Development of Novel Silica-Based Formulation of α-Lipoic Acid: Evaluation of Photo and Thermal Stability of the Encapsulated Drug
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Syntheses of LA Composites with Different Silica Materials
2.2.1. Sol-Gel Synthesis of Composites of LA with Unmodified Silica (UMS) at pH 3 and 7
2.2.2. Sol-Gel Synthesis of Composites of LA with Mercaptopropyl (MPMS), Aminopropyl (APMS) and Methyl (MMS) Modified Silica Materials at pH 3 and 7
2.2.3. Sol-Gel Synthesis of Silica Matrixes (UMS, MMS, MPMS and APMS) at pH 3 and 7
2.3. Photodegradation Experiment and Analysis of Obtained Data
2.4. Thermal Degradation Experiment
2.5. Fourier Transform Infrared (FTIR) Spectra
2.6. Zeta Potential Measurements of Synthesized Silica Matrixes
2.7. Scanning Electron Microscopy
2.8. Statistics
3. Results
3.1. FTIR Spectroscopy
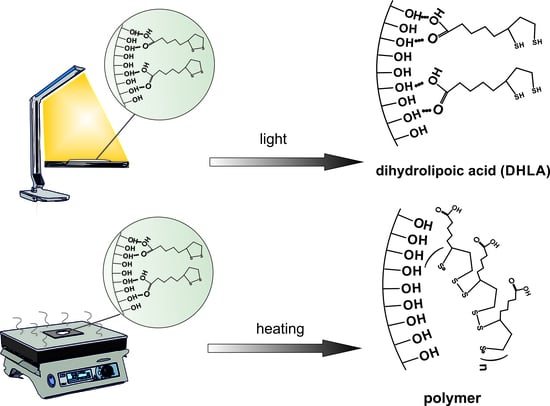
3.2. Study of Photodegradation of LA in the Composites
3.3. Study of Thermal Degradation of LA in the Composites
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maleki, A.; Kettiger, H.; Schoubben, A.; Rosenholm, J.M.; Ambrogi, V.; Hamidi, M. Mesoporous silica materials: From physico-chemical properties to enhanced dissolution of poorly water-soluble drugs. J. Control Release 2017, 262, 329–347. [Google Scholar] [CrossRef] [PubMed]
- Meka, A.K.; Jenkins, L.J.; Dàvalos-Salas, M.; Pujara, N.; Wong, K.Y.; Kumeria, T.; Mariadason, J.M.; Popat, A. Enhanced solubility, permeability and anticancer activity of vorinostat using tailored mesoporous silica nanoparticles. Pharmaceutics 2018, 10, 283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Zheng, K.; Zhang, Z.; Shi, W.; Jing, S.; Wang, L.; Zheng, W.; Zhao, D.; Xu, J.; Zhang, P. Adsorption and protection of plasmid DNA on mesoporous silica nanoparticles modified with various amounts of organosilane. J. Colloid Interface Sci. 2012, 369, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Berlier, G.; Gastaldi, L.; Sapino, S.; Miletto, I.; Bottinelli, E.; Chirio, D.; Ugazio, E. MCM-41 as a useful vector for rutin topical formulations: Synthesis, characterization and testing. Int. J. Pharm. 2013, 457, 177–186. [Google Scholar] [CrossRef]
- Ruiz-Rico, M.; Daubenschüz, H.; Pérez-Esteve, E.; Marcos, M.D.; Amorós, P.; Martínez-Máñez, R.; Barat, J.M. Protective effect of mesoporous silica particles on encapsulated folates. Eur. J. Pharm. Biopharm. 2016, 105, 9–17. [Google Scholar] [CrossRef]
- Czuryszkiewicz, T.; Areva, S.; Honkanen, M.; Lindén, M. Synthesis of sol-gel silica materials providing a slow release of biphosphonate. Colloid Surf. A Physicochem. Eng. 2005, 254, 69–74. [Google Scholar] [CrossRef]
- Albarran, L.; López, T.; Quintana, P.; Chagoya, V. Controlled release of IFC-305 encapsulated in silica nanoparticles for liver cancer synthesized by sol-gel. Colloid Surf. A Phys. Eng. 2011, 384, 131–136. [Google Scholar] [CrossRef]
- Parfenyuk, E.V.; Dolinina, E.S. Development of novel delivery system for cardiovascular drug molsidomine: Influence of synthesis method and conditions on molsidomine release from its composites with hydrophilic silica in vitro. J. Pharm. Sci. 2016, 105, 1952–1959. [Google Scholar]
- Szewczyk, A.; Prokopowicz, M.; Sawicki, W.; Majda, D.; Walker, G. Aminopropyl-functionalized mesoporous silica SBA-15 as drug carrier for cefazolin: Adsorption profiles, release studies, and mineralization potential. Micropor. Mesopor. Mater. 2019, 274, 113–126. [Google Scholar] [CrossRef]
- Gomes, M.B.; Negrato, C.A. Alpha-lipoic acid as a pleiotropic compound with potential therapeutic use in diabetes and other chronic diseases. Diabetol. Metab. Syndr. 2014, 6, 80. [Google Scholar] [CrossRef] [Green Version]
- Thioctic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/864 (accessed on 15 February 2020).
- Perricone, N.V. Topical 5% alpha lipoic acid cream in the treatment of cutaneous rhytids. Aesthet. Surg. J. 2000, 20, 218–222. [Google Scholar] [CrossRef] [Green Version]
- Leysen, J.; Aerts, O. Further evidence of thioctic acid (α-lipoic acid) being a strong cosmetics sensitizer. Contact Dermat. 2016, 74, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Matsugo, S.; Han, D.; Tritschler, H.J.; Packer, L. Decomposition of α-lipoic acid derivatives by photoirradaition—Formation of dihydrolipoic acid from α-lipoic acid. Biochem. Mol. Biol. Int. 1996, 38, 51–59. [Google Scholar] [PubMed]
- Wada, N.; Wakami, H.; Konishi, T.; Matsugo, S. The degradation and regeneration of α-lipoic acid under the irradiation of UV light in the existence of homocysteine. J. Clin. Biochem. Nutr. 2009, 44, 218–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsugo, S.; Bito, T.; Konishi, T. Photochemical stability of lipoic acid and its impact on skin ageing. Free Radic. Res. 2011, 45, 918–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, P.R.; Edwards, J.O. Effect of solvent on the photolysis of α-lipoic acid. J. Org. Chem. 1969, 34, 3131–3134. [Google Scholar] [CrossRef]
- Wagner, A.F.; Walton, E.; Boxer, G.E.; Pruss, M.P.; Holly, F.W.; Folkers, K. Properties and derivatives of a-lipoic acid. J. Am. Chem. Soc. 1956, 78, 5079–5081. [Google Scholar] [CrossRef]
- Thomas, R.C.; Reed, L.J. Disulfide polymers of DL-α-lipoic acid. J. Am. Chem. Soc. 1956, 78, 6148–6149. [Google Scholar] [CrossRef]
- Kisanuki, A.; Kimpara, Y.; Oikado, Y.; Kado, N.; Matsumoto, M.; Endo, K. Ring-opening polymerization of lipoic acid and characterization of the polymer. J. Polym. Sci. A 2010, 48, 5247–5253. [Google Scholar] [CrossRef]
- Park, C.H.; Lee, J. PH-dependent sustained release characteristics of disulfide polymers prepared by simple thermal polymerization. J. Biomater. Sci. Polym. Ed. 2013, 24, 1848–1857. [Google Scholar] [CrossRef]
- Berlier, G.; Gastaldi, L.; Ugazio, E.; Miletto, I.; Iliade, P.; Sapino, S. Stabilization of quercetin flavonoid in MCM-41 mesoporous silica: Positive effect of surface functionalization. J. Colloid Interface Sci. 2013, 393, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Sapino, S.; Ugazio, E.; Gastaldi, L.; Miletto, I.; Berlier, G.; Zonari, D.; Oliaro-Bosso, S. Mesoporous silica as topical nanocarriers for quercetin: Characterization and in vitro studies. Eur. J. Pharm. Biopharm. 2015, 89, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yan, K.; Hu, C.; Zhao, Y.; Chen, Z.; Zhu, X.; Möller, M. Encapsulation of enzymes in silica nanocapsules formed by an amphiphilic precursor polymer in water. J. Mater. Chem. B 2015, 3, 1261–1267. [Google Scholar] [CrossRef]
- Takahashi, H.; Bungo, Y.; Mikuni, K. The aqueous solubility and thermal stability of α-lipoic acid are enhanced by cyclodextrin. Biosci. Biotechnol. Biochem. 2011, 75, 633–637. [Google Scholar] [CrossRef]
- Li, Y.-X.; Park, E.Y.; Lim, S.-T. Stabilization of alpha-lipoic acid by complex formation with octenylsuccinylated high amylose starch. Food Chem. 2018, 242, 389–394. [Google Scholar] [CrossRef]
- Kofuji, K.; Isobe, T.; Murata, Y. Controlled release of alpha-lipoic acid through incorporation into natural polysaccharide-based gel beads. Food Chem. 2009, 115, 483–487. [Google Scholar] [CrossRef]
- Wang, J.; Tang, J.; Zhou, X.; Xia, Q. Physicochemical characterization, identification and improved photo-stability of alpha-lipoic acid-loaded nanostructured lipid carrier. Drug Dev. Ind. Pharm. 2014, 40, 201–210. [Google Scholar] [CrossRef]
- Substances Added to Food (Formerly EAFUS). Available online: https://www.accessdata.fda.gov/scripts/fdcc/?set=FoodSubstances (accessed on 15 February 2020).
- Jang, D.-J.; Jeong, E.J.; Lee, H.-M.; Kim, B.-C.; Lim, C.-J.; Kim, C.-K. Improvement of bioavailability and photostability of amlodipine using redispersible dry emulsion. Eur. J. Pharm. Sci. 2006, 28, 405–411. [Google Scholar] [CrossRef]
- Raza, K.; Singh, B.; Lohan, S.; Sharma, G.; Negi, P.; Yachha, Y.; Katare, O.P. Nano-lipoidal carriers of tretinoin with enhanced percutaneous absorption, photostability, biocompatibility and anti-psoriatic activity. Int. J. Pharm. 2013, 456, 65–72. [Google Scholar] [CrossRef]
- Coelho, L.; Almeida, I.F.; Sousa Lobo, J.M.; Sousa Silva, J.P. Photostabilization strategies of photosensitive drugs. Int. J. Pharm. 2018, 541, 19–25. [Google Scholar] [CrossRef]
- Ahmad, I.; Arsalan, A.; Ali, S.A.; Bano, R.; Munir, I.; Sahar, A. Formulation and stabilization of norfloxacin in liposomal preparations. Eur. J. Pharm. Sci. 2016, 91, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.G.; Detoni, C.B.; Lima da Silva, T.; Colome, L.M.; Pohlmann, A.R.; Guterres, S.S. α-Tocopherol acetate-loaded chitosan microparticles: Stability during spray drying process, photostability and swelling evaluation. J. Drug Deliv. Sci. Technol. 2015, 30, 220–222. [Google Scholar] [CrossRef]
- Cappello, B.; Maio, C.D.; Iervolono, M.; Miro, A. Improvement of solubility and stability of valsartan by hydroxypropyl-β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2006, 54, 289–294. [Google Scholar] [CrossRef]
- Akimsheva, E.Y.; Dolinina, E.S.; Parfenyuk, E.V. Interactions of sol-gel encapsulated acyclovir with silica matrix. Colloid Surf. B Biointerfaces 2019, 178, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Morais, E.C.; Correa, G.G.; Brambilla, R.; Radtke, C.; Baibich, I.M.; dos Santos, J.H.Z. The interaction of encapsulated pharmaceutical drugs with a silica matrix. Colloids Surf. B Biointerfaces 2013, 103, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Al-Oweini, R.; El-Rassy, H. Synthesis and characterization by FTIR spectroscopy of silica aerogels prepared using several Si(OR)4 and R’’ Si(OR’ )3 precursors. J. Mol. Struct. 2009, 919, 140–145. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Bassler, G.C.; Morrill, T.C. Spectrometric Identification of Organic Compounds, 3rd ed.; John Willey and Sons, Inc.: New York, NY, USA, 1974. [Google Scholar]
- Ryu, I.S.; Liu, X.; Jin, Y.; Sun, J.; Lee, Y.J. Stoichiometric analysis of competing intermolecular hydrogen bonds using infrared spectroscopy. RSC Adv. 2018, 8, 23481. [Google Scholar] [CrossRef] [Green Version]
- Basyuk, V.A. Infrared spectra of carboxylic compounds on silica surfaces at 1500–1800 cm−1. J. Appl. Spectrosc. 1994, 60, 29–33. [Google Scholar] [CrossRef]
- Larkin, P.J.I.R.; Spectroscopy, R. Principles and Spectral Interpretation; Elsevier: San Diego, CA, USA, 2011. [Google Scholar]
- Max, J.-J.; Chapados, C. Infrared spectroscopy of aqueous carboxylic acids: Comparison between different acids and their salts. J. Phys. Chem. A 2004, 108, 3324–3337. [Google Scholar] [CrossRef]
- Sun, L.; Crooks, R.M. Molecular interactions between organized, surface-confined monolayers and vapor-phase probe molecules. 5. Acid-base interactions. Langmuir 1993, 9, 1775–1780. [Google Scholar] [CrossRef]
- Nikolić, R.S.; Krstić, N.S.; Nikolić, G.M.; Kocić, G.M.; Cakić, M.D.; Andelković, D.H. Molecular mechanisms of beneficial effects of lipoic acid in copper intoxicated rats assessment by FTIR and ESI-MS. Polyhedron 2014, 80, 223–227. [Google Scholar] [CrossRef]
- Morita, S. Hydrogen-bonds structure in poly(2-hydroxyethyl methacrylate) studied by temperature-dependent infrared spectroscopy. Front. Chem. 2014, 2, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, M.; Stievano, L.; Lambert, J.F. Adsorption and thermal condensation mechanisms of amino acids on oxide supports. 1. Glycine on silica. Langmuir 2004, 20, 914–923. [Google Scholar] [CrossRef]
- Ioele, F.; Oliverio, I.; Andreu, M.; De Luca, M.A.; Miranda, G.R. Different photodegradation behavior of barnidipine under natural and forced irradiation. J. Photochem. Photobiol. A Chem. 2010, 215, 205–213. [Google Scholar] [CrossRef]
- Godlewska, M.; Odachowska, A.; Turkowicz, M.; Karpinska, J. Analysis of reaction between α-lipoic acid and 2-chloro-1-methylquinolinium tetrafluoroborate used as a precolumn derivatization technique in chromatographic determination of α-lipoic acid. J. Anal. Methods Chem. 2015, 2015, 535387. [Google Scholar] [CrossRef] [Green Version]
- Onoue, S.; Uchida, A.; Kuriyama, K.; Nakamura, T.; Seto, Y.; Kato, M.; Hatanaka, J.; Tanaka, T.; Miyoshi, H.; Yamada, S. Novel solid self-emulsifying drug delivery system of coenzyme Q10 with improved photochemical and pharmacokinetic behaviors. Eur. J. Pharm. Sci. 2012, 46, 492–499. [Google Scholar] [CrossRef]
- Carey, F.A. Organic Chemistry, 6th ed.; McGraw-Hill: New York, NY, USA, 2005. [Google Scholar]









| Designation of Samples | Precursor | Synthesis pH | Drug Loading, mg/g |
|---|---|---|---|
| LA-UMS (pH 3) | TEOS | 3 | 60 |
| LA-UMS (pH 7) | TEOS | 7 | 57 |
| LA-MPMS (pH 3) | TEOS + MPTMOS | 3 | 59 |
| LA-MPMS (pH 7) | TEOS + MPTMOS | 7 | 58 |
| LA-APMS (pH 3) | TEOS + APTEOS | 3 | 46 |
| LA-APMS (pH 7) | TEOS + APTEOS | 7 | 56 |
| LA-MMS (pH 3) | TEOS + MTMOS | 3 | 60 |
| LA-MMS (pH 7) | TEOS + MTMOS | 7 | 61 |
| Band, cm−1 | Assignment | References |
|---|---|---|
| 2928, 2856, 2844 | asymmetric and symmetric ν (C–H) | [38,39] |
| 1770 | ν(C=O) | [39,40,41] |
| 1697 | ν(C=O) | [39,42,43] |
| 1467 | δ(CH2) scissoring | [38,39,43] |
| 1428, 1407 | [39,44] | |
| ν(C–O)/δ(OH) out-of-plane | [42,44] | |
| 1306 | δ(C–H) | [39,42] |
| 1250 | ν(C–O)/δ(OH) out-of-plane | [39,42,43] |
| 1202 | [39] | |
| 1078 | [39,42,43] | |
| 946, 932 | δ(O–H) out-of-plane | [39,42,45] |
| 734 | δ(C–H) | [38,39] |
| 674 | ν(C–S) | [39,45] |
| 521, 496, 449 | ν(S–S) | [39,45] |
| Zeta-Potentials, mV | ||
|---|---|---|
| Silica Matrix | pH 3 | pH 7 |
| UMS (pH 3) | −0.68 | - |
| UMS (pH 7) | - | −38.0 |
| MMS (pH 3) | −4,78 | - |
| MMS (pH 7) | - | −32.0 |
| APMS (pH 3) | 28.9 | - |
| APMS (pH 7) | - | 30.8 |
| MPMS (pH 3) | −3.28 | - |
| MPMS (pH 7) | - | −48.5 |
| Composite | Zero-Order Model | First-Order Model | Second-Order Model |
|---|---|---|---|
| LA | k0 = 7.30 h−1; t0.5 = 6.9 h R2 = 0.8891 | k1 = 0.09 h−1; t0.5 = 7.6 h R2 = 0.9338 | k2 = 1.1 × 10−3 h−1; t0.5 = 9.1 h R2 = 0.9793 |
| LA-UMS (pH 3) | k0 = 8.30 h−1; t0.5 = 6.0 h R2 = 0.9030 | k1 = 0.01 h−1; t0.5 = 6.8 h R2 = 0.9393 | k2 = 1.3 × 10−3 h−1; t0.5 = 7.7 h R2 = 0.9844 |
| LA-UMS (pH 7) | k0 = 7.45 h−1; t0.5 = 6.8 h R2 = 0.9396 | k1 = 0.09 h−1; t0.5 = 7.8 h R2 = 0.9554 | k2 = 0.9 × 10−3 h−1; t0.5 = 9.1 h R2 = 0.9776 |
| LA-MMS (pH 3) | k0 = 8.42 h−1; t0.5 = 5.9 h R2 = 0.9153 | k1 = 0.11 h−1; t0.5 = 6.3 h R2 = 0.9497 | k2 = 38 × 10−3 h−1; t0.5 = 2.6 h R2 = 0.9871 |
| LA-MMS (pH 7) | k0 = 14.6 h−1; t0.5 = 3.4 h R2 = 0.8645 | k1 = 0.23 h−1; t0.5 = 3.0 h R2 = 0.9767 | k2 = 1.4 × 10−3 h−1; t0.5 = 7.1 h R2 = 0.9740 |
| LA-MPMS (pH 3) | k0 = 8.25 h−1; t0.5 = 6.0 h R2 = 0.9322 | k1 = 0.11 h−1; t0.5 = 3.0 h R2 = 0.9767 | k2 = 1.4 × 10−3 h−1; t0.5 = 7.1 h R2 = 0.9730 |
| LA-MPMS (pH 7) | k0 = 7.36 h−1; t0.5 = 6.8 h R2 = 0.9332 | k1 = 0.09 h−1; t0.5 = 7.9 h R2 = 0.9451 | k2 = 0.9 × 10−3 h−1; t0.5 = 9.0 h R2 = 0.9745 |
| LA-APMS (pH 3) | k0 = 16.72 h−1; t0.5 = 2.9 h R2 = 0.5972 | k1 = 0.53 h−1; t0.5 = 1.3 h R2 = 0.8339 | k2 = 24 × 10−3 h−1; t0.5 = 0.4 h R2 = 0.9753 |
| LA-APMS (pH 7) | k0 = 15.22 h−1; t0.5 = 3.3 h R2 = 0.4551 | k1 = 0.67 h−1; t0.5 = 1.1 h R2 = 0.7860 | k2 = 57 × 10−3 h−1; t0.5 = 0.2 h R2 = 0.9751 |
| Composite | First Stage | Second Stage | ||||||
|---|---|---|---|---|---|---|---|---|
| k1, h−1 | R2 | t1, h | Q1, % | k2, h−1 | R2 | Q2, % | t2, h | |
| LA-UMS (pH 3) | 133.4 | 0.9303 | 0.21 | 74.9 | 4.2 | 0.9690 | 58.2 | 3.79 |
| LA-UMS (pH 7) | 20.5 | 0.9708 | 0.41 | 91.4 | 4.0 | 0.9656 | 77.5 | 3.59 |
| LA-MMS (pH 3) | 101.1 | 0.9398 | 0.41 | 60.6 | 5.6 | 0.9688 | 42.6 | 3.59 |
| LA-MMS (pH 7) | 33.2 | 0.9701 | 0.33 | 89.4 | 1.2 | 0.9441 | 85.6 | 3.67 |
| LA-APMS (pH 3) | 73.4 | 0.9730 | 1.05 | 33.5 | 4.4 | 0.9516 | 19.3 | 2.95 |
| LA-APMS (pH 7) | 127.6 | 0.9213 | 0.32 | 61.8 | 6.7 | 0.9690 | 39.8 | 3.68 |
| LA-MPMS (pH 3) | 74.2 | 0.9806 | 0.67 | 49.9 | 4.9 | 0.9140 | 35.1 | 3.33 |
| LA-MPMS (pH 7) | 59.3 | 0.9206 | 0.50 | 72.7 | 2.4 | 0.9333 | 64.0 | 3.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolinina, E.S.; Akimsheva, E.Y.; Parfenyuk, E.V. Development of Novel Silica-Based Formulation of α-Lipoic Acid: Evaluation of Photo and Thermal Stability of the Encapsulated Drug. Pharmaceutics 2020, 12, 228. https://doi.org/10.3390/pharmaceutics12030228
Dolinina ES, Akimsheva EY, Parfenyuk EV. Development of Novel Silica-Based Formulation of α-Lipoic Acid: Evaluation of Photo and Thermal Stability of the Encapsulated Drug. Pharmaceutics. 2020; 12(3):228. https://doi.org/10.3390/pharmaceutics12030228
Chicago/Turabian StyleDolinina, Ekaterina S., Elizaveta Yu. Akimsheva, and Elena V. Parfenyuk. 2020. "Development of Novel Silica-Based Formulation of α-Lipoic Acid: Evaluation of Photo and Thermal Stability of the Encapsulated Drug" Pharmaceutics 12, no. 3: 228. https://doi.org/10.3390/pharmaceutics12030228
APA StyleDolinina, E. S., Akimsheva, E. Y., & Parfenyuk, E. V. (2020). Development of Novel Silica-Based Formulation of α-Lipoic Acid: Evaluation of Photo and Thermal Stability of the Encapsulated Drug. Pharmaceutics, 12(3), 228. https://doi.org/10.3390/pharmaceutics12030228