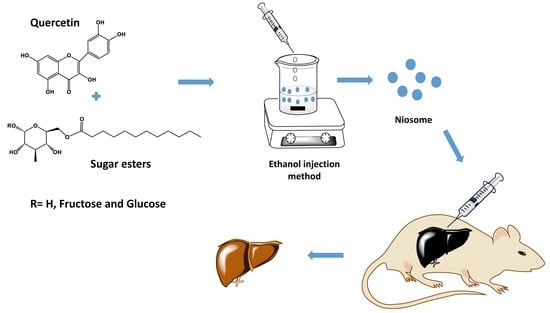
Quercetin Loaded Monolaurate Sugar Esters-Based Niosomes: Sustained Release and Mutual Antioxidant—Hepatoprotective Interplay
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Sugar Esters
2.1.1. Synthesis of Glucose Monolaurate

2.1.2. Synthesis of Trehalose Monolaurate



2.2. Preparation of Niosomes
2.3. Determination of Encapsulation Efficiency (EE%)
2.4. Size and Zeta-Potential Measurements
2.5. In Vitro Release Profile
2.6. Differential Scanning Calorimetry
2.7. Fourier Transform Infrared (FT-IR) Spectroscopy
2.8. Morphology of Niosomes
2.9. Physical Stability Study
2.10. In Vitro Protective Effect of Quercetin Niosomes
2.10.1. Cell Viability Assay
2.10.2. Protective Effect on H2O2-Induced Oxidative Stress in HepG2 Cells
2.11. In Vivo Studies
2.11.1. CCl4-Induced Hepatotoxicity
- Group I: a negative control group that received normal saline;
- Group II: a positive control group that received a single dose of CCl4 in corn oil (1:1 v/v), injected intraperitoneally, at a dose of 1 mL/kg on the fifth day;
- Group III: received free quercetin in normal saline solution daily at a dose of 30 mg/kg [36];
- Group IV: received optimum quercetin–niosomes (containing an equivalent concentration of 30 mg/kg of quercetin).
2.11.2. Assessment of Oxidative Stress Parameters
2.11.3. Histopathological Assessment of Liver Tissues
2.12. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of Quercetin Loaded Niosomes
3.2. In Vitro Drug Release
3.3. TEM Imaging of Niosomes
3.4. DSC Studies
3.5. FT-IR Studies
3.6. Stability Studies
3.7. In Vitro Protective Effect of Quercetin Niosomes
3.7.1. Cell Viability Assay
3.7.2. Protective Effect on H2O2-Induced Oxidative Stress in HepG2 Cells
3.8. In Vivo Studies
3.8.1. CCl4-Induced Hepatotoxicity
3.8.2. Assessment of Oxidative Stress Parameters
3.8.3. Histopathology
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [Green Version]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial Activity of Some Flavonoids and Organic Acids Widely Distributed in Plants. J. Clin. Med. 2020, 9, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Paramás, A.M.; Ayuda-Durán, B.; Martínez, S.; González-Manzano, S.; Santos-Buelga, C. The mechanisms behind the biological activity of flavonoids. Curr. Med. Chem. 2019, 26, 6976–6990. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.; Diwan, A.; Chandra, S. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Banjarnahor, S.D.; Artanti, N. Antioxidant properties of flavonoids. Med. J. Indones. 2014, 23, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Eftekhari, A.; Ahmadian, E.; Panahi-Azar, V.; Hosseini, H.; Tabibiazar, M.; Maleki Dizaj, S. Hepatoprotective and free radical scavenging actions of quercetin nanoparticles on aflatoxin B1-induced liver damage: In vitro/in vivo studies. Artif. Cells Nanomed. Biotechnol. 2018, 46, 411–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amanzadeh, E.; Esmaeili, A.; Abadi, R.E.N.; Kazemipour, N.; Pahlevanneshan, Z.; Beheshti, S. Quercetin conjugated with superparamagnetic iron oxide nanoparticles improves learning and memory better than free quercetin via interacting with proteins involved in LTP. Sci. Rep. 2019, 9, 6876. [Google Scholar] [CrossRef]
- Mokbel, K.; Mokbel, K. Chemoprevention of Breast Cancer With Vitamins and Micronutrients: A Concise Review. In Vivo 2019, 33, 983–997. [Google Scholar] [CrossRef] [Green Version]
- Bentz, A.B. A Review of Quercetin: Chemistry, Antioxident Properties, and Bioavailability. J. Young Investig. 2017. Available online: http://www.jyi.org/research/re.php?id=3416 (accessed on 31 December 2019).
- Khan, H.; Ullah, H.; Aschner, M.; Cheang, W.S.; Akkol, E.K. Neuroprotective Effects of Quercetin in Alzheimer’s Disease. Biomolecules 2020, 10, 59. [Google Scholar] [CrossRef] [Green Version]
- Mozafari, M.R.; Flanagan, J.; Matia-Merino, L.; Awati, A.; Omri, A.; Suntres, Z.E.; Singh, H. Recent trends in the lipid-based nanoencapsulation of antioxidants and their role in foods. J. Sci. Food Agric. 2006, 86, 2038–2045. [Google Scholar] [CrossRef]
- Ghadi, Z.S.; Dinarvand, R.; Asemi, N.; Amiri, F.T.; Ebrahimnejad, P. Preparation, characterization and in vivo evaluation of novel hyaluronan containing niosomes tailored by Box-Behnken design to co-encapsulate curcumin and quercetin. Eur. J. Pharm. Sci. 2019, 130, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Bokkenheuser, V.D.; Shackleton, C.; Winter, J. Hydrolysis of dietary flavonoid glycosides by strains of intestinal Bacteroides from humans. Biochem. J. 1987, 248, 953–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogundajo, A.T.; Imoru, J.O.; Asaolu, F. Quercetin potentiates hepatoprotective and antioxidant response to intraperitoneal, intravenous, subcutaneous and oral administration in Wistar rats. Asian J. Biomed. Pharm. Sci. 2014, 4, 57. [Google Scholar]
- Li, D.; Liu, A.; Liu, M.; Li, X.; Guo, H.; Zuo, C.; Li, Y. The intestine-responsive lysozyme nanoparticles-in-oxidized starch microgels with mucoadhesive and penetrating properties for improved epithelium absorption of quercetin. Food Hydrocoll. 2020, 99, 105309. [Google Scholar] [CrossRef]
- Mouhid, L.; Corzo-Martínez, M.; Torres, C.; Vázquez, L.; Reglero, G.; Fornari, T.; Ramírez de Molina, A. Improving in vivo efficacy of bioactive molecules: An overview of potentially antitumor phytochemicals and currently available lipid-based delivery systems. J. Oncol. 2017, 2017, 7351976. [Google Scholar] [CrossRef] [Green Version]
- Roy, P.; Parveen, S.; Ghosh, P.; Ghatak, K.; Dasgupta, S. Flavonoid loaded nanoparticles as an effective measure to combat oxidative stress in Ribonuclease A. Biochimie 2019, 162, 185–197. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Q.; Chu, L.; Xia, Q. Liposome-chitosan hydrogel bead delivery system for the encapsulation of linseed oil and quercetin: Preparation and in vitro characterization studies. LWT 2020, 117, 108615. [Google Scholar] [CrossRef]
- Bagheri, A.; Chu, B.S.; Yaakob, H. Niosomal drug delivery systems: Formulation, preparation and applications. World Appl. Sci. J. 2014, 32, 1671–1685. [Google Scholar]
- Ghafelehbashi, R.; Akbarzadeh, I.; Yaraki, M.T.; Lajevardi, A.; Fatemizadeh, M.; Saremi, L.H. Preparation, physicochemical properties, in vitro evaluation and release behavior of cephalexin-loaded niosomes. Int. J. Pharm. 2019, 569, 118580. [Google Scholar] [CrossRef]
- Moghassemi, S.; Hadjizadeh, A. Nano-niosomes as nanoscale drug delivery systems: An illustrated review. J. Control. Release 2014, 185, 22–36. [Google Scholar] [CrossRef] [PubMed]
- García-Manrique, P.; Machado, N.D.; Fernández, M.A.; Blanco-López, M.C.; Matos, M.; Gutiérrez, G. Effect of drug molecular weight on niosomes size and encapsulation efficiency. Colloids Surf. B Biointerfaces 2020, 186, 110711. [Google Scholar] [CrossRef] [PubMed]
- Nielloud, F. Pharmaceutical Emulsions and Suspensions: Revised and Expanded; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Uchegbu, I.F.; Vyas, S.P. Non-ionic surfactant based vesicles (niosomes) in drug delivery. Int. J. Pharm. 1998, 172, 33–70. [Google Scholar] [CrossRef]
- Kumar, G.P.; Rajeshwarrao, P. Nonionic surfactant vesicular systems for effective drug delivery—An overview. Acta Pharm. Sin. B 2011, 1, 208–219. [Google Scholar] [CrossRef] [Green Version]
- Neta, N.S.; Teixeira, J.A.; Rodrigues, L.R. Sugar ester surfactants: Enzymatic synthesis and applications in food industry. Crit. Rev. Food Sci. Nutr. 2015, 55, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Becerra, N.; Toro, C.; Zanocco, A.L.; Lemp, E.; Günther, G. Characterization of micelles formed by sucrose 6-O-monoesters. Colloids Surf. A Physicochem. Eng. Asp. 2008, 327, 134–139. [Google Scholar] [CrossRef]
- Campana, R.; Merli, A.; Verboni, M.; Biondo, F.; Favi, G.; Duranti, A.; Lucarini, S. Synthesis and Evaluation of Saccharide-Based Aliphatic and Aromatic Esters as Antimicrobial and Antibiofilm Agents. Pharmaceuticals. 2019, 12, 186. [Google Scholar] [CrossRef] [Green Version]
- Sarpe, V.A.; Kulkarni, S.S. Synthesis of maradolipid. J. Org. Chem. 2011, 76, 6866–6870. [Google Scholar] [CrossRef]
- Schiefelbein, L.; Keller, M.; Weissmann, F.; Luber, M.; Bracher, F.; Frieß, W. Synthesis, characterization and assessment of suitability of trehalose fatty acid esters as alternatives for polysorbates in protein formulation. Eur. J. Pharm. Biopharm. 2010, 76, 342–350. [Google Scholar] [CrossRef]
- Stocker, B.L.; Khan, A.A.; Chee, S.H.; Kamena, F.; Timmer, M.S. On One Leg: Trehalose Monoesters Activate Macrophages in a Mincle—Dependent Manner. ChemBioChem 2014, 15, 382–388. [Google Scholar] [CrossRef]
- Griffin, W.C. Calculation of HLB values of non-ionic surfactants. J. Soc. Cosmet. Chem. 1954, 5, 249–256. [Google Scholar]
- El-Gogary, R.I.; Rubio, N.; Wang, J.T.W.; Al-Jamal, W.T.; Bourgognon, M.; Kafa, H.; Naeem, M.; Klippstein, R.; Abbate, V.; Leroux, F.; et al. Polyethylene glycol conjugated polymeric nanocapsules for targeted delivery of quercetin to folate-expressing cancer cells in vitro and in vivo. ACS Nano 2014, 8, 1384–1401. [Google Scholar] [CrossRef] [PubMed]
- Elmowafy, E.; Gad, H.; Biondo, F.; Casettari, L.; Soliman, M.E. Exploring optimized methoxy poly (ethylene glycol)-block-poly (ε-caprolactone) crystalline cored micelles in anti-glaucoma pharmacotherapy. Int. J. Pharm. 2019, 566, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.E.; Elmowafy, E.; Casettari, L.; Alexander, C. Star-shaped poly (oligoethylene glycol) copolymer-based gels: Thermo-responsive behaviour and bioapplicability for risedronate intranasal delivery. Int. J. Pharm. 2018, 543, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Galho, A.R.; Cordeiro, M.F.; Ribeiro, S.A.; Marques, M.S.; Antunes, M.F.D.; Luz, D.C.; Hädrich, G.; Muccillo-Baisch, A.L.; Barros, D.M.; Lima, J.V.; et al. Protective role of free and quercetin-loaded nanoemulsion against damage induced by intracerebral haemorrhage in rats. Nanotechnology 2016, 27, 175101. [Google Scholar] [CrossRef] [PubMed]
- Shaker, S.; Gardouh, A.R.; Ghorab, M.M. Factors affecting liposomes particle size prepared by ethanol injection method. Res. Pharm. Sci. 2017, 12, 346. [Google Scholar] [CrossRef] [PubMed]
- Mali, N.; Darandale, S.; Vavia, P. Niosomes as a vesicular carrier for topical administration of minoxidil: Formulation and in vitro assessment. Drug Deliv. Transl. Res. 2013, 3, 587–592. [Google Scholar] [CrossRef]
- Gugleva, V.; Titeva, S.; Rangelov, S.; Momekova, D. Design and in vitro evaluation of doxycycline hyclate niosomes as a potential ocular delivery system. Int. J. Pharm. 2019, 567, 118431. [Google Scholar] [CrossRef]
- Kulkarni, P.; Rawtani, D. Application of Box-Behnken Design in the Preparation, Optimization, and In Vitro Evaluation of Self-assembly–based Tamoxifen-and Doxorubicin-loaded and Dual Drug–loaded Niosomes for Combinatorial Breast Cancer Treatment. J. Pharm. Sci. 2019, 108, 2643–2653. [Google Scholar] [CrossRef]
- Gilani, S.J.; Imam, S.S.; Ahmed, A.; Chauhan, S.; Mirza, M.A.; Taleuzzaman, M. Formulation and evaluation of thymoquinone niosomes: Application of developed and validated RP-HPLC method in delivery system. Drug Dev. Ind. Pharm. 2019, 45, 1799–1806. [Google Scholar] [CrossRef]
- Kulkarni, P.; Rawtani, D.; Barot, T. Formulation and optimization of long acting dual niosomes using box-Behnken experimental design method for combinative delivery of ethionamide and D-cycloserine in tuberculosis treatment. Colloids Surf. A Physicochem. Eng. Asp. 2019, 565, 131–142. [Google Scholar] [CrossRef]
- Sezgin-Bayindir, Z.; Antep, M.N.; Yuksel, N. Development and characterization of mixed niosomes for oral delivery using candesartan cilexetil as a model poorly water-soluble drug. AAPS PharmSciTech 2015, 16, 108–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savic, I.M.; Nikolic, V.D.; Savic-Gajic, I.; Nikolic, L.B.; Radovanovic, B.C.; Mladenovic, J.D. Investigation of properties and structural characterization of the quercetin inclusion complex with (2-hydroxypropyl)-β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2015, 82, 383–394. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Tang, K.; Hu, X.; Zou, G. Physicochemical characterization and antioxidant activity of quercetin-loaded chitosan nanoparticles. J. Appl. Polym. Sci. 2008, 107, 891–897. [Google Scholar] [CrossRef]
- Milanezi, F.G.; Meireles, L.M.; de Christo Scherer, M.M.; de Oliveira, J.P.; da Silva, A.R.; de Araujo, M.L.; Endringer, D.C.; Fronza, M.; Guimarães, M.C.C.; Scherer, R.; et al. Antioxidant, antimicrobial and cytotoxic activities of gold nanoparticles capped with quercetin. Saudi Pharm. J. 2019, 27, 968–974. [Google Scholar] [CrossRef]
- Anbarasan, B.; Rekha, S.; Elango, K.; Shriya, B.; Ramaprabhu, S. Optimization of the formulation and in-vitro evaluation of Capecitabine Niosomes for the treatment of Colon Cancer. Int. J. Pharm. Sci. Res. 2013, 4, 1504. [Google Scholar]
- Gupta, U.; Singh, V.K.; Kumar, V.; Khajuria, Y. Spectroscopic studies of cholesterol: Fourier transform infra-red and vibrational frequency analysis. Mater. Focus 2014, 3, 211–217. [Google Scholar] [CrossRef]
- Purwono, B. Chemical Synthesis of Monosaccharide Lauric Acid Esters as Antibacterial and Antifungal Agents in Materials Science Forum; Trans Tech Publication: Bäch, Switzerland, 2019. [Google Scholar]
- Khan, M.I.; Madni, A.; Ahmad, S.; Khan, A.; Rehmanand, M.; Mahmood, M.A. ATR-FTIR Based Pre and Post Formulation Compatibility Studies for the Design of Niosomal Drug Delivery System Containing Nonionic Amphiphiles and Chondroprotective Drug. J. Chem. Soc. Pak. 2015, 37, 527–534. [Google Scholar]
- Li, D. Development and Evaluation of a Niosome Carrier for Topical Use of Antioxidant; ResearchSpace@ Auckland: Auckland, New Zealand, 2014. [Google Scholar]
- Shilakari Asthana, G.; PSharma, K.; Asthana, A. In vitro and in vivo evaluation of niosomal formulation for controlled delivery of clarithromycin. Scientifica 2016, 2016, 6492953. [Google Scholar] [CrossRef] [Green Version]
- Ghani, N.A.A.; Channip, A.A.; Chok Hwee Hwa, P.; Ja’afar, F.; Yasin, H.M.; Usman, A. Physicochemical properties, antioxidant capacities, and metal contents of virgin coconut oil produced by wet and dry processes. Food Sci. Nutr. 2018, 6, 1298–1306. [Google Scholar] [CrossRef]
- Sobeh, M.; Esmat, A.; Petruk, G.; Abdelfattah, M.A.; Dmirieh, M.; Monti, D.M.; Abdel-Naim, A.B.; Wink, M. Phenolic compounds from Syzygium jambos (Myrtaceae) exhibit distinct antioxidant and hepatoprotective activities in vivo. J. Funct. Foods 2018, 41, 223–231. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.Q.; Shi, L.; Xu, X.N.; Huang, S.C.; Lu, B.; Ji, L.L.; Wang, Z.T. Therapeutic detoxification of quercetin against carbon tetrachloride-induced acute liver injury in mice and its mechanism. J. Zhejiang Univ. Sci. B 2014, 15, 1039–1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livingstone, C.; Davis, J. Targeting therapeutics against glutathione depletion in diabetes and its complications. Br. J. Diabetes Vasc. Dis. 2007, 7, 258–265. [Google Scholar] [CrossRef]
- Recknagel, R.O.; Glende, E.A., Jr.; Dolak, J.A.; Waller, R.L. Mechanisms of carbon tetrachloride toxicity. Pharmacol. Ther. 1989, 43, 139–154. [Google Scholar] [CrossRef]
- Oyenihi, O.R.; Brooks, N.L.; Oguntibeju, O.O. Effects of kolaviron on hepatic oxidative stress in streptozotocin induced diabetes. BMC Complementary Altern. Med. 2015, 15, 236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fouad, D.; Badr, A.; Attia, H.A. Hepatoprotective activity of raspberry ketone is mediated via inhibition of the NF-κB/TNF-α/caspase axis and mitochondrial apoptosis in chemically induced acute liver injury. Toxicol. Res. 2019, 8, 663–676. [Google Scholar] [CrossRef] [PubMed]








| Surfactant (C12 Chain) | HLB a | Mw |
|---|---|---|
| Sorbitan monolaurate (Span® 20) | 8.6 | 346.5 |
| Glucose monolaurate | 9.89 | 362.5 |
| Sucrose monolaurate | 13.01 | 524.6 |
| Trehalose monolaurate | 13.01 | 524.6 |
| Formula Code | Surfactant Type | Surfactant: Cholesterol Ratio | PS (nm) | PDI | ZP (mV) | EE% |
|---|---|---|---|---|---|---|
| F1 | Sorbitan C12 (Span® 20) | 1:1 | 177.4 ± 1.89 | 0.19 ± 0.07 | −26.23 ± 1.56 | 80.85 ± 2.00 |
| F2 | Glucose C12 | 1:1 | 161.0 ± 4.61 | 0.09 ± 0.01 | −28.75 ± 0.07 | 83.59 ± 3.66 |
| F3 | Sucrose C12 | 1:1 | 179.0 ± 2.99 | 0.24 ± 0.02 | −32.2 ± 0.42 | 54.60 ± 2.72 |
| F4 | Trehalose C12 | 1:1 | 174.1 ± 9.28 | 0.25 ± 0.03 | −25.25 ± 0.07 | 62.13 ± 2.64 |
| F5 | Sorbitan C12 (Span® 20) | 2:1 | 188.9 ± 3.61 | 0.11 ± 0.03 | −27.54 ± 2.35 | 55.44 ± 1.30 |
| F6 | Glucose C12 | 2:1 | 217.4 ± 3.52 | 0.12 ± 0.08 | −25.3 ± 3.53 | 61.26 ± 2.21 |
| F7 | Sucrose C12 | 2:1 | 224.4 ± 4.38 | 0.34 ± 0.04 | −35.25 ± 1.20 | 56.15 ± 1.49 |
| F8 | Trehalose C12 | 2:1 | 222.9 ± 8.74 | 0.26 ± 0.02 | −33.05 ± 1.22 | 62.14 ± 5.63 |
| Normal Untreated Control | Positive CCl4 Treated Control | Free Quercetin | Quercetin Niosomes | ||
|---|---|---|---|---|---|
| Serum biomarker enzymes | ALT (IU/L) | 37.5 ± 1.5 | 164.78 * ± 5.19 | 94.93 ± 7.35 | 63.80 *,+ ± 7.52 |
| AST (IU/L) | 34.07 ± 0.76 | 136.52 * ± 1.23 | 85.02 ± 2.04 | 69.49 *,+ ± 6.02 | |
| Serum biochemical parameters | ALP (IU/L) | 118.38 ± 3.13 | 191.37 * ± 3.5 | 138.24 ± 2.68 | 128.57 *,+ ± 4.29 |
| Total protein (g/dL) | 6.84 ± 0.07 | 7.63 * ± 0.27 | 6.59 ± 0.15 | 6.71 *,+ ± 0.06 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmowafy, E.; El-Derany, M.O.; Biondo, F.; Tiboni, M.; Casettari, L.; Soliman, M.E. Quercetin Loaded Monolaurate Sugar Esters-Based Niosomes: Sustained Release and Mutual Antioxidant—Hepatoprotective Interplay. Pharmaceutics 2020, 12, 143. https://doi.org/10.3390/pharmaceutics12020143
Elmowafy E, El-Derany MO, Biondo F, Tiboni M, Casettari L, Soliman ME. Quercetin Loaded Monolaurate Sugar Esters-Based Niosomes: Sustained Release and Mutual Antioxidant—Hepatoprotective Interplay. Pharmaceutics. 2020; 12(2):143. https://doi.org/10.3390/pharmaceutics12020143
Chicago/Turabian StyleElmowafy, Enas, Marwa O. El-Derany, Francesca Biondo, Mattia Tiboni, Luca Casettari, and Mahmoud E. Soliman. 2020. "Quercetin Loaded Monolaurate Sugar Esters-Based Niosomes: Sustained Release and Mutual Antioxidant—Hepatoprotective Interplay" Pharmaceutics 12, no. 2: 143. https://doi.org/10.3390/pharmaceutics12020143