An Assessment of Mesoporous Silica Nanoparticle Architectures as Antigen Carriers
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis Protocol of MSNPs
2.2. Scanning Electron Microscopy (SEM)
2.3. Transmission Electron Microscopy (TEM)
2.4. Surface Physical Properties Measurement
2.5. Disc Centrifuge Measurement
2.6. Surface Mobility Measurement
2.7. Antigen Loading onto MSNPs
2.7.1. Loading Temperature Variation
2.7.2. Assessment of Loading Efficiency
2.8. Assessment of Release of the Diphtheria Toxoid from the MSNPs
2.9. Stability Test
2.10. Protein Concentration Determination
2.11. SDS-PAGE
2.12. Haemotoxicity Assay
3. Results
3.1. Physical Characterization of the MSNPs
3.2. Antigen Loading
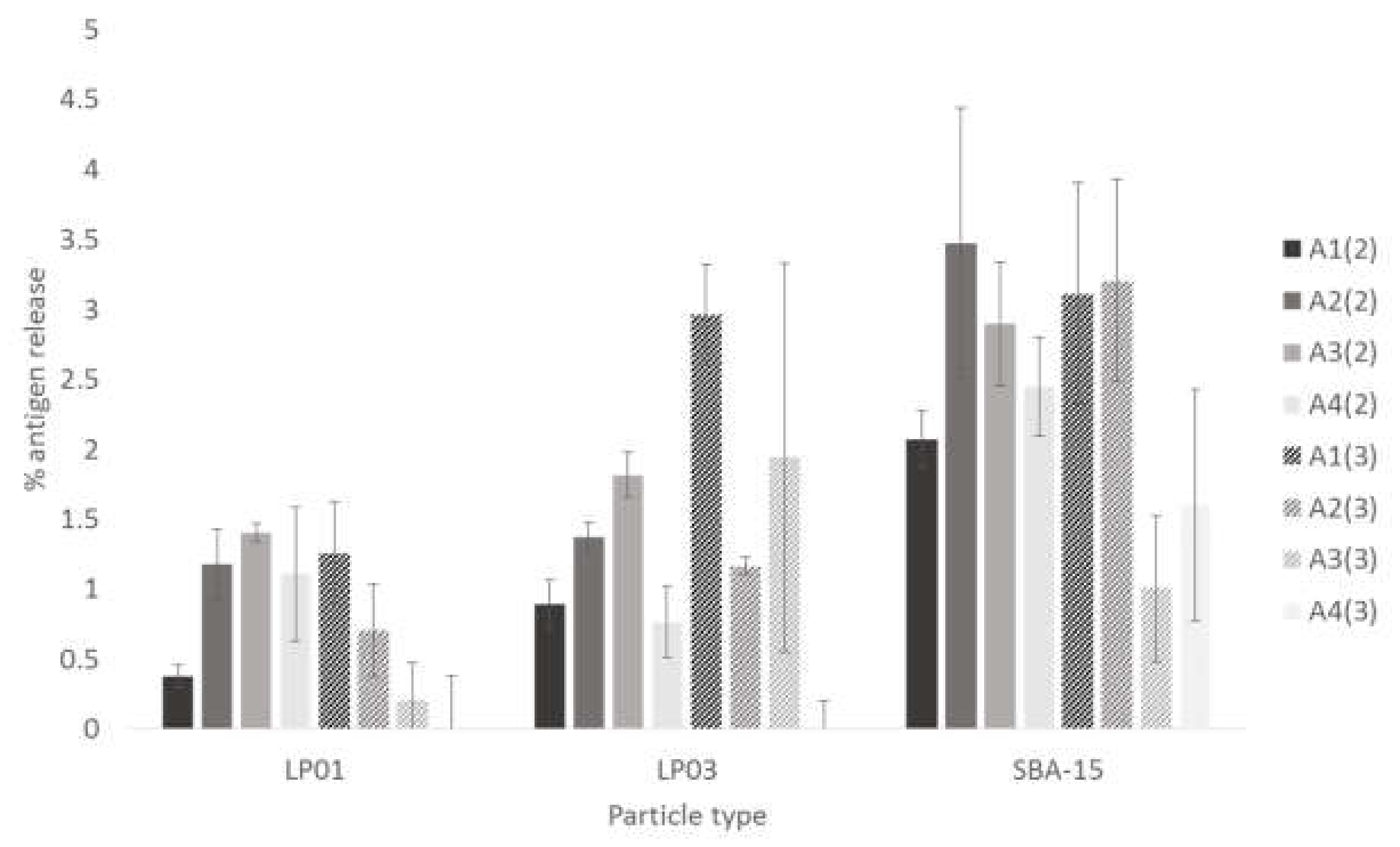
3.3. Antigen Unloading from MSNPs
3.4. Stability of the MSNP: Antigen Complex
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Van der Laan, J.; Gould, S.; Tanir, J. Safety of vaccine adjuvants: Focus on immunity. Vaccine 2015, 33, 1507–1514. [Google Scholar] [CrossRef] [Green Version]
- Guimarães, L.; Baker, B.; Perricone, C.; Shoenfeld, Y. V accines adjuvants and autoimmunity. Pharmacol. Res. 2015, 100, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Gellin, B.; Salisbury, D. Communicating the role and value of vaccine adjuvants. Vaccine 2015, 33 (Suppl. S2), B44–B46. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomedicine 2015, 11, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Mody, K.; Popat, A.; Mahony, D.; Cavallaro, A.S.; Yu, C.; Mitter, N. Mesoporous silica nanoparticles as antigen carriers and adjuvants for vaccine delivery. Nanoscale 2013, 5, 5167. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, T.; Zhao, Q.; Wang, S.; Zou, M.; Cheng, G. Enhanced mucosal and systemic immune responses obtained by porous silica nanoparticles used as an oral vaccine adjuvant: Effect of silica architecture on immunological properties. Int. J. Pharm. 2012, 436, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Coffman, R.; Sher, A.; Seder, R. Vaccine adjuvants: Putting Innate Immunity to work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef] [Green Version]
- Mohan, T.; Verma, P.; Rao, D. Novel adjuvants & delivery vehicles for vaccines development: A road ahead. Indian J. Med. 2013, 138, 779–795. [Google Scholar]
- Beran, J. Safety and immunogenicity of a new hepatitis B vaccine for the protection of patients with renal insufficiency including pre-haemodialysis and haemodialysis patients. Expert Opin. Biol. Ther. 2008, 8, 235–247. [Google Scholar] [CrossRef]
- Podda, A. The adjuvanted influenza vaccines with novel adjuvants: Experience with the MF-59 adjuvanted vaccine. Vaccine 2001, 19, 2673–2680. [Google Scholar] [CrossRef]
- Banzhoff, A.; Gasparini, R.; Laghi-Pasini, F.; Staniscia, T.; Durando, P.; Montomoli, E.; Capecchi, P.; Di Giovanni, P.; Sticchi, L.; Gentile, C.; et al. MF59-Adjuvanted H5N1 vaccine induces immunologic memory and heterotypic antibody responses in non-elderly and elderly adults. PLoS ONE 2009, 4, e4384. [Google Scholar] [CrossRef]
- Halperin, S.A.; Dobson, S.; McNeil, S.A.; Langley, J.M.; Smith, B.; McCall-Sani, R.; Levitt, D.; Van Nest, G.; Gennevois, D.; Eiden, J.J. Comparison of the safety and immunogenicity of hepatitis B virus surface antigen co-administered with an immunostimulatory phosphorothioate oligonucleotide and a licensed hepatitis B vaccine in healthy young adults. Vaccine 2006, 24, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, T.F.; Horacek, T.; Knuf, M.; Damman, H.-G.; Roman, F.; Dramé, M.; Gillard, P.; Jilg, W. Single dose vaccination with AS03-adjuvanted H5N1 vaccines in a randomized trial induces strong and broad immune responsiveness to booster vaccination in adults. Vaccine 2009, 27, 6284–6290. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Cai, W.; Jin, F. A novel oil-in-water emulsion as a potential adjuvant for influenza virus: Development, characterization, stability and in vivo evaluation. Int. J. Pharm. 2014, 468, 187–195. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D.; Fox, C. New generation adjuvants- from empiricism to rational design. Vaccine 2015, 33, B14–B20. [Google Scholar] [CrossRef] [PubMed]
- Clapp, T.; Munks, M.; Trivedi, R.; Kompella, U.; Braun, L. Freeze-thaw stress of alhydrogel alone is sufficient to reduce the immunogenicity of a recombinant hepatitis B vaccine containing native antigen. Vaccine 2014, 32, 3765–3771. [Google Scholar] [CrossRef] [PubMed]
- Tomljenovic, L.; Shaw, C. Aluminium vaccine adjuvants: Are they safe? Curr. Med. Chem. 2011, 18, 2630–2637. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, L.P.; Carvalho, L.V.; Lima, F.A.; Quayle, C.; Fantini, M.C.; Tanaka, G.S.; Cabrera, W.H.; Furtado, M.F.; Tambourgi, D.V.; Matos, J.D.R.; et al. Ordered Mesoporous Silica SBA-15: A New Effective Adjuvant to Induce Antibody Response. Small 2006, 2, 254–256. [Google Scholar] [CrossRef]
- Sun, B.; Xia, T. Nanomaterial-based vaccine adjuvants. J. Mater. Chem. B 2016, 4, 5496–5509. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, R.; Nie, G. Applications of nanomaterials as vaccine adjuvants. Hum. Vaccin. Immunother. 2014, 10, 2761–2774. [Google Scholar] [CrossRef] [Green Version]
- Ge, Y.; Zhang, Y.; Xia, J.; Ma, M.; He, S.; Nie, F.; Gu, N. Effect of surface charge and agglomerate degree of magnetic iron oxide nanoparticles on KB cellular uptake in vitro. Colloids Surf. B Biointerfaces 2009, 73, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Liu, Y.; Wang, L.; Xu, L.; Bai, R.; Ji, Y.; Wu, X.; Zhao, Y.; Li, Y.; Chen, C. Surface chemistry and aspect ratio mediated cellular uptake of Au nanorods. Biomaterials 2010, 31, 7606–7619. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Paixao Cavalcante, D.; Townley, H.E. Macrophage-like THP-1 cells show effective uptake of silica nanoparticles carrying inactivated diphtheria toxoid for vaccination. J. Nanopartic. Res. 2020, 22, 23. [Google Scholar] [CrossRef] [Green Version]
- Brunauer, S.; Emmett, P.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.; Joyner, L.; Halenda, P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Fan, J.; Lei, J.; Wang, L.; Yu, C.; Tu, B.; Zhao, D. Rapid and high-capacity immobilization of enzymes based on mesoporous silicas with controlled morphologies. Chem. Commun. 2003, 17, 2140–2141. [Google Scholar] [CrossRef] [PubMed]
- Pappenheimer, A. Diphtheria toxin. I. Isolation and characterization of a toxic protein from culture filtrates of C. diptheriae. J. Biol. Chem. 1937, 120, 543–553. [Google Scholar]
- Sigma-Aldrich. Sigma-Aldrich Product Specification; Sigma-Aldrich: St. Louis, MO, USA, 14 June 2019. [Google Scholar]
- Kisler, J.; Dahler, A.; Steves, G.; O’Connor, A. Separation of biological molecules using mesoporous molecular sieves. Microporous Mesoporous Mater. 2001, 44, 769–774. [Google Scholar] [CrossRef]
- Guo, H.; Feng, X.-M.; Sun, S.; Wei, Y.-Q.; Sun, D.-H.; Liu, X.; Liu, Z.; Luo, J.; Yin, H. Immunization of mice by Hollow Mesoporous Silica Nanoparticles as carriers of Porcine Circovirus Type 2 ORF2 Protein. Virol. J. 2012, 9, 108. [Google Scholar] [CrossRef] [Green Version]
- Hartono, S.B.; Qiao, S.; Liu, J.; Jack, K.; Ladewig, B.P.; Hao, Z.; Lu, G. Functionalized Mesoporous Silica with Very Large Pores for Cellulase Immobilization. J. Phys. Chem. C 2010, 114, 8353–8362. [Google Scholar] [CrossRef]
- McUmber, A.; Randolph, T.; Schwartz, D. Electrostatic Interactions Influence Protein Adsorption (but Not Desorption) at the Silica–Aqueous Interface. J. Phys. Chem. Lett. 2015, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Pan, F.; Garcia-Gancedo, L.; Flewitt, A.; Ashley, G.; Luo, J.; Lu, J. Interfacial recognition of human prostate-specific antigen by immobilized monoclonal antibody: Effects of solution conditions and surface chemistry. J. R. Soc. Interface 2012, 9, 2457–2467. [Google Scholar] [CrossRef] [PubMed]
- Buijs, J.; van-den-Berg, P.; Lichtenbelt, J.; Norde, W.; Lyklema, J. Adsorption dynamics of IgG and Its F(ab’)2 and Fc fragments studied by reflectometry. J. Colloid Interface Sci. 1996, 178, 594–605. [Google Scholar] [CrossRef]
- Elofsson, U.; Paulsson, M.; Arnebrant, T. Adsorption of ß-lactoglobulin A and B in relation to self-association: Effect of concentration and pH. Langmuir 1997, 13, 1695–1700. [Google Scholar] [CrossRef]
- Choe, S.; Bennett, M.J.; Fujii, G.; Curmi, P.; Kantardjieff, K.A.; Collier, R.J.; Eisenberg, D. The crystal structure of diphtheria toxin. Nature 1992, 357, 216–222. [Google Scholar] [CrossRef]
- Lu, J.; Liong, M.; Zink, J.I.; Tamanoi, F. Tamanoi, Mesoporous silica nanoparticles as a delivery system for hydrophobic anticancer drugs. Small 2007, 3, 1341–1346. [Google Scholar] [CrossRef]
- Slowing, I.; Trewyn, B.; Lin, V. Effect of surface functionalization of MCM-41-type mesoporous silica nanoparticles on the endocytosis by human cancer cells. J. Am. Chem. Soc. 2006, 128, 14792–14793. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Liong, M.; Li, Z.; Zink, J.I.; Tamanoi, F. Biocompatibility, biodistribution and drug-delivery efficiency of mesoporous silica nanoparticles for cancer therapy in animals. Small 2010, 6, 1794–1805. [Google Scholar] [CrossRef] [Green Version]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [Green Version]
- Donhowe, E.G.; Flores, F.P.; Kerr, W.L.; Wicker, L.; Kong, F. Characterization and in vitro bioavailability of β-carotene: Effects of microencapsulation method and food matrix. LWT Food Sci. Technol. 2014, 57, 42–48. [Google Scholar] [CrossRef]
- Huang, X.; Young, N.; Townley, H. Characterization and Comparison of Mesoporous Silica Particles for Optimized Drug Delivery. Nanomater. Nanotechnol. 2014, 4, 2. [Google Scholar] [CrossRef]





| DT:MSNP | 1:2 | 1:5 | 1:10 | 1:20 | 1:50 | 1:100 |
|---|---|---|---|---|---|---|
| Volume of DT stock solution (µL) | 10 | 10 | 10 | 10 | 10 | 10 |
| Volume of MSNPs suspension (µL) | 4 | 10 | 20 | 40 | 100 | 200 |
| Volume of PBS (µL) | 386 | 380 | 370 | 350 | 290 | 190 |
| Total volume (µL) | 400 | 400 | 400 | 400 | 400 | 400 |
| Nominal DT concentration (µg/mL) | 250 | 250 | 250 | 250 | 250 | 250 |
| MSNPs concentration (µg/mL) | 500 | 1250 | 2500 | 5000 | 12500 | 25000 |
| Morphology | Average Yield (%) | Size (nm) | Surface Properties | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ζ Potential (mV) | BET Surface are (m2/g) | Average Pore Size (nm) | Pore Peak Size (nm) | Pore Volume (cm3/g) | |||||
| Ads. | Des. | Ads. | Des. | ||||||
| SP1 | 62.4 | 1509 ± 269 | −26.8 | 992.4 | 2.6 | 2.7 | 2.6 | 2.7 | 0.78 |
| SP2 | 80.6 | 138 ± 26 | −20.7 | 1110.9 | 2.9 | 2.9 | 2.6 | 2.8 | 1.02 |
| SP3 | 96.5 | 496 ± 13 | −29.5 | 162.3 | 2.9 | 2.9 | 2.4 | 2.7 | 0.13 |
| SP4 | 88.5 | 202 ± 19 | −24.0 | 632.1 | 5.7 | 4.7 | 5.5 | 3.8 | 0.77 |
| LP1 | 88.6 | 462 ± 25 | −20.6 | 701.9 | 11.1 | 10.2 | 4 & 70 | 4 & 70 | 1.90 |
| LP2 | 60.1 | 217 ± 22 | −22.9 | 650.0 | 12.4 | 13.0 | 3.5 & 40 | 3.5 & 40 | 1.80 |
| LP3 | 91.9 | 217 ± 5 | −16.8 | 660.0 | 16.5 | 16.2 | 3.5 & 70 | 3.5 & 70 | 2.40 |
| SBA-15 | N/A | N/A | −19.7 | 794.0 | 7.9 | 7.7 | 10.0 | 10.0 | 1.30 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Townley, H.E. An Assessment of Mesoporous Silica Nanoparticle Architectures as Antigen Carriers. Pharmaceutics 2020, 12, 294. https://doi.org/10.3390/pharmaceutics12030294
Huang X, Townley HE. An Assessment of Mesoporous Silica Nanoparticle Architectures as Antigen Carriers. Pharmaceutics. 2020; 12(3):294. https://doi.org/10.3390/pharmaceutics12030294
Chicago/Turabian StyleHuang, Xinyue, and Helen E Townley. 2020. "An Assessment of Mesoporous Silica Nanoparticle Architectures as Antigen Carriers" Pharmaceutics 12, no. 3: 294. https://doi.org/10.3390/pharmaceutics12030294
APA StyleHuang, X., & Townley, H. E. (2020). An Assessment of Mesoporous Silica Nanoparticle Architectures as Antigen Carriers. Pharmaceutics, 12(3), 294. https://doi.org/10.3390/pharmaceutics12030294




