RETRACTED: Omega-3 Self-Nanoemulsion Role in Gastroprotection against Indomethacin-Induced Gastric Injury in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Formulation of Omega-3 SNEDDS
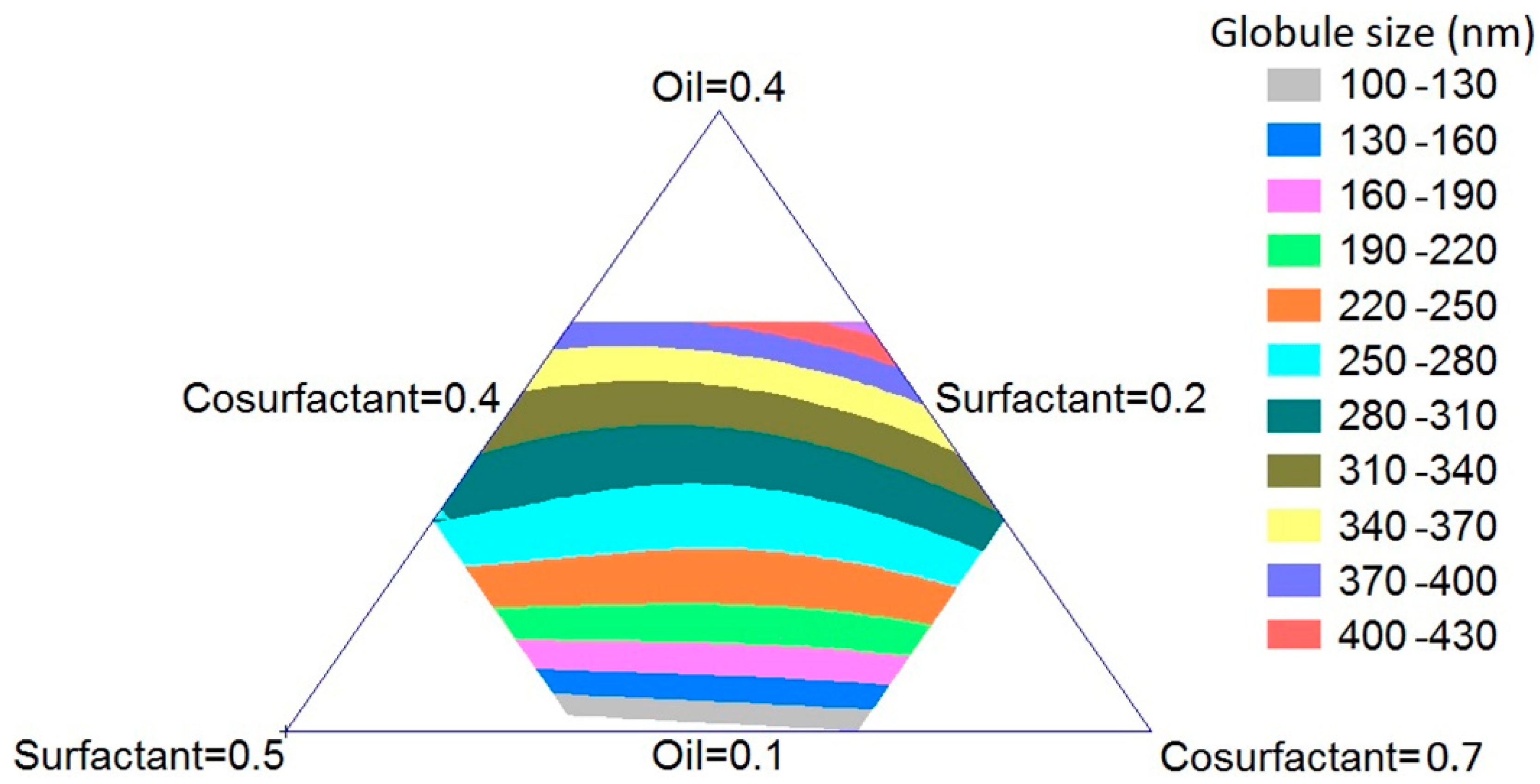
2.2. Optimization of Omega-3 SNEDDS
2.3. Omega-3 SNEDDS Globule Size Determination
2.4. Prediction and Preparation of Optimized Omega-3 SNEDDS Formulation
2.5. In Vivo Evaluation of Optimized Omega-3 SNEDDS Formulation
2.5.1. Animals
2.5.2. Assessment of Gastric Mucosal Lesions
2.5.3. Determination of Gastric Mucosa Oxidative Stress Parameters
2.6. Statistical Analysis
3. Results and Discussion
3.1. Formulation and Optimization of Omega-3 SNEDDS Preparations
3.2. Validation of the Optimized Omega-3 SNEDDS Formulation
3.3. In Vivo Evaluation of Optimized Omega-3 SNEDDS Formulation
3.3.1. Effect of Pure Omega-3 Oil and SNEDDS Formula on Indomethacin-Induced Gastric Lesions
3.3.2. Effect of Pure Omega-3 Oil and SNEDDS Formula on Gastric Mucosal Oxidative Stress Parameters
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kuna, L.; Jakab, J.; Smolic, R.; Raguz-Lucic, N.; Vcev, A.; Smolic, M. Peptic Ulcer Disease: A Brief Review of Conventional Therapy and Herbal Treatment Options. J. Clin. Med. 2019, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Yang, S.; Xu, R.; Chen, G. Association between Poor Sleep Quality and Subsequent Peptic Ulcer Recurrence in Older Patients with Mild Cognitive Impairment: Examining the Role of Social Engagement. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.H.; Baeg, M.K.; Ko, S.Y.; Han, K. Do Women Who Sleep More Have Reduced Risk of Peptic Ulcer Disease; Korean National Health and Nutrition Examination Survey (2008–2009). Sci. Rep. 2016, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Snowden, F.M. Emerging and reemerging diseases: A historical perspective. Immunol. Rev. 2008, 225, 9–26. [Google Scholar] [CrossRef]
- Wongrakpanich, S.; Wongrakpanich, A.; Melhado, K.; Rangaswami, J. A Comprehensive Review of Non-Steroidal Anti-Inflammatory Drug Use in The Elderly. Aging Dis. 2018, 9, 143–150. [Google Scholar] [CrossRef]
- Xiao, X.; Nakatsu, G.; Jin, Y.; Wong, S.; Yu, J.; Lau, J.Y.W. Gut Microbiota Mediates Protection Against Enteropathy Induced by Indomethacin. Sci. Rep. 2017, 7, 40317. [Google Scholar] [CrossRef]
- Lucas, S. The Pharmacology of Indomethacin. Headache J. Head Face Pain 2016, 56, 436–446. [Google Scholar] [CrossRef]
- Shahin, N.N.; Abdelkader, N.F.; Safar, M.M. A Novel Role of Irbesartan in Gastroprotection against Indomethacin-Induced Gastric Injury in Rats: Targeting DDAH/ADMA and EGFR/ERK Signaling. Sci. Rep. 2018, 8, 4280. [Google Scholar] [CrossRef]
- Al-Harbi, M.; Islam, M.; Al-Shabanah, O.; Al-Gharably, N. Effect of acute administration of fish oil (omega-3 marine triglyceride) on gastric ulceration and secretion induced by various ulcerogenic and necrotizing agents in rats. Food Chem. Toxicol. 1995, 33, 553–558. [Google Scholar] [CrossRef]
- Brinton, E.A.; Mason, R.P. Prescription omega-3 fatty acid products containing highly purified eicosapentaenoic acid (EPA). Lipids Health Dis. 2017, 16, 23. [Google Scholar] [CrossRef]
- Güzel, C.; Ulak, G.; Sermet, A.; Ciçek, R.; Ulak, M. Effect of fish oil on indometacin-induced gastric lesions in rats. Arzneimittelforschung 1995, 45, 1172–1173. [Google Scholar]
- Shahparast, Y.; Eskandani, M.; Rajaei, A.; Khosroushahi, A.Y. Preparation, Physicochemical Characterization and Oxidative Stability of Omega-3 Fish Oil/α-Tocopherol-co-Loaded Nanostructured Lipidic Carriers. Adv. Pharm. Bull. 2019, 9, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Sreedhar, R.; Kumar, V.S.; Pillai, A.K.B.; Mangalathillam, S. Omega-3 Fatty Acid Based Nanolipid Formulation of Atorvastatin for Treating Hyperlipidemia. Adv. Pharm. Bull. 2019, 9, 271–280. [Google Scholar] [CrossRef]
- Mayurasakorn, K.; Williams, J.J.; Ten, V.S.; Deckelbaum, R.J. Docosahexaenoic acid: Brain accretion and roles in neuroprotection after brain hypoxia and ischemia. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 158–167. [Google Scholar] [CrossRef]
- Cho, Y.H.; Lee, S.Y.; Jeong, D.W.; Choi, E.J.; Kim, Y.J.; Lee, J.G.; Yi, Y.H.; Cha, H.S. Effect of Pumpkin Seed Oil on Hair Growth in Men with Androgenetic Alopecia: A Randomized, Double-Blind, Placebo-Controlled Trial. Evidence-Based Complement. Altern. Med. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Mustafa, G.; Khan, Z.; Bansal, T.; Talegaonkar, S. Preparation and Characterization of Oil in Water Nano-Reservoir Systems for Improved Oral Delivery of Atorvastatin. Curr. Nanosci. 2012, 5, 428–440. [Google Scholar] [CrossRef]
- Bu, H.; He, X.; Zhang, Z.; Yin, Q.; Yu, H.; Li, Y. A TPGS-incorporating nanoemulsion of paclitaxel circumvents drug resistance in breast cancer. Int. J. Pharm. 2014, 471, 206–213. [Google Scholar] [CrossRef]
- Yang, C.; Wu, T.; Qi, Y.; Zhang, Z. Recent Advances in the Application of Vitamin E TPGS for Drug Delivery. Theranostics 2018, 8, 464–485. [Google Scholar] [CrossRef]
- Guo, Y.; Luo, J.; Tan, S.-W.; Otieno, B.O.; Zhang, Z. The applications of Vitamin E TPGS in drug delivery. Eur. J. Pharm. Sci. 2013, 49, 175–186. [Google Scholar] [CrossRef]
- Ujhelyi, Z.; Kalantari, A.; Vecsernyés, M.; Roka, E.; Fenyvesi, F.; Poka, R.; Kozma, B.; Bácskay, I. The Enhanced Inhibitory Effect of Different Antitumor Agents in Self-Microemulsifying Drug Delivery Systems on Human Cervical Cancer HeLa Cells. Molecules 2015, 20, 13226–13239. [Google Scholar] [CrossRef]
- Ujhelyi, Z.; Fenyvesi, F.; Varadi, J.; Fehér, P.; Kiss, T.; Veszelka, S.; Deli, M.; Vecsernyés, M.; Bácskay, I. Evaluation of cytotoxicity of surfactants used in self-micro emulsifying drug delivery systems and their effects on paracellular transport in Caco-2 cell monolayer. Eur. J. Pharm. Sci. 2012, 47, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Date, A.A.; Desai, N.; Dixit, R.; Nagarsenker, M. Self-nanoemulsifying drug delivery systems: Formulation insights, applications and advances. Nanomedicine 2010, 5, 1595–1616. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, U.A.; Ahmed, O.A.A.; Hosny, K.M. Development and Evaluation of Avanafil Self-nanoemulsifying Drug Delivery System with Rapid Onset of Action and Enhanced Bioavailability. AAPS PharmSciTech 2014, 16, 53–58. [Google Scholar] [CrossRef] [PubMed]
- El-Say, K.M.; Ahmed, T.A.; Ahmed, O.A.; Hosny, K.M.; Abd-Allah, F.I. Self-Nanoemulsifying Lyophilized Tablets for Flash Oral Transmucosal Delivery of Vitamin K: Development and Clinical Evaluation. J. Pharm. Sci. 2017, 106, 2447–2456. [Google Scholar] [CrossRef]
- Szabo, S.; Hollander, D. Pathways of gastrointestinal protection and repair: Mechanisms of action of sucralfate. Am. J. Med. 1989, 86, 23–31. [Google Scholar] [CrossRef]
- Uchiyama, M.; Mihara, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Fossati, P.; Prencipe, L.; Berti, G. Use of 3,5-dichloro-2-hydroxybenzenesulfonic acid/4-aminophenazone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clin. Chem. 1980, 26, 227–231. [Google Scholar] [CrossRef]
- Konturek, S.J.; Konturek, P.C.; Pawlik, T.; Sliwowski, Z.; Ochmański, W.; Hahn, E.G. Duodenal mucosal protection by bicarbonate secretion and its mechanisms. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2004, 55, 55. [Google Scholar]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.Q.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 Is an Omega-3 Fatty Acid Receptor Mediating Potent Anti-inflammatory and Insulin-Sensitizing Effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef]
- Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Fatty acids from fish: The anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 2010, 68, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.J.; Spite, M. Resolvins: Anti-Inflammatory and Proresolving Mediators Derived from Omega-3 Polyunsaturated Fatty Acids. Annu. Rev. Nutr. 2012, 32, 203–227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hu, X.; Yang, W.; Gao, Y.; Chen, J. Omega-3 polyunsaturated fatty acid supplementation confers long-term neuroprotection against neonatal hypoxic-ischemic brain injury through anti-inflammatory actions. Stroke 2010, 41, 2341–2347. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.X.; Weylandt, K.H. Modulation of Inflammatory Cytokines by Omega-3 Fatty Acids. Membr. Biog. 2008, 49, 133–143. [Google Scholar]
- Singer, P.; Shapiro, H.; Theilla, M.; Anbar, R.; Singer, J.; Cohen, J. Anti-inflammatory properties of omega-3 fatty acids in critical illness: Novel mechanisms and an integrative perspective. Intensiv. Care Med. 2008, 34, 1580–1592. [Google Scholar] [CrossRef]
- Londhe, P.; Guttridge, D.C. Inflammation induced loss of skeletal muscle. Bone 2015, 80, 131–142. [Google Scholar] [CrossRef]



| Omega-3 SNEDDS Formula # | Factors (X1–X3) | Response | |||
|---|---|---|---|---|---|
| Omega-3 (%) | Surfactant Mixture (%) | Transcutol (%) | Globule Size (nm) | ||
| Observed | Fitted | ||||
| 1 | 30 | 30 | 40 | 412.0 | 401.5 |
| 2 | 30 | 20 | 50 | 458.0 | 451.8 |
| 3 | 20 | 40 | 40 | 278.0 | 279.4 |
| 4 | 10 | 40 | 50 | 78.0 | 74.7 |
| 5 | 20 | 20 | 60 | 291.0 | 304.7 |
| 6 | 10 | 30 | 60 | 98.0 | 100.5 |
| 7 | 25 | 30 | 45 | 325.0 | 312.8 |
| 8 | 25 | 25 | 50 | 342.0 | 321.7 |
| 9 | 20 | 35 | 45 | 281.0 | 266.7 |
| 10 | 15 | 35 | 50 | 207.0 | 202.7 |
| 11 | 20 | 25 | 55 | 261.0 | 272.1 |
| 12 | 15 | 30 | 55 | 211.0 | 205.4 |
| 13 | 30 | 25 | 45 | 380.0 | 409.8 |
| 14 | 25 | 25 | 40 | 310.0 | 324.6 |
| 15 | 20 | 30 | 50 | 255.0 | 261.8 |
| 16 | 20 | 30 | 50 | 258.0 | 261.8 |
| 17 | 25 | 20 | 55 | 367.0 | 358.7 |
| 18 | 15 | 40 | 45 | 201.0 | 202.1 |
| 19 | 20 | 30 | 50 | 259.0 | 261.8 |
| 20 | 10 | 35 | 55 | 82.0 | 89.3 |
| 21 | 15 | 25 | 60 | 235.0 | 217.7 |
| 22 | 20 | 30 | 50 | 248.0 | 261.8 |
| 23 | 30 | 30 | 40 | 408.0 | 401.5 |
| Factor | Optimum Level | Low Level | High Level | |
|---|---|---|---|---|
| X1 | 0.1 | 0.1 | 0.3 | |
| X2 | 0.4 | 0.2 | 0.4 | |
| X3 | 0.5 | 0.4 | 0.6 | |
| Response | Prediction | Actual | Residual | |
| Globule Size | 74.7 nm | 77.2 nm | 2.5 | |
| R2 | Adj R2 | SEE | MAE | |
| 98.6% | 97.63% | 15.49 | 9.46 | |
| Df | Mean Square | F-Ratio | p-Value | |
| 9 | 24,400.5 | 101.64 | 0.00001 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, O.A.A.; Fahmy, U.A.; Bakhaidar, R.; El-Moselhy, M.A.; Okbazghi, S.Z.; Ahmed, A.-S.F.; Hammad, A.S.A.; Alhakamy, N.A. RETRACTED: Omega-3 Self-Nanoemulsion Role in Gastroprotection against Indomethacin-Induced Gastric Injury in Rats. Pharmaceutics 2020, 12, 140. https://doi.org/10.3390/pharmaceutics12020140
Ahmed OAA, Fahmy UA, Bakhaidar R, El-Moselhy MA, Okbazghi SZ, Ahmed A-SF, Hammad ASA, Alhakamy NA. RETRACTED: Omega-3 Self-Nanoemulsion Role in Gastroprotection against Indomethacin-Induced Gastric Injury in Rats. Pharmaceutics. 2020; 12(2):140. https://doi.org/10.3390/pharmaceutics12020140
Chicago/Turabian StyleAhmed, Osama A. A., Usama A. Fahmy, Rana Bakhaidar, Mohamed A. El-Moselhy, Solomon Z. Okbazghi, Al-Shaimaa F. Ahmed, Asmaa S. A. Hammad, and Nabil A. Alhakamy. 2020. "RETRACTED: Omega-3 Self-Nanoemulsion Role in Gastroprotection against Indomethacin-Induced Gastric Injury in Rats" Pharmaceutics 12, no. 2: 140. https://doi.org/10.3390/pharmaceutics12020140
APA StyleAhmed, O. A. A., Fahmy, U. A., Bakhaidar, R., El-Moselhy, M. A., Okbazghi, S. Z., Ahmed, A.-S. F., Hammad, A. S. A., & Alhakamy, N. A. (2020). RETRACTED: Omega-3 Self-Nanoemulsion Role in Gastroprotection against Indomethacin-Induced Gastric Injury in Rats. Pharmaceutics, 12(2), 140. https://doi.org/10.3390/pharmaceutics12020140





