Kayser, O.; Müller, R. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef]
- Hao, J.; Wang, F.; Wang, X.; Zhang, D.; Bi, Y.; Gao, Y.; Zhao, X.; Zhang, Q. Development and optimization of baicalin-loaded solid lipid nanoparticles prepared by coacervation method using central composite design. Eur. J. Pharm. Sci. 2012, 47, 497–505. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, X.; Wu, H.; Li, J.; Shu, L.; Liu, R.; Li, L.; Li, N. Preparation and evaluation of solid lipid nanoparticles of baicalin for ocular drug delivery system in vitro and in vivo. Drug Dev. Industrial Pharm. 2011, 37, 475–481. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, H.; Shu, L.; Zhang, Y.; Okeke, C.; Zhang, L.; Li, J.; Li, N. Preparation and evaluation of Baicalin-loaded cationic solid lipid nanoparticles conjugated with OX26 for improved delivery across the BBB. Drug Dev. Industrial Pharm. 2015, 41, 353–361. [Google Scholar] [CrossRef]
- Haider, M.; Hassan, M.A.; Ahmed, I.S.; Shamma, R. Thermogelling Platform for Baicalin Delivery for Versatile Biomedical Applications. Mol. Pharm. 2018, 15, 3478–3488. [Google Scholar] [CrossRef]
- Azab, A.K.; Doviner, V.; Orkin, B.; Kleinstern, J.; Srebnik, M.; Nissan, A.; Rubinstein, A. Biocompatibility evaluation of crosslinked chitosan hydrogels after subcutaneous and intraperitoneal implantation in the rat. J. Biomed. Mater. Res. A 2007, 83, 414–422. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.; Xue, Z.Y.; Cheng, H.W.; Huang, F.W.; Zhuo, R.X.; Zhang, X.Z. Fabrication of lactobionic-loaded chitosan microcapsules as potential drug carriers targeting the liver. Acta. Biomater. 2011, 7, 1665–1673. [Google Scholar] [CrossRef]
- Suh, J.K.; Matthew, H.W. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: A review. Biomaterials 2000, 21, 2589–2598. [Google Scholar]
- Kawase, M.; Michibayashi, N.; Nakashima, Y.; Kurikawa, N.; Yagi, K.; Mizoguchi, T. Application of glutaraldehyde-crosslinked chitosan as a scaffold for hepatocyte attachment. Biol. Pharm. Bull. 1997, 20, 708–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Z.; Levy-Nissenbaum, E.; Alexis, F.; Luptak, A.; Teply, B.A.; Chan, J.M.; Shi, J.; Digga, E.; Cheng, J.; Langer, R.; et al. Engineering of targeted nanoparticles for cancer therapy using internalizing aptamers isolated by cell-uptake selection. ACS Nano 2012, 6, 696–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lammers, T.; Kiessling, F.; Hennink, W.E.; Storm, G. Drug targeting to tumors: Principles, pitfalls and (pre-) clinical progress. J. Control Release 2012, 161, 175–187. [Google Scholar] [CrossRef]
- Kamaly, N.; Xiao, Z.; Valencia, P.M.; Radovic-Moreno, A.F.; Farokhzad, O.C. Targeted polymeric therapeutic nanoparticles: Design, development and clinical translation. Chem. Soc. Rev. 2012, 41, 2971–3010. [Google Scholar] [CrossRef]
- Choi, J.Y.; Ramasamy, T.; Kim, S.Y.; Kim, J.; Ku, S.K.; Youn, Y.S.; Kim, J.R.; Jeong, J.H.; Choi, H.G.; Yong, C.S.; et al. PEGylated lipid bilayer-supported mesoporous silica nanoparticle composite for synergistic co-delivery of axitinib and celastrol in multi-targeted cancer therapy. Acta. Biomater. 2016, 39, 94–105. [Google Scholar] [CrossRef]
- Petros, R.A.; DeSimone, J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef]
- Richard, C.; Chaumet-Riffaud, P.; Belland, A.; Parat, A.; Contino-Pepin, C.; Bessodes, M.; Scherman, D.; Pucci, B.; Mignet, N. Amphiphilic perfluoroalkyl carbohydrates as new tools for liver imaging. Int. J. Pharm. 2009, 379, 301–308. [Google Scholar] [CrossRef]
- Rigopoulou, E.I.; Roggenbuck, D.; Smyk, D.S.; Liaskos, C.; Mytilinaiou, M.G.; Feist, E.; Conrad, K.; Bogdanos, D.P. Asialoglycoprotein receptor (ASGPR) as target autoantigen in liver autoimmunity: Lost and found. Autoimmun. Rev. 2012, 12, 260–269. [Google Scholar] [CrossRef]
- Stockert, R.J. The asialoglycoprotein receptor: Relationships between structure, function, and expression. Physiol. Rev. 1995, 75, 591–609. [Google Scholar] [CrossRef]
- Eisenberg, C.; Seta, N.; Appel, M.; Feldmann, G.; Durand, G.; Feger, J. Asialoglycoprotein receptor in human isolated hepatocytes from normal liver and its apparent increase in liver with histological alterations. J. Hepatol. 1991, 13, 305–309. [Google Scholar] [CrossRef]
- Chaumet-Riffaud, P.; Martinez-Duncker, I.; Marty, A.L.; Richard, C.; Prigent, A.; Moati, F.; Sarda-Mantel, L.; Scherman, D.; Bessodes, M.; Mignet, N. Synthesis and application of lactosylated, 99mTc chelating albumin for measurement of liver function. Bioconjug. Chem. 2010, 21, 589–596. [Google Scholar] [CrossRef]
- Pan, Q.; Lv, Y.; Williams, G.R.; Tao, L.; Yang, H.; Li, H.; Zhu, L. Lactobionic acid and carboxymethyl chitosan functionalized graphene oxide nanocomposites as targeted anticancer drug delivery systems. Carbohydr. Polym. 2016, 151, 812–820. [Google Scholar] [CrossRef]
- Liu, H.; Wang, H.; Xu, Y.; Guo, R.; Wen, S.; Huang, Y.; Liu, W.; Shen, M.; Zhao, J.; Zhang, G.; et al. Lactobionic acid-modified dendrimer-entrapped gold nanoparticles for targeted computed tomography imaging of human hepatocellular carcinoma. ACS Appl. Mater. Interfaces 2014, 6, 6944–6953. [Google Scholar] [CrossRef] [PubMed]
- Lapitsky, Y. Ionically crosslinked polyelectrolyte nanocarriers: Recent advances and open problems. Curr. Opin. Colloid Interface Sci. 2014, 19, 122–130. [Google Scholar] [CrossRef]
- Chen, G.; Li, D.; Li, J.; Luo, Y.; Wang, J.; Shi, X.; Guo, R. Targeted delivery of doxorubicin by lactobionic acid-modified laponite to hepatocarcinoma cells. J. Control Release 2015, 213, e34. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Clarke, C.P.; Starkey, Y.Y.L.; Clarke, G.D. Comparison of a novel fast-dissolving acetaminophen tablet formulation (FD-APAP) and standard acetaminophen tablets using gamma scintigraphy and pharmacokinetic studies. Drug Dev. Ind. Pharm. 2011, 37, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Stevens, H.; Voelker, M.; Gow, L.; MacDougall, F.; Bieri, G. In-vivo disintegration and absorption of two fast acting aspirin tablet formulations compared to ibuprofen tablets using pharmacoscintigraphy. J. Drug Delivery Sci. Technol. 2019, 51, 535–541. [Google Scholar] [CrossRef]
- Nasr, T.; Bondock, S.; Rashed, H.M.; Fayad, W.; Youns, M.; Sakr, T.M. Novel hydrazide-hydrazone and amide substituted coumarin derivatives: Synthesis, cytotoxicity screening, microarray, radiolabeling and in vivo pharmacokinetic studies. Eur. J. Med. Chem. 2018, 151, 723–739. [Google Scholar] [CrossRef]
- Weitschies, W.; Wilson, C.G. In vivo imaging of drug delivery systems in the gastrointestinal tract. Int. J. pharm. 2011, 417, 216–226. [Google Scholar] [CrossRef]
- Singh, A.; Bhardwaj, N.; Bhatnagar, A. Pharmacoscintigraphy: An unexplored modality in India. Indian J. Pharm. Sci. 2004, 66, 18. [Google Scholar]
- Rashed, H.M.; Shamma, R.N.; Basalious, E.B. Contribution of both olfactory and systemic pathways for brain targeting of nimodipine-loaded lipo-pluronics micelles: In vitro characterization and in vivo biodistribution study after intranasal and intravenous delivery. Drug Delivery 2017, 24, 181–187. [Google Scholar] [CrossRef] [Green Version]
- Wrobel, N.; Schinkinger, M.; Mirsky, V.M. A Novel Ultraviolet Assay for Testing Side Reactions of Carbodiimides. Anal. Biochem. 2002, 305, 135–138. [Google Scholar] [CrossRef]
- Williams, A.; Ibrahim, I.T. A new mechanism involving cyclic tautomers for the reaction with nucleophiles of the water-soluble peptide coupling reagent 1-ethyl-3-(3′-(dimethylamino) propyl) carbodiimide (EDC). J. Am. Chem. Soc. 1981, 103, 7090–7095. [Google Scholar] [CrossRef]
- Mattson, G.; Conklin, E.; Desai, S.; Nielander, G.; Savage, M.D.; Morgensen, S. A practical approach to crosslinking. Mol. Biol. R 1993, 17, 167–183. [Google Scholar] [CrossRef]
- Calvo, P.; Remuñán-López, C.; Vila-Jato, J.L.; Alonso, M.J. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Bansal, D.; Yadav, K.; Pandey, V.; Ganeshpurkar, A.; Agnihotri, A.; Dubey, N. Lactobionic acid coupled liposomes: An innovative strategy for targeting hepatocellular carcinoma. Drug Delivery 2016, 23, 140–146. [Google Scholar] [CrossRef]
- Bagari, R.; Bansal, D.; Gulbake, A.; Jain, A.; Soni, V.; Jain, S.K. Chondroitin sulfate functionalized liposomes for solid tumor targeting. J. Drug Targeting 2011, 19, 251–257. [Google Scholar] [CrossRef]
- Singh, G.; Pai, R.S.; Devi, V.K. Optimization of pellets containing solid dispersion prepared by extrusion/spheronization using central composite design and desirability function. J. Young Pharm. 2012, 4, 146–156. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, D.; Kaur, I.P. Improved pharmacodynamics of timolol maleate from a mucoadhesive niosomal ophthalmic drug delivery system. Int. J. Pharm. 2005, 290, 155–159. [Google Scholar] [CrossRef]
- Abouhussein, D.M.; Khattab, A.; Bayoumi, N.A.; Mahmoud, A.F.; Sakr, T.M. Brain targeted rivastigmine mucoadhesive thermosensitive In situ gel: Optimization, in vitro evaluation, radiolabeling, in vivo pharmacokinetics and biodistribution. J. Drug Delivery Sci. Technol. 2018, 43, 129–140. [Google Scholar] [CrossRef]
- Sakr, T.; Khedr, M.; Rashed, H.; Mohamed, M. In silico-based repositioning of phosphinothricin as a novel technetium-99m imaging probe with potential anti-cancer activity. Molecules 2018, 23, 496. [Google Scholar] [CrossRef] [Green Version]
- Rashed, H.M.; Shamma, R.N.; El-Sabagh, H.A. Preparation of 99mTc-levetiracetam intranasal microemulsion as the first radiotracer for SPECT imaging of the Synaptic Vesicle Protein SV2A. Eur. J. Pharm. Sci. 2018, 121, 29–33. [Google Scholar] [CrossRef]
- Motaleb, M.; Ibrahim, I.; Rizq, R.A.; Elzanfaly, E. Preparation, chromatographic evaluation and biodistribution of 99mTc-procainamide as a radiopharmaceutical for heart imaging. Radiochim. Acta 2017, 105, 215–223. [Google Scholar] [CrossRef]
- Sakr, T.; El-Safoury, D.; Awad, G.A.; Motaleb, M. Biodistribution of 99mTc-Sunitinib as a potential radiotracer for tumor hypoxia imaging. J. Labelled Compd. Radiopharm. 2013, 56, 392–395. [Google Scholar] [CrossRef]
- Saddar, E.; El-Tawoosy, M.; Motaleb, H. Preparation and biological evaluation of radioiodinated risperidone and lamotrigine as models for brain imaging agents. J. Radioanal. and Nucl. Chem. 2014, 301, 189–196. [Google Scholar] [CrossRef]
- Lai, L.F.; Guo, H.X. Preparation of new 5-fluorouracil-loaded zein nanoparticles for liver targeting. Int. J. Pharm. 2011, 404, 317–323. [Google Scholar] [CrossRef]
- Kumari, S.; Kumar Annamareddy, S.H.; Abanti, S.; Kumar Rath, P. Physicochemical properties and characterization of chitosan synthesized from fish scales, crab and shrimp shells. Int. J. Biol. Macromol. 2017, 104, 1697–1705. [Google Scholar] [CrossRef]
- Al-Mahallawi, A.M.; Abdelbary, A.A.; Aburahma, M.H. Investigating the potential of employing bilosomes as a novel vesicular carrier for transdermal delivery of tenoxicam. Int. J. Pharm. 2015, 485, 329–340. [Google Scholar] [CrossRef]
- Ahmed, I.S.; El Hosary, R.; Hassan, M.A.; Haider, M.; Abd-Rabo, M.M. Efficacy and Safety Profiles of Oral Atorvastatin-Loaded Nanoparticles: Effect of Size Modulation on Biodistribution. Mol. Pharm. 2018, 15, 247–255. [Google Scholar] [CrossRef]
- Ahmed, I.S.; El-Hosary, R.; Shalaby, S.; Abd-Rabo, M.M.; Elkhateeb, D.G.; Nour, S. PD-PK evaluation of freeze-dried atorvastatin calcium-loaded poly-epsilon-caprolactone nanoparticles. Int. J. Pharm. 2016, 504, 70–79. [Google Scholar] [CrossRef]
- Boseila, A.A.; Rashed, H.M.; Sakr, T.M.; Abdel-Reheem, A.Y.; Basalious, E.B. Superiority of DEAE-Dx-Stabilized Cationic Bile-Based Vesicles over Conventional Vesicles for Enhanced Hepatic Delivery of Daclatasvir. Mol. Pharm. 2019, 16, 4190–4199. [Google Scholar] [CrossRef] [PubMed]







| Formulation Code | LA Addition | LA Conc (mg/L) | EE (%) | PS (nm) | ZP (mV) | PDI |
|---|---|---|---|---|---|---|
| F1 | After | 50 | 80.7 ± 0.9 | 713 ± 65 | 51.0 ± 0.78 | 0.72 ± 0.0 |
| F2 | After | 75 | 84.3 ± 2.5 | 882 ± 74 | 47.8 ± 1.98 | 0.76 ± 0.0 |
| F3 | After | 100 | 80.4 ± 0.9 | 1141 ± 24 | 47.9 ± 0.21 | 0.91 ± 0.0 |
| F4 | Before | 50 | 93.1 ± 2.4 | 621 ± 49 | 48.9 ± 0.04 | 0.77 ± 0.1 |
| F5 | Before | 75 | 85.8 ± 0.2 | 1160 ± 182 | 48.7 ± 0.04 | 0.67 ± 0.0 |
| F6 | Before | 100 | 86.2 ± 5.3 | 2252 ± 394 | 48.4 ± 1.06 | 0.51 ± 0.0 |
| BA-loaded-LA-free NPs | - | - | 77.4 ± 3.5 | 485 ± 25 | 51.6 ± 0.05 | 0.78 ± 0.0 |
| Response | R2 | Adjusted R2 | Predicted R2 | Adequate Precision | Significant Terms |
|---|---|---|---|---|---|
| EE% | 0.938 | 0.886 | 80.7±0.98 | 10.363 | X1 (p = 0.0001), X1 × X2 (p = 0.0343) |
| PS (nm) | 0.976 | 0.995 | 84.3±2.54 | 18.572 | X1 (p = 0.0004), X2 (p < 0.0001), X1 × X2 (p = 0.0014) |
| ZP (mV) | 0.893 | 0.804 | 77.4±3.52 | 7.787 | X2 (p = 0.0016) |
| Formulation | EE% | PS (nm) | ZP (mV) | PDI |
|---|---|---|---|---|
| BA-loaded-CL-NPs | ||||
| No Sonication | 77.4 ± 3.52 | 485 ± 25 | 51.6 ± 0.05 | 0.78 ± 0.02 |
| Sonication | 76.6 ± 6.52 | 435 ± 19 | 51.4 ± 0.08 | 0.75 ± 0.02 |
| BA-loaded-LA-modified-CL-NPs | ||||
| No Sonication | 93.2 ± 2.41 | 621 ± 79 | 48.9 ± 0.04 | 0.77 ± 0.12 |
| Sonication | 93.7 ± 3.21 | 490 ± 39 | 48.1 ± 0.04 | 0.31 ± 0.23 |
| BA-NPs | Storage Conditions | PS (nm) | EE (%) | ZP (mV) |
|---|---|---|---|---|
| LA-free | Fresh | 435 ± 19 | 76.6 ± 6.5 | 41.4 ± 0.08 |
| Room temp. | 645 ± 59 ∗ | 78.2 ± 6.6 | 37.2 ± 0.05 ∗ | |
| 4 °C | 449 ± 15 | 75.3 ± 3.2 | 40.3 ± 0.06 | |
| LA-modified | Fresh | 490 ± 39 | 93.7 ± 3.2 | 48.1 ± 0.04 |
| Room temp. | 805 ± 67 ∗ | 94.6 ± 4.3 | 41.3± 0.04 ∗ | |
| 4 °C | 482 ± 24 | 92.4 ± 5.1 | 47.1 ± 0.02 |
| Parameter | LA-Modified-NPs | LA-Free-NPs |
|---|---|---|
| DTI (1h) | 2.25 | - |
| DTI (2h) | 3.8 | - |
| DTI (4h) | 1 | - |
| DTI (6h) | 2.6 | - |
| DTI (24h) | 2 | - |
| DTE | 4.22 | 1.45 |
| RTE | 2.24 | - |
| Blood | Cmax (%AD/g) | AUC0–24 (%AD/g) |
| 99mTc-BA solution | 0.04 ± 0.006 | 0.1 ± 0.001 |
| 99mTc-BA-loaded-LA-free-CL-NPs | 0.16 ± 0.011 *,a | 2.43 ± 0.34 *,a |
| 99mTc-BA-loaded-LA-modifie-CL-NPs | 0.14 ± 0.014 *,a | 1.87 ± 0.025 *,a,b |
| Liver | Cmax (%AD/g) | AUC0-24 (%AD/g) |
| 99mTc-BA solution | 0.07 ± 0.01 | 0.30 ± 0.04 |
| 99mTc-BA-loaded-LA-free-CL-NPs | 0.24 ± 0.02 *,a | 3.53 ± 0.51 *,a |
| 99mTc-BA-loaded-LA-modified-CL-NPs | 0.52 ± 0.06 *,a,b | 7.9 ± 0.94 *,a,b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, I.S.; Rashed, H.M.; Fayez, H.; Farouk, F.; Shamma, R.N. Nanoparticle-Mediated Dual Targeting: An Approach for Enhanced Baicalin Delivery to the Liver. Pharmaceutics 2020, 12, 107. https://doi.org/10.3390/pharmaceutics12020107
Ahmed IS, Rashed HM, Fayez H, Farouk F, Shamma RN. Nanoparticle-Mediated Dual Targeting: An Approach for Enhanced Baicalin Delivery to the Liver. Pharmaceutics. 2020; 12(2):107. https://doi.org/10.3390/pharmaceutics12020107
Chicago/Turabian StyleAhmed, Iman Saad, Hassan Medhat Rashed, Hend Fayez, Faten Farouk, and Rehab Nabil Shamma. 2020. "Nanoparticle-Mediated Dual Targeting: An Approach for Enhanced Baicalin Delivery to the Liver" Pharmaceutics 12, no. 2: 107. https://doi.org/10.3390/pharmaceutics12020107
APA StyleAhmed, I. S., Rashed, H. M., Fayez, H., Farouk, F., & Shamma, R. N. (2020). Nanoparticle-Mediated Dual Targeting: An Approach for Enhanced Baicalin Delivery to the Liver. Pharmaceutics, 12(2), 107. https://doi.org/10.3390/pharmaceutics12020107






