Cannabinoids, Blood–Brain Barrier, and Brain Disposition
Abstract
:1. Introduction
2. Methods: Search Strategy and Data Selection
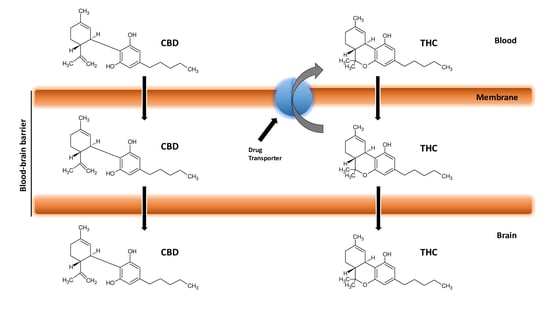
3. Blood–Brain Barrier
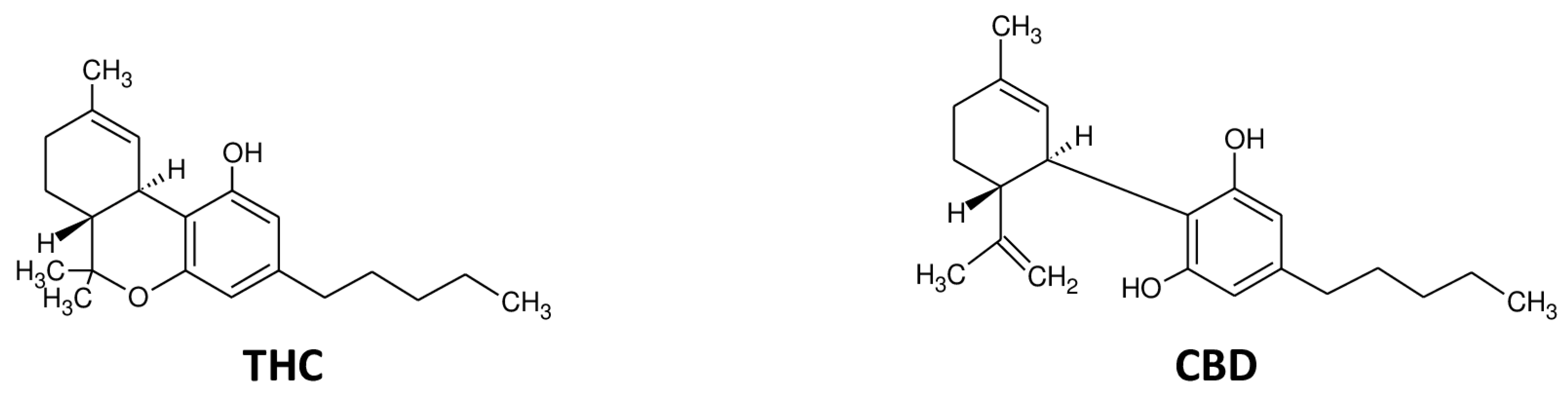
4. Cannabinoids and Blood–Brain Barrier
5. Cannabidiol and Diseases Involving Blood–Brain Barrier Breakdown
6. Cannabinoid Receptors and Blood–Brain Barrier
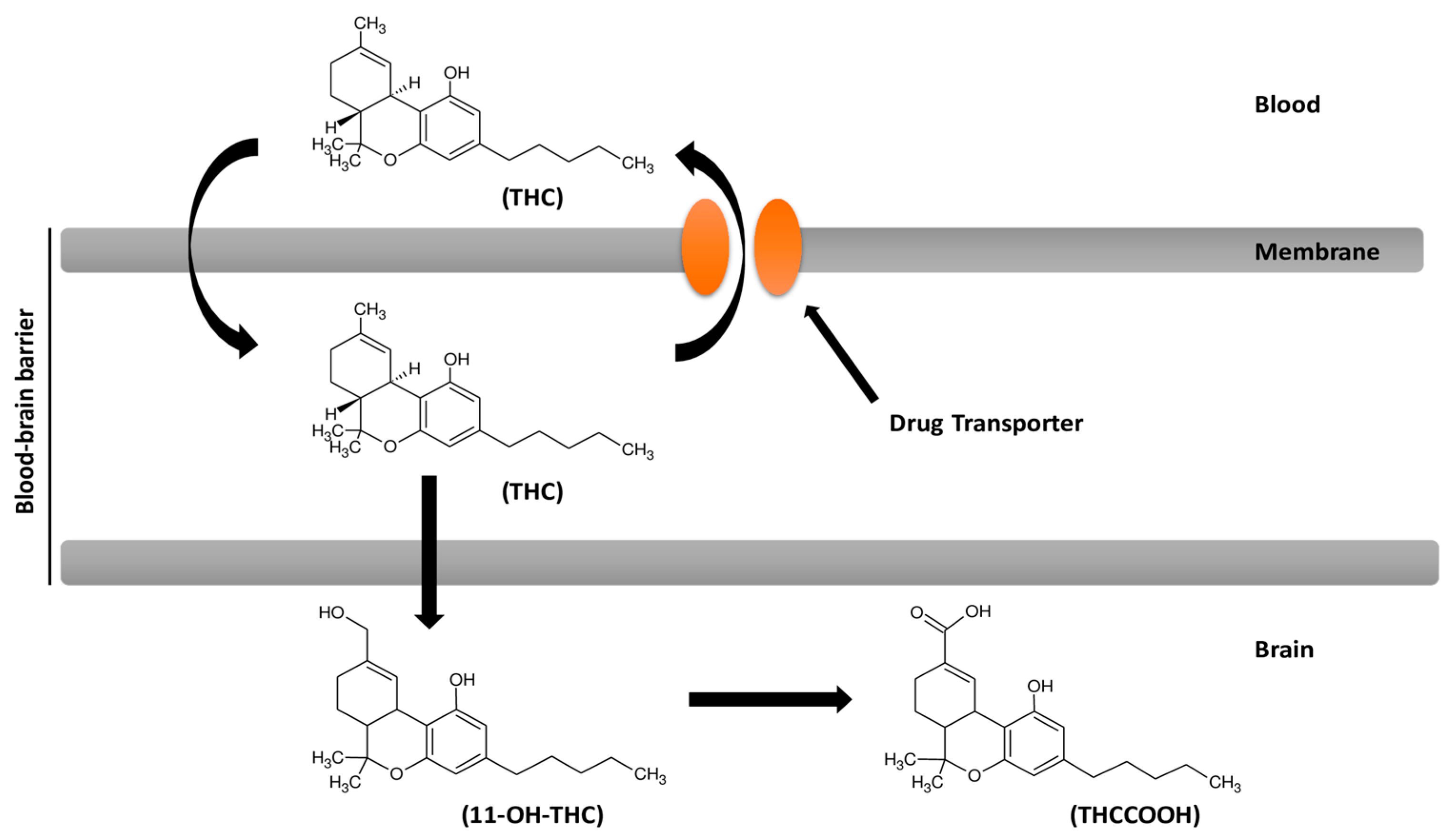
7. Cannabinoids Pharmacokinetics and their Delivery in the Brain
8. Cannabinoids and Efflux Pumps in the Blood–Brain Barrier
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ATPase | adenosinetriphosphatase |
| ABC | adenosine triphosphate-binding cassette |
| BBB | blood–brain barrier |
| BMEC | brain microvascular endothelial cells |
| Bcrp | breast cancer resistance protein |
| CBC | cannabichromene |
| CBD | cannabidiol |
| CB1R | cannabinoid receptor 1 |
| CB2R | cannabinoid receptor 2 |
| CCL2 | C-C motif chemokine ligand 2 |
| CCL5 | C-C motif chemokine ligand 5 |
| CNS | central nervous system |
| GPCR | G protein-coupled receptor |
| HIV | human immunodeficiency virus |
| ICAM-1 | intercellular adhesion molecule-1 |
| i.p. | intraperitoneally |
| LNCs | lipid nanocapsules |
| LPS | lipopolysaccharide |
| MDR1 | Multi-Drug Resistance 1 |
| Mg-ATPase | Magnesium-ATPase |
| P-gp | P-glycoprotein |
| PPARγ | peroxisome proliferator-activated receptor gamma |
| Na-ATPase | Sodium-ATPase |
| THC | delta-9-tetrahydrocannabinol |
| 11-OH-THC | 11-hydroxy-delta-9-THC |
| THCCOOH | 11-nor-9-carboxy-delta-9-THC |
| TRPV1 | transient receptor potential subfamily V member 1 |
| 5HT1A | serotonin 1A receptor |
| VCAM-1 | vascular cell adhesion molecule-1 |
References
- Vučković, S.; Srebro, D.; Vujović, K.S.; Vučetić, Č.; Prostran, M. Cannabinoids and pain: New insights from old molecules. Front. Pharmacol. 2018, 9, 1259. [Google Scholar] [CrossRef] [Green Version]
- Milando, R.; Friedman, A. Cannabinoids: Potential role in inflammatory and neoplastic skin diseases. Am. J. Clin. Dermatol. 2019, 20, 167–180. [Google Scholar] [CrossRef]
- Hanus, L.O.; Meyer, S.M.; Munoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A unified critical inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef] [Green Version]
- Alexander, S.P. Therapeutic potential of cannabis-related drugs. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 64, 157–166. [Google Scholar] [CrossRef]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; Di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S.; et al. Cannabinoids for medical use: A systematic review and meta-analysis. JAMA 2015, 313, 2456–2473. [Google Scholar] [CrossRef]
- Freeman, A.M.; Petrilli, K.; Lees, R.; Hindocha, C.; Mokrysz, C.; Curran, H.V.; Saunders, R.; Freeman, T.P. How does cannabidiol (CBD) influence the acute effects of delta-9-tetrahydrocannabinol (THC) in humans? A systematic review. Neurosci. Biobehav. Rev. 2019, 107, 696–712. [Google Scholar] [CrossRef]
- Calapai, G.; Mannucci, C.; Chinou, I.; Cardia, L.; Calapai, F.; Sorbara, E.E.; Firenzuoli, B.; Ricca, V.; Gensini, G.F.; Firenzuoli, F. Preclinical and clinical evidence supporting use of cannabidiol in psychiatry. Evid. Based Complement. Altern. Med. 2019, 2019, 2509129. [Google Scholar] [CrossRef] [Green Version]
- Klumpers, L.E.; Thacker, D.L. A brief background on cannabis: From plant to medical indications. J. AOAC Int. 2019, 102, 412–420. [Google Scholar] [CrossRef]
- Pertwee, R.G. Receptors and channels targeted by synthetic cannabinoid receptor agonists and antagonists. Curr. Med. Chem. 2010, 17, 1360–1381. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, E.D.; Dutra, R.C. Cannabinoid receptors as therapeutic targets for autoimmune diseases: Where do we stand? Drug Discov. Today 2019, 24, 1845–1853. [Google Scholar] [CrossRef]
- Grotenhermen, F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin. Pharmacokinet. 2003, 42, 327–360. [Google Scholar] [CrossRef]
- Bloomfield, M.A.P.; Hindocha, C.; Green, S.F.; Wall, M.B.; Lees, R.; Petrilli, K.; Costello, H.; Olabisi Ogunbiyi, M.; Bossong, M.G.; Freeman, T.P. The neuropsychopharmacology of cannabis: A review of human imaging studies. Pharmacol. Ther. 2019, 195, 132–161. [Google Scholar] [CrossRef]
- Sharma, P.; Murthy, P.; Bharath, M.M. Chemistry, metabolism, and toxicology of cannabis: Clinical implications. Iran J. Psychiatry 2012, 7, 149–156. [Google Scholar]
- Boggs, D.L.; Peckham, A.; Boggs, A.; Ranganathan, M. Delta-9-tetrahydrocannabinol and cannabidiol: Separating the chemicals from the “weed,” a pharmacodynamic discussion. Ment. Health Clin. 2016, 6, 277–284. [Google Scholar] [CrossRef]
- Grotenhermen, F.; Russo, E.; Zuardi, A.W. Even high doses of oral cannabidiol do not cause thc-like effects in humans: Comment on merrick et al. Cannabis Cannabinoid Res. 2016, 1, 102–112. [Google Scholar] [CrossRef] [Green Version]
- Renard, J.; Norris, C.; Rushlow, W.; Laviolette, S.R. Neuronal and molecular effects of cannabidiol on the mesolimbic dopamine system: Implications for novel schizophrenia treatments. Neurosci. Biobehav. Rev. 2017, 75, 157–165. [Google Scholar] [CrossRef]
- McPartland, J.M.; Duncan, M.; Di Marzo, V.; Pertwee, R.G. Are cannabidiol and Δ-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br. J. Pharmacol. 2015, 172, 737–753. [Google Scholar] [CrossRef] [Green Version]
- Arnold, J.C.; Boucher, A.A.; Karl, T. The yin and yang of cannabis-induced psychosis: The actions of Δ-tetrahydrocannabinol and cannabidiol in rodent models of schizophrenia. Curr. Pharm. Des. 2012, 18, 5113–5130. [Google Scholar] [CrossRef]
- Mao, K.; You, C.; Lei, D.; Zhang, H. High dosage of cannabidiol (CBD) alleviates pentylenetetrazole-induced epilepsy in rats by exerting an anticonvulsive effect. Int. J. Clin. Exp. Med. 2015, 8, 8820–8827. [Google Scholar]
- Bumb, J.M.; Enning, F.; Leweke, F.M. Drug repurposing and emerging adjunctive treatments for schizophrenia. Expert Opin. Pharmacother. 2015, 16, 1049–1067. [Google Scholar] [CrossRef]
- Iseger, T.A.; Bossong, M.G. A systematic review of the antipsychotic properties of cannabidiol in humans. Schizophr. Res. 2015, 162, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Mannucci, C.; Navarra, M.; Calapai, F.; Spagnolo, E.V.; Busardò, F.P.; Cas, R.D.; Ippolito, F.M.; Calapai, G. Neurological aspects of medical use of cannabidiol. CNS Neurol. Disord. Drug Targets 2017, 16, 541–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberg, E.C.; Tsien, R.W.; Whalley, B.J.; Devinsky, O. Cannabinoids and epilepsy. Neurotherapeutics 2015, 12, 747–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberg, E.C.; Louik, J.; Conway, E.; Devinsky, O.; Friedman, D. Quality of Life in Childhood Epilepsy in pediatric patients enrolled in a prospective, open-label clinical study with cannabidiol. Epilepsia 2017, 58, e96–e100. [Google Scholar] [CrossRef]
- Pertwee, R.G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef] [Green Version]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.; Denovan-Wright, E.M. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef] [Green Version]
- Hassan, S.; Eldeeb, K.; Millns, P.J.; Bennett, A.J.; Alexander, S.P.; Kendall, D.A. Cannabidiol enhances microglial phagocytosis via transient receptor potential (TRP) channel activation. Br. J. Pharmacol. 2014, 171, 2426–2439. [Google Scholar] [CrossRef] [Green Version]
- Ryberg, E.; Larsson, N.; Sjögren, S.; Hjorth, S.; Hermansson, N.O.; Leonova, J.; Elebring, T.; Nilsson, K.; Drmota, T.; Greasley, P.J. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 2007, 152, 1092–1101. [Google Scholar] [CrossRef]
- Fogaça, M.V.; Campos, A.C.; Coelho, L.D.; Duman, R.S.; Guimarães, F.S. The anxiolytic effects of cannabidiol in chronically stressed mice are mediated by the endocannabinoid system: Role of neurogenesis and dendritic remodeling. Neuropharmacology 2018, 135, 22–33. [Google Scholar] [CrossRef]
- Scuderi, C.; Steardo, L.; Esposito, G. Cannabidiol promotes amyloid precursor protein ubiquitination and reduction of beta amyloid expression in SHSY5YAPP+ cells through PPARγ involvement. Phytother. Res. 2014, 28, 1007–1013. [Google Scholar] [CrossRef]
- Sonego, A.B.; Gomes, F.V.; Del Bel, E.A.; Guimaraes, F.S. Cannabidiol attenuates haloperidol-induced catalepsy and c-Fos protein expression in the dorsolateral striatum via 5-HT1A receptors in mice. Behav. Brain Res. 2016, 309, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Kathmann, M.; Flau, K.; Redmer, A.; Tränkle, C.; Schlicker, E. Cannabidiol is an allosteric modulator at mu- and delta-opioid receptors. Naunyn Schmiedebergs Arch. Pharmacol. 2006, 372, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.R.; Ali, D.W. Pharmacology of medical cannabis. Adv. Exp. Med. Biol. 2019, 1162, 151–165. [Google Scholar] [PubMed]
- Fernández-Ruiz, J.; Sagredo, O.; Pazos, M.R.; García, C.; Pertwee, R.; Mechoulam, R.; Martínez-Orgado, J. Cannabidiol for neurodegenerative disorders: Important new clinical applications for this phytocannabinoid? Br. J. Clin. Pharmacol. 2013, 75, 323–333. [Google Scholar] [CrossRef]
- Deiana, S.; Watanabe, A.; Yamasaki, Y.; Amada, N.; Arthur, M.; Fleming, S.; Woodcock, H.; Dorward, P.; Pigliacampo, B.; Close, S.; et al. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Δ⁹-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive-compulsive behaviour. Psychopharmacology 2012, 219, 859–873. [Google Scholar]
- Suzuki, Y.; Nagai, N.; Umemura, K. A review of the mechanisms of blood-brain barrier permeability by tissue-type plasminogen activator treatment for cerebral ischemia. Front. Cell. Neurosci. 2016, 10, 2. [Google Scholar] [CrossRef] [Green Version]
- Zlokovic, B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [Green Version]
- Hladky, S.B.; Barrand, M.A. Fluid and ion transfer across the blood-brain and blood-cerebrospinal fluid barriers; a comparative account of mechanisms and roles. Fluids Barriers CNS 2016, 13, 19. [Google Scholar] [CrossRef] [Green Version]
- Garcia, C.M.; Darland, D.C.; Massingham, L.J.; D’Amore, P.A. Endothelial cell-astrocyte interactions and TGF beta are required for induction of blood-neural barrier properties. Brain Res. Dev. Brain Res. 2004, 152, 25–38. [Google Scholar] [CrossRef]
- Almutairi, M.M.; Gong, C.; Xu, Y.G.; Chang, Y.; Shi, H. Factors controlling permeability of the blood-brain barrier. Cell Mol. Life Sci. 2016, 73, 57–77. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J. Blood-brain barrier structure and function and the challenges for CNS drug delivery. J. Inherit. Metab. Dis. 2013, 36, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Brook, E.; Mamo, J.; Wong, R.; Al-Salami, H.; Falasca, M.; Lam, V.; Takechi, R. Blood-brain barrier disturbances in diabetes-associated dementia: Therapeutic potential for cannabinoids. Pharmacol. Res. 2019, 141, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Vendel, E.; de Lange, E.C. Functions of the CB1 and CB 2 receptors in neuroprotection at the level of the blood-brain barrier. Neuromol. Med. 2014, 16, 620–642. [Google Scholar] [CrossRef]
- Muoio, V.; Persson, P.B.; Sendeski, M.M. The neurovascular unit—Concept review. Acta Physiol. (Oxf.) 2014, 210, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Neuwelt, E.A. Mechanisms of disease: The blood-brain barrier. Neurosurgery 2004, 54, 131–140. [Google Scholar] [CrossRef]
- Persidsky, Y.; Ramirez, S.H.; Haorah, J.; Kanmogne, G.D. Blood-brain barrier: Structural components and function under physiologic and pathologic conditions. J. Neuroimmune Pharmacol. 2006, 1, 223–236. [Google Scholar] [CrossRef]
- Mecha, M.; Feliú, A.; Iñigo, P.M.; Mestre, L.; Carrillo-Salinas, F.J.; Guaza, C. Cannabidiol provides long-lasting protection against the deleterious effects of inflammation in a viral model of multiple sclerosis: A role for A2A receptors. Neurobiol. Dis. 2013, 59, 141–150. [Google Scholar] [CrossRef]
- Hind, W.H.; England, T.J.; O’Sullivan, S.E. Cannabidiol protects an in vitro model of the blood-brain barrier from oxygen-glucose deprivation via PPARγ and 5-HT1A receptors. Br. J. Pharmacol. 2016, 173, 815–825. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.; Rossi, E.; Saubamea, B.; Chasseigneaux, S.; Cochois, V.; Choublier, N.; Smirnova, M.; Glacial, F.; Perrière, N.; Bourdoulous, S.; et al. Cannabidiol increases proliferation, migration, tubulogenesis, and integrity of human brain endothelial cells through trpv2 activation. Mol. Pharm. 2019, 16, 1312–1326. [Google Scholar] [CrossRef]
- Tsou, Y.H.; Zhang, X.Q.; Zhu, H.; Syed, S.; Xu, X. Drug delivery to the brain across the blood-brain barrier using nanomaterials. Small 2018, 14, e1801588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aparicio-Blanco, J.; Romero, I.A.; Male, D.K.; Slowing, K.; García-García, L.; Torres-Suárez, A.I. Cannabidiol enhances the passage of lipid nanocapsules across the blood-brain barrier both in vitro and in vivo. Mol. Pharm. 2019, 16, 1999–2010. [Google Scholar] [CrossRef] [PubMed]
- Pytel, P.; Alexander, J.J. Pathogenesis of septic encephalopathy. Curr. Opin. Neurol. 2009, 22, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Valdepeñas, L.; Martínez-Orgado, J.A.; Benito, C.; Millán, A.; Tolón, R.M.; Romero, J. Cannabidiol reduces lipopolysaccharide-induced vascular changes and inflammation in the mouse brain: An intravital microscopy study. J. Neuroinflamm. 2011, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Haskó, G.; Liaudet, L.; Drel, V.R.; Obrosova, I.G.; Pacher, P. Cannabidiol attenuates high glucose-induced endothelial cell inflammatory response and barrier disruption. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H610–H619. [Google Scholar] [CrossRef] [Green Version]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Patel, V.; Saito, K.; Matsumoto, S.; Kashiwaya, Y.; Horváth, B.; Mukhopadhyay, B.; Becker, L.; et al. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J. Am. Coll. Cardiol. 2010, 56, 2115–2125. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Chen, Q.; Guo, J.; Yang, L.; Tao, Y.; Li, L.; Miao, H.; Feng, H.; Chen, Z.; Zhu, G. Minocycline attenuates neonatal germinal-matrix-hemorrhage-induced neuroinflammation and brain edema by activating cannabinoid receptor 2. Mol. Neurobiol. 2016, 53, 1935–1948. [Google Scholar] [CrossRef]
- Chen, D.J.; Gao, M.; Gao, F.F.; Su, Q.X.; Wu, J. Brain cannabinoid receptor 2: Expression, function and modulation. Acta Pharmacol. Sin. 2017, 38, 312–316. [Google Scholar] [CrossRef]
- Persidsky, Y.; Ho, W.; Ramirez, S.H.; Potula, R.; Abood, M.E.; Unterwald, E.; Tuma, R. HIV-1 infection and alcohol abuse: Neurocognitive impairment, mechanisms of neurodegeneration and therapeutic interventions. Brain Behav. Immun. 2011, 25, S61–S70. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, S.H.; Haskó, J.; Skuba, A.; Fan, S.; Dykstra, H.; McCormick, R.; Reichenbach, N.; Krizbai, I.; Mahadevan, A.; Zhang, M.; et al. Activation of cannabinoid receptor 2 attenuates leukocyte-endothelial cell interactions and blood-brain barrier dysfunction under inflammatory conditions. J. Neurosci. 2012, 32, 4004–4016. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M.; Fiori, A.; Bari, M.; Granata, F.; Gasperi, V.; De Stefano, M.E.; Finazzi-Agrò, A.; Strom, R. Regulation by cannabinoid receptors of anandamide transport across the blood-brain barrier and through other endothelial cells. Thromb. Haemost. 2006, 95, 117–127. [Google Scholar] [PubMed] [Green Version]
- Benyó, Z.; Ruisanchez, É.; Leszl-Ishiguro, M.; Sándor, P.; Pacher, P. Endocannabinoids in cerebrovascular regulation. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H785–H801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maresz, K.; Pryce, G.; Ponomarev, E.D.; Marsicano, G.; Croxford, J.L.; Shriver, L.P.; Ledent, C.; Cheng, X.; Carrier, E.J.; Mann, M.K.; et al. Direct suppression of CNS autoimmune inflammation via the cannabinoid receptor CB1 on neurons and CB2 on autoreactive T cells. Nat. Med. 2007, 13, 492–497. [Google Scholar] [CrossRef]
- Molina-Holgado, F.; Molina-Holgado, E.; Guaza, C. The endogenous cannabinoid anandamide potentiates interleukin-6 production by astrocytes infected with Theiler’s murine encephalomyelitis virus by a receptor-mediated pathway. FEBS Lett. 1998, 433, 139–142. [Google Scholar] [CrossRef]
- Marsicano, G.; Goodenough, S.; Monory, K.; Hermann, H.; Eder, M.; Cannich, A.; Azad, S.C.; Cascio, M.G.; Gutiérrez, S.O.; van der Stelt, M.; et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 2003, 302, 84–88. [Google Scholar] [CrossRef] [Green Version]
- Mestre, L.; Iñigo, P.M.; Mecha, M.; Correa, F.G.; Hernangómez-Herrero, M.; Loría, F.; Docagne, F.; Borrell, J.; Guaza, C. Anandamide inhibits Theiler’s virus induced VCAM-1 in brain endothelial cells and reduces leukocyte transmigration in a model of blood brain barrier by activation of CB receptors. J. Neuroinflamm. 2011, 8, 102. [Google Scholar] [CrossRef] [Green Version]
- Cabral, G.A.; Jamerson, M. Marijuana use and brain immune mechanisms. Int. Rev. Neurobiol. 2014, 118, 199–230. [Google Scholar]
- Schou, J.; Prockop, L.D.; Dahlstrom, G.; Rohde, C. Penetration of delta-9-tetrahydrocannabinol and 11-OH-delta-9-tetrahydrocannabinol through the blood-brain barrier. Acta Pharmacol. Toxicol. (Cph.) 1977, 41, 33–38. [Google Scholar] [CrossRef]
- McGilveray, I.J. Pharmacokinetics of cannabinoids. Pain Res. Manag. 2005, 10, 15A–22A. [Google Scholar] [CrossRef] [Green Version]
- Hunault, C.C.; van Eijkeren, J.C.; Mensinga, T.T.; de Vries, I.; Leenders, M.E.; Meulenbelt, J. Disposition of smoked cannabis with high Δ-tetrahydrocannabinol content: A kinetic model. Toxicol. Appl. Pharmacol. 2010, 246, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, J.E.; Ohlsson, A.; Agurell, S.; Hollister, L.; Gillespie, H. Clinical effects and plasma levels of delta 9-tetrahydrocannabinol (delta 9-THC) in heavy and light users of cannabis. Psychopharmacology 1981, 74, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Huestis, M.A.; Henningfield, J.E.; Cone, E.J. Blood cannabinoids. I. Absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuana. J. Anal. Toxicol. 1992, 16, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Law, B.; Mason, P.A.; Moffat, A.C.; Gleadle, R.I.; King, L.J. Forensic aspects of the metabolism and excretion of cannabinoids following oral ingestion of cannabis resin. J. Pharm. Pharmacol. 1984, 36, 289–294. [Google Scholar] [CrossRef]
- McIsaac, W.; Fritchie, G.E.; Idänpään-Heikkilä, J.E.; Ho, B.T.; Englert, L.F. Distribution of marihuana in monkey brain and concomitant behavioural effects. Nature 1971, 230, 593–594. [Google Scholar] [CrossRef]
- Nahas, G.G.; Frick, H.C.; Lattimer, J.K.; Latour, C.; Harvey, D. Pharmacokinetics of THC in brain and testis, male gametotoxicity and premature apoptosis of spermatozoa. Hum. Psychopharmacol. 2002, 17, 103–113. [Google Scholar] [CrossRef]
- Nahas, G.G. The pharmacokinetics of THC in fat and brain: Resulting functional responses to marihuana smoking. Hum. Psychopharmacol. 2001, 16, 247–255. [Google Scholar] [CrossRef]
- Nahas, G.; Leger, C.; Tocque, B.; Hoellinger, H. The kinetics of cannabinoid distribution and storage with special reference to the brain and testis. J. Clin. Pharmacol. 1981, 21, 208S–214S. [Google Scholar] [CrossRef]
- Huestis, M.A.; Henningfield, J.E.; Cone, E.J. Blood cannabinoids. II. Models for the prediction of time of marijuana exposure from plasma concentrations of delta-9-tetrahydrocannabinol (THC) and 11-nor-9-carboxy-delta 9-tetrahydrocannabinol (THCCOOH). J. Anal. Toxicol. 1992, 16, 283–290. [Google Scholar] [CrossRef]
- Huestis, M.A. Pharmacokinetics and metabolism of the plant cannabinoids, delta9-tetrahydrocannabinol, cannabidiol and cannabinol. Handb. Exp. Pharmacol. 2005, 168, 657–690. [Google Scholar]
- Poklis, J.L.; Thompson, C.C.; Long, K.A.; Lichtman, A.H.; Poklis, A. Disposition of cannabichromene, cannabidiol, and Δ⁹-tetrahydrocannabinol and its metabolites in mouse brain following marijuana inhalation determined by high-performance liquid chromatography-tandem mass spectrometry. J. Anal. Toxicol. 2010, 34, 516–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hložek, T.; Uttl, L.; Kadeřábek, L.; Balíková, M.; Lhotková, E.; Horsley, R.R.; Nováková, P.; Šíchová, K.; Štefková, K.; Tylš, F.; et al. Pharmacokinetic and behavioural profile of THC, CBD, and THC+CBD combination after pulmonary, oral, and subcutaneous administration in rats and confirmation of conversion in vivo of CBD to THC. Eur. Neuropsychopharmacol. 2017, 27, 1223–1237. [Google Scholar] [CrossRef] [PubMed]
- Saenz, S.R.; Lewis, R.J.; Angier, M.K.; Wagner, J.R. Postmortem fluid and tissue concentrations of THC, 11-OH-THC and THC-COOH. J. Anal. Toxicol. 2017, 41, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Hoosain, F.G.; Choonara, Y.E.; Tomar, L.K.; Kumar, P.; Tyagi, C.; du Toit, L.C.; Pillay, V. Bypassing P-glycoprotein drug efflux mechanisms: Possible applications in pharmacoresistant schizophrenia therapy. Biomed. Res. Int. 2015, 2015, 484963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elkhayat, H.A.; Aly, R.H.; Elagouza, I.A.; El-Kabarity, R.H.; Galal, Y.I. Role of P-glycoprotein inhibitors in children with drug-resistant epilepsy. Acta Neurol. Scand. 2017, 136, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.F.; Pokharel, D.; Bebawy, M. A novel mechanism governing the transcriptional regulation of ABC transporters in MDR cancer cells. Drug Deliv. Transl. Res. 2017, 7, 276–285. [Google Scholar] [CrossRef]
- Kathawala, R.J.; Gupta, P.; Ashby, C.R., Jr.; Chen, Z.S. The modulation of ABC transporter-mediated multidrug resistance in cancer: A review of the past decade. Drug Resist. Updat. 2015, 18, 1–17. [Google Scholar] [CrossRef]
- Miller, D.S. Regulation of ABC transporters at the blood-brain barrier. Clin. Pharmacol. Ther. 2015, 97, 395–403. [Google Scholar] [CrossRef]
- Lazarowski, A.; Czornyj, L.; Lubienieki, F.; Girardi, E.; Vazquez, S.; D’Giano, C. ABC transporters during epilepsy and mechanisms underlying multidrug resistance in refractory epilepsy. Epilepsia 2007, 48, 140–149. [Google Scholar] [CrossRef]
- Löscher, W.; Potschka, H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat. Rev. Neurosci. 2005, 6, 591–602. [Google Scholar] [CrossRef]
- Bonhomme-Faivre, L.; Benyamina, A.; Reynaud, M.; Farinotti, R.; Abbara, C. Disposition of Delta tetrahydrocannabinol in CF1 mice deficient in mdr1a P-glycoprotein. Addict. Biol. 2008, 13, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Spiro, A.S.; Wong, A.; Boucher, A.A.; Arnold, J.C. Enhanced brain disposition and effects of Δ9-tetrahydrocannabinol in P-glycoprotein and breast cancer resistance protein knockout mice. PLoS ONE 2012, 7, e35937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdullahi, W.; Davis, T.P.; Ronaldson, P.T. Functional expression of P-glycoprotein and organic anion transporting polypeptides at the blood-brain barrier: Understanding transport mechanisms for improved cns drug delivery? AAPS J. 2017, 19, 931–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brzozowska, N.; Li, K.M.; Wang, X.S.; Booth, J.; Stuart, J.; McGregor, I.S.; Arnold, J.C. ABC transporters P-gp and Bcrp do not limit the brain uptake of the novel antipsychotic and anticonvulsant drug cannabidiol in mice. PeerJ 2016, 4, e2081. [Google Scholar] [CrossRef]
- Feinshtein, V.; Erez, O.; Ben-Zvi, Z.; Erez, N.; Eshkoli, T.; Sheizaf, B.; Sheiner, E.; Huleihel, M.; Holcberg, G. Cannabidiol changes P-gp and BCRP expression in trophoblast cell lines. PeerJ 2013, 1, e153. [Google Scholar] [CrossRef] [Green Version]
- Doran, A.; Obach, R.S.; Smith, B.J.; Hosea, N.A.; Becker, S.; Callegari, E.; Chen, C.; Chen, X.; Choo, E.; Cianfrogna, J.; et al. The impact of P-glycoprotein on the disposition of drugs targeted for indications of the central nervous system: Evaluation using the MDR1A/1B knockout mouse model. Drug Metab. Dispos. 2005, 33, 165–174. [Google Scholar] [CrossRef] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calapai, F.; Cardia, L.; Sorbara, E.E.; Navarra, M.; Gangemi, S.; Calapai, G.; Mannucci, C. Cannabinoids, Blood–Brain Barrier, and Brain Disposition. Pharmaceutics 2020, 12, 265. https://doi.org/10.3390/pharmaceutics12030265
Calapai F, Cardia L, Sorbara EE, Navarra M, Gangemi S, Calapai G, Mannucci C. Cannabinoids, Blood–Brain Barrier, and Brain Disposition. Pharmaceutics. 2020; 12(3):265. https://doi.org/10.3390/pharmaceutics12030265
Chicago/Turabian StyleCalapai, Fabrizio, Luigi Cardia, Emanuela Elisa Sorbara, Michele Navarra, Sebastiano Gangemi, Gioacchino Calapai, and Carmen Mannucci. 2020. "Cannabinoids, Blood–Brain Barrier, and Brain Disposition" Pharmaceutics 12, no. 3: 265. https://doi.org/10.3390/pharmaceutics12030265
APA StyleCalapai, F., Cardia, L., Sorbara, E. E., Navarra, M., Gangemi, S., Calapai, G., & Mannucci, C. (2020). Cannabinoids, Blood–Brain Barrier, and Brain Disposition. Pharmaceutics, 12(3), 265. https://doi.org/10.3390/pharmaceutics12030265