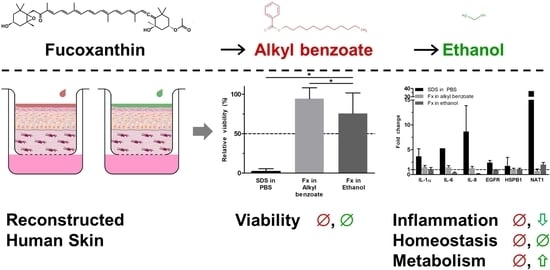
Skin Irritation Testing beyond Tissue Viability: Fucoxanthin Effects on Inflammation, Homeostasis, and Metabolism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reconstructed Human Skin
2.2. Test Substance Application
2.3. Viability Assay
2.4. Morphology and Immunofluorescence
2.5. Gene Expression
2.6. Statistical Analysis
3. Results
3.1. Fucoxanthin Effects on RHS Morphology
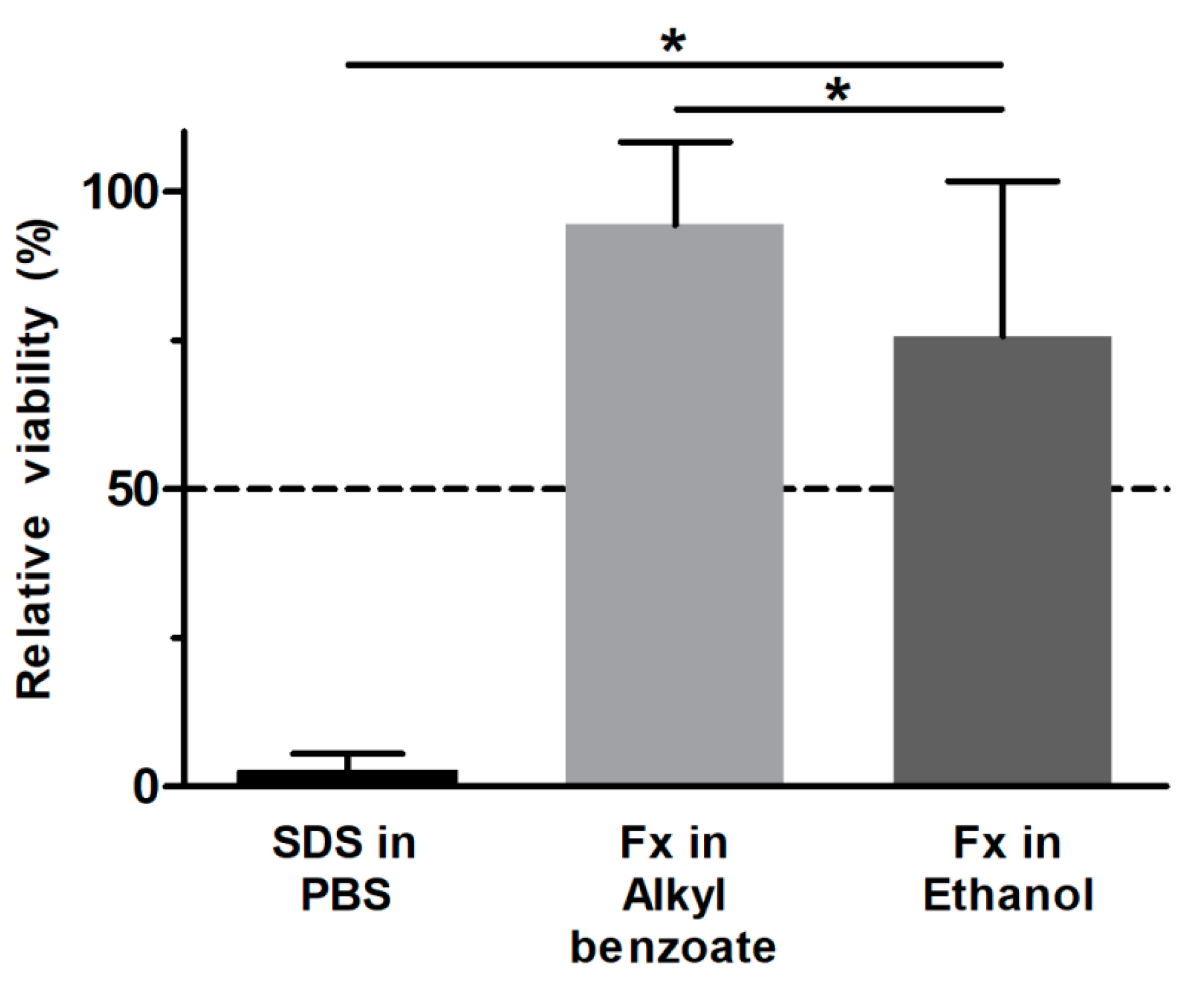
3.2. Fucoxanthin Effects on RHS Viability
3.3. Fucoxanthin Effects on RHS Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Flament, F.; Bazin, R.; Laquieze, S.; Rubert, V.; Simonpietri, E.; Piot, B. Effect of the sun on visible clinical signs of aging in caucasian skin. Clin. Cosmet. Investig. Dermatol. 2013, 6, 221–232. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Yan, S.; Wu, M.; Li, F.; Xu, X.; Song, W.; Zhao, J.; Xu, J.; Kan, H. Ambient ozone pollution as a risk factor for skin disorders. Br. J. Dermatol. 2011, 165, 224–225. [Google Scholar] [CrossRef]
- Nishisgori, C. Current concept of photocarcinogenesis. Photochem. Photobiol. Sci. 2015, 14, 1713–1721. [Google Scholar] [CrossRef] [PubMed]
- Awad, F.; Assrawi, E.; Louvrier, C.; Jumeau, C.; Giurgea, I.; Amselem, S.; Karabina, S.A. Photoaging and skin cancer: Is the inflammasome the missing link? Mech. Ageing Dev. 2018, 172, 131–137. [Google Scholar] [CrossRef]
- Burke, K.E. Mechanisms of aging and development—A new understanding of environmental damage to the skin and prevention with topical antioxidants. Mech. Ageing Dev. 2018, 172, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Muresan, X.M.; Sticozzi, C.; Belmonte, G.; Pecorelli, A.; Cervellati, F.; Demaude, J.; Krol, Y.; Oresajo, C. Ozone-induced damage in 3d-skin model is prevented by topical vitamin c and vitamin e compound mixtures application. J. Dermatol. Sci. 2016, 82, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, C.; Jürgensen, J.S.; Degen, A.; Hackethal, M.; Ulrich, M.; Patel, M.J.; Eberle, J.; Terhorst, D.; Sterry, W.; Stockfleth, E. Prevention of non-melanoma skin cancer in organ transplant patients by regular use of a sunscreen: A 24 months, prospective, case–control study. Br. J. Dermatol. 2009, 161, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Heerfordt, I.M.; Torsnes, L.R.; Philipsen, P.A.; Wulf, H.C. Sunscreen use optimized by two consecutive applications. PLoS ONE 2018, 13, e0193916. [Google Scholar] [CrossRef]
- Autier, P. Sunscreen abuse for intentional sun exposure. Br. J. Dermatol. 2009, 161 (Suppl. 3), 40–45. [Google Scholar] [CrossRef]
- Lohan, S.B.; Muller, R.; Albrecht, S.; Mink, K.; Tscherch, K.; Ismaeel, F.; Lademann, J.; Rohn, S.; Meinke, M.C. Free radicals induced by sunlight in different spectral regions—In vivo versus ex vivo study. Exp. Dermatol. 2016, 25, 380–385. [Google Scholar] [CrossRef]
- Thiele, J.J.; Schroeter, C.; Hsieh, S.N.; Podda, M.; Packer, L. The antioxidant network of the stratum corneum. Curr. Probl. Dermatol. 2001, 29, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Rhie, G.; Shin, M.H.; Seo, J.Y.; Choi, W.W.; Cho, K.H.; Kim, K.H.; Park, K.C.; Eun, H.C.; Chung, J.H. Aging- and photoaging-dependent changes of enzymic and nonenzymic antioxidants in the epidermis and dermis of human skin in vivo. J. Investig. Dermatol. 2001, 117, 1212–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Sanchez, A.; Barrajon-Catalan, E.; Herranz-Lopez, M.; Micol, V. Nutraceuticals for skin care: A comprehensive review of human clinical studies. Nutrients 2018, 10, 403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, J.; Yuan, J.-P.; Wu, C.-F.; Wang, J.-H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef] [PubMed]
- Sachindra, N.M.; Sato, E.; Maeda, H.; Hosokawa, M.; Niwano, Y.; Kohno, M.; Miyashita, K. Radical scavenging and singlet oxygen quenching activity of marine carotenoid fucoxanthin and its metabolites. J. Agric. Food Chem. 2007, 55, 8516–8522. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Nakashima, M.; Koshiba, T.; Maoka, T.; Nishino, H.; Yano, M.; Sumida, T.; Kim, O.K.; Koshimizu, K.; Ohigashi, H. Modifying effects of carotenoids on superoxide and nitric oxide generation from stimulated leukocytes. Cancer Lett. 2000, 149, 115–123. [Google Scholar] [CrossRef]
- Hashimoto, T.; Ozaki, Y.; Taminato, M.; Das, S.K.; Mizuno, M.; Yoshimura, K.; Maoka, T.; Kanazawa, K. The distribution and accumulation of fucoxanthin and its metabolites after oral administration in mice. Br. J. Nutr. 2009, 102, 242–248. [Google Scholar] [CrossRef] [Green Version]
- Gaspar, L.R.; Tharmann, J.; Maia Campos, P.M.; Liebsch, M. Skin phototoxicity of cosmetic formulations containing photounstable and photostable uv-filters and vitamin a palmitate. Toxicol. In Vitro 2013, 27, 418–425. [Google Scholar] [CrossRef] [Green Version]
- Lane, M.E. Skin penetration enhancers. Int. J. Pharm. 2013, 447, 12–21. [Google Scholar] [CrossRef]
- OECD 439. Test no. 439: In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method. In Oecd Guidelines for the Testing of Chemicals, Section 4: Health Effects. 2019. Available online: https://doi.org/10.1787/9789264242845-en (accessed on 12 January 2020).
- Kandárová, H.; Hayden, P.; Klausner, M.; Kubilus, J.; Sheasgreen, J. An in vitro skin irritation test (sit) using the epiderm reconstructed human epidermal (rhe) model. JoVE 2009, e1366. [Google Scholar] [CrossRef] [Green Version]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. Imagej2: Imagej for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- Hübner, J.; Raschke, M.; Rutschle, I.; Grassle, S.; Hasenberg, T.; Schirrmann, K.; Lorenz, A.; Schnurre, S.; Lauster, R.; Maschmeyer, I.; et al. Simultaneous evaluation of anti-egfr-induced tumour and adverse skin effects in a microfluidic human 3d co-culture model. Sci. Rep. 2018, 8, 15010. [Google Scholar] [CrossRef] [PubMed]
- Kent, W.J.; Sugnet, C.W.; Furey, T.S.; Roskin, K.M.; Pringle, T.H.; Zahler, A.M.; Haussler, D. The human genome browser at ucsc. Genome Res. 2002, 12, 996–1006. [Google Scholar] [CrossRef] [Green Version]
- Maas-Szabowski, N.; Stark, H.J.; Fusenig, N.E. Keratinocyte growth regulation in defined organotypic cultures through il-1-induced keratinocyte growth factor expression in resting fibroblasts. J. Investig. Dermatol. 2000, 114, 1075–1084. [Google Scholar] [CrossRef] [Green Version]
- Berroth, A.; Kühnl, J.; Kurschat, N.; Schwarz, A.; Stäb, F.; Schwarz, T.; Wenck, H.; Fölster-Holst, R.; Neufang, G. Role of fibroblasts in the pathogenesis of atopic dermatitis. J. Allergy Clin. Immun. 2013, 131, 1547–1554.e1546. [Google Scholar] [CrossRef]
- The Brazilian National Cancer Institute. Non-Melanoma Skin Cancer. In Cancer Types. 2008. Available online: https://www.inca.gov.br/tipos-de-cancer/cancer-de-pele-nao-melanoma (accessed on 12 January 2020).
- International Agency for Research on Cancer. World Cancer Report 2014; World Health Organization: Geneva, Switzerland, 2014; Available online: http://apps.who.int/bookorders/anglais/detart1.jsp?codlan=1&codcol=80&codcch=275 (accessed on 12 January 2020).
- Parrado, C.; Mercado-Saenz, S.; Perez-Davo, A.; Gilaberte, Y.; Gonzalez, S.; Juarranz, A. Environmental stressors on skin aging. Mechanistic insights. Front. Pharmacol. 2019, 10, 759. [Google Scholar] [CrossRef]
- Hüls, A.; Vierkotter, A.; Gao, W.; Kramer, U.; Yang, Y.; Ding, A.; Stolz, S.; Matsui, M.; Kan, H.; Wang, S.; et al. Traffic-related air pollution contributes to development of facial lentigines: Further epidemiological evidence from caucasians and asians. J. Investig. Dermatol. 2016, 136, 1053–1056. [Google Scholar] [CrossRef] [Green Version]
- Mistry, N. Guidelines for formulating anti-pollution products. Cosmetics 2017, 4, 57. [Google Scholar] [CrossRef] [Green Version]
- Becker, L.C.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety assessment of alkyl benzoates as used in cosmetics. Int. J. Toxicol. 2012, 31, 342s–372s. [Google Scholar] [CrossRef]
- Kandárová, H.; Bendova, H.; Letasiova, S.; Coleman, K.P.; De Jong, W.H.; Jirova, D. Evaluation of the medical devices benchmark materials in the controlled human patch testing and in the rhe in vitro skin irritation protocol. Toxicol. In Vitro 2018, 50, 433–438. [Google Scholar] [CrossRef]
- Wanjiku, B.; Yamamoto, K.; Klossek, A.; Schumacher, F.; Pischon, H.; Mundhenk, L.; Rancan, F.; Judd, M.M.; Ahmed, M.; Zoschke, C.; et al. Qualifying X-ray and stimulated raman spectromicroscopy for mapping cutaneous drug penetration. Anal. Chem. 2019, 91, 7208–7214. [Google Scholar] [CrossRef] [PubMed]
- Li, L.N.; Margolis, L.B.; Hoffman, R.M. Skin toxicity determined in vitro by three-dimensional, native-state histoculture. Proc. Natl. Acad. Sci. USA 1991, 88, 1908–1912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zukowski, P.; Maciejczyk, M.; Waszkiel, D. Sources of free radicals and oxidative stress in the oral cavity. Arch. Oral Biol. 2018, 92, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Jung, E.C.; Zhu, H.; Zou, Y.; Hui, X.; Maibach, H. Vehicle effects on human stratum corneum absorption and skin penetration. Toxicol. Ind. Health 2017, 33, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.Y.; Ingelman-Sundberg, M.; Neve, E.; Matsumoto, H.; Nishitani, Y.; Minowa, Y.; Fukui, Y.; Bailey, S.M.; Patel, V.B.; Cunningham, C.C.; et al. Ethanol and oxidative stress. Alcohol. Clin. Exp. Res. 2001, 25, 237s–243s. [Google Scholar] [CrossRef]
- Frankart, A.; Coquette, A.; Schroeder, K.R.; Poumay, Y. Studies of cell signaling in a reconstructed human epidermis exposed to sensitizers: Il-8 synthesis and release depend on egfr activation. Arch. Dermatol. Res. 2012, 304, 289–303. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Perspective article: Growth factors and cytokines in wound healing. Wound Rep. Reg. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Verschuure, P.; Tatard, C.; Boelens, W.C.; Grongnet, J.F.; David, J.C. Expression of small heat shock proteins hspb2, hspb8, hsp20 and cvhsp in different tissues of the perinatal developing pig. Eur. J. Cell Biol. 2003, 82, 523–530. [Google Scholar] [CrossRef]
- Rodríguez-Luna, A.; Ávila-Román, J.; González-Rodríguez, M.L.; Cózar, M.J.; Rabasco, A.M.; Motilva, V.; Talero, E. Fucoxanthin-containing cream prevents epidermal hyperplasia and uvb-induced skin erythema in mice. Mar. Drugs 2018, 16, 378. [Google Scholar] [CrossRef] [Green Version]
- Bailey, J.; Thew, M.; Balls, M. An analysis of the use of animal models in predicting human toxicology and drug safety. Altern. Lab. Anim. 2014, 42, 181–199. [Google Scholar] [CrossRef] [Green Version]
- Hausmann, C.; Vogt, A.; Kerscher, M.; Ghoreschi, K.; Schäfer-Korting, M.; Zoschke, C. Optimizing skin pharmacotherapy for older patients: The future is at hand but are we ready for it? Drug Discov. Today 2020. [Google Scholar] [CrossRef] [PubMed]
- De Vecchi, R.; Dakic, V.; Mattos, G.; Rigaudeau, A.-S.; Oliveira, V.; Garcia, C.; Alépée, N.; Bouez, C. Implementation, availability and regulatory status of an OECD accepted reconstructed human epidermis model in brazil. Vigilância Sanitária Debate 2018, 6, 64. [Google Scholar] [CrossRef]
- Maschmeyer, I.; Hasenberg, T.; Jaenicke, A.; Lindner, M.; Lorenz, A.K.; Zech, J.; Garbe, L.A.; Sonntag, F.; Hayden, P.; Ayehunie, S.; et al. Chip-based human liver-intestine and liver-skin co-cultures—A first step toward systemic repeated dose substance testing in vitro. Eur. J. Pharm. Biopharm. 2015, 95, 77–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Gene Name | Used Primer Sequence | |
|---|---|---|
| Forward | Reverse | |
| ACTB | CCACCATGTACCCTGGCATT | GCTTGCTGATCCACATCTGCT |
| EGFR | GCCGACAGCTATGAGATGGAG | TGGAGGTGCAGTTTTTGAAGTG |
| HSPB1 | GACCCCACCCAAGTTTCCTC | TCGGATTTTGCAGCTTCTGG |
| IL-1α | GTGACTGCCCAAGATGAAGACC | TGCCAAGCACACCCAGTAGTC |
| IL-6 | CTGGATCAGGACTTTTGTACTCATCT | CCAATCTGGATTCAATGAGGAGACT |
| IL-8 | GTGGAGAAGTTTTTGAAGAGGGC | TCTGGCAACCCTACAACAGAC |
| NAT1 | ATCCGAGCTGTTCCCTTTGAG | AACATACCCTCCCAACATCGTG |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spagolla Napoleão Tavares, R.; Stuchi Maria-Engler, S.; Colepicolo, P.; Debonsi, H.M.; Schäfer-Korting, M.; Marx, U.; Rigo Gaspar, L.; Zoschke, C. Skin Irritation Testing beyond Tissue Viability: Fucoxanthin Effects on Inflammation, Homeostasis, and Metabolism. Pharmaceutics 2020, 12, 136. https://doi.org/10.3390/pharmaceutics12020136
Spagolla Napoleão Tavares R, Stuchi Maria-Engler S, Colepicolo P, Debonsi HM, Schäfer-Korting M, Marx U, Rigo Gaspar L, Zoschke C. Skin Irritation Testing beyond Tissue Viability: Fucoxanthin Effects on Inflammation, Homeostasis, and Metabolism. Pharmaceutics. 2020; 12(2):136. https://doi.org/10.3390/pharmaceutics12020136
Chicago/Turabian StyleSpagolla Napoleão Tavares, Renata, Silvya Stuchi Maria-Engler, Pio Colepicolo, Hosana Maria Debonsi, Monika Schäfer-Korting, Uwe Marx, Lorena Rigo Gaspar, and Christian Zoschke. 2020. "Skin Irritation Testing beyond Tissue Viability: Fucoxanthin Effects on Inflammation, Homeostasis, and Metabolism" Pharmaceutics 12, no. 2: 136. https://doi.org/10.3390/pharmaceutics12020136
APA StyleSpagolla Napoleão Tavares, R., Stuchi Maria-Engler, S., Colepicolo, P., Debonsi, H. M., Schäfer-Korting, M., Marx, U., Rigo Gaspar, L., & Zoschke, C. (2020). Skin Irritation Testing beyond Tissue Viability: Fucoxanthin Effects on Inflammation, Homeostasis, and Metabolism. Pharmaceutics, 12(2), 136. https://doi.org/10.3390/pharmaceutics12020136