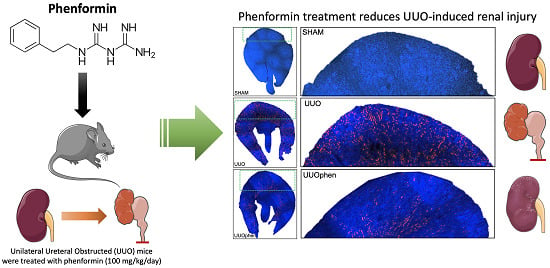
Phenformin Attenuates Renal Injury in Unilateral Ureteral Obstructed Mice without Affecting Immune Cell Infiltration
Abstract
:1. Introduction
2. Methods
2.1. Housing and Biguanide Treatment
2.2. Unilateral Ureteral Obstruction
2.3. Cell Preparation and Flow Cytometry
2.4. Western Blot
2.5. Quantitative PCR
2.6. Histology
2.7. Immunofluorescence Staining
2.8. Statistical Analysis
3. Results
3.1. Phenformin Induces Elevated Blood Lactate Levels in UUO Mice
3.2. Biguanides Attenuate Tubular Injury in Response to 3dUUO
3.3. Effects of Biguanides on Oxidative Stress in Response to 3dUUO
3.4. Biguanides Differentially Impact the Inflammatory Response in UUO Mice
3.5. Biguanides Affect Protein Expression of the pSTAT3 Pathway
3.6. Impact of Metformin and Phenformin on Immune Cell Infiltration
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Luft, D.; Schmülling, R.M.; Eggstein, M. Originals Lactic Acidosis in Biguanide-Treated Diabetics a Review of 330 Cases; Diabetologia: Bristol, UK, 1978; pp. 75–87. [Google Scholar]
- Sanchez-Rangel, E.; Inzucchi, S.E. Metformin: Clinical use in type 2 diabetes. Diabetologia 2017, 60, 1586–1593. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, T.I.; Jeon, S.M.; Hong, S.P.; Cheon, J.H.; Kim, W.H. The effects of metformin on the survival of colorectal cancer patients with diabetes mellitus. Int. J. Cancer 2012, 131, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Neven, E.; Vervaet, B.A.; Brand, K.; Gottwald-Hostalek, U.; Opdebeeck, B.; De Maré, A.; Verhulst, A.; Lalau, J.-D.; Kamel, S.; De Broe, M.E.; et al. Metformin prevents the development of severe chronic kidney disease and its associated mineral and bone disorder. Kidney Int. 2018, 94, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Garamaleki, M.N.; Kazemi, D.; Heydarinegad, H.; Safarmashaei, S. Effect of metformine (Glucophage) on renal function after complete unilateral ureteral obstruction in dog. J. Toxicol. Sci. 2012, 4, 6–10. [Google Scholar]
- Christensen, M.; Jensen, J.B.; Jakobsen, S.; Jessen, N.; Frøkiær, J.; Kemp, B.E.; Marciszyn, A.L.; Li, H.; Pastor-Soler, N.M.; Hallows, K.R.; et al. Renoprotective effects of metformin are independent of organic cation transporters 1 & 2 and AMP-activated protein kinase in the kidney. Sci. Rep. 2016, 6, 35952. [Google Scholar]
- Wang, D.-S.; Jonker, J.W.; Kato, Y.; Kusuhara, H.; Schinkel, A.H.; Sugiyama, Y. Involvement of organic cation transporter 1 in hepatic and intestinal distribution of metformin. J. Pharmacol. Exp. Ther. 2002, 302, 510–515. [Google Scholar] [CrossRef] [Green Version]
- Sogame, Y.; Kitamura, A.; Yabuki, M.; Komuro, S. A comparison of uptake of metformin and phenformin mediated by hOCT1 in human hepatocytes. Biopharm. Drug Dispos. 2009, 30, 476–484. [Google Scholar] [CrossRef] [Green Version]
- Thakur, S.; Viswanadhapalli, S.; Kopp, J.B.; Shi, Q.; Barnes, J.L.; Block, K.; Gorin, Y.; Abboud, H.E. Activation of AMP-activated protein kinase prevents tgf-β1–induced epithelial-mesenchymal transition and myofibroblast activation. Am. J. Pathol. 2015, 185, 2168–2180. [Google Scholar] [CrossRef] [Green Version]
- Appleyard, V.; Murray, K.E.; Coates, P.J.; Wullschleger, S.; Bray, S.E.; Kernohan, N.M.; Fleming, S.; Alessi, D.R.; Thompson, A.M. Phenformin as prophylaxis and therapy in breast cancer xenografts. Br. J. Cancer 2012, 106, 1117–1122. [Google Scholar] [CrossRef]
- Gürtler, A.; Kunz, N.; Gomolka, M.; Hornhardt, S.; Friedl, A.A.; McDonald, K.; Kohn, J.E.; Posch, A. Stain-Free technology as a normalization tool in Western blot analysis. Anal. Biochem. 2013, 433, 105–111. [Google Scholar] [CrossRef]
- Han, W.K.; Bailly, V.; Abichandani, R.; Thadhani, R.; Bonventre, J.V. Kidney injury molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 2002, 62, 237–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawley, S.A.; Ross, F.A.; Chevtzoff, C.; Green, K.A.; Evans, A.; Fogarty, S.; Towler, M.C.; Brown, L.J.; Ogunbayo, O.A.; Evans, A.M.; et al. Use of cells expressing γ subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010, 11, 554–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christensen, M.; Nørgård, M.Ø.; Jensen, M.S.; Møller, B.K.; Nørregaard, R. Metformin modulates immune cell infiltration into the kidney during unilateral ureteral obstruction in mice. Physiol. Rep. 2019, 7, e14141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dendooven, A.; Ishola, D.A.; Nguyen, T.Q.; Van Der Giezen, D.M.; Kok, R.J.; Goldschmeding, R.; Joles, J.A. Oxidative stress in obstructive nephropathy. Int. J. Exp. Pathol. 2011, 92, 202–210. [Google Scholar] [CrossRef]
- Vasamsetti, S.B.; Karnewar, S.; Kanugula, A.K.; Thatipalli, A.R.; Kumar, J.M.; Kotamraju, S. Metformin inhibits monocyte-to-macrophage differentiation via ampk-mediated inhibition of STAT3 activation: Potential role in atherosclerosis. Diabetes 2015, 64, 2028–2041. [Google Scholar] [CrossRef] [Green Version]
- Ramachandran, P.; Pellicoro, A.; Vernon, M.A.; Boulter, L.; Aucott, R.L.; Ali, A.; Hartland, S.N.; Snowdon, V.K.; Cappon, A.; Gordon-Walker, T.T.; et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc. Natl. Acad. Sci. USA 2012, 109, E3186–E3195. [Google Scholar] [CrossRef] [Green Version]
- Zeisberg, M.; Neilson, E.G. Mechanisms of tubulointerstitial fibrosis. J. Am. Soc. Nephrol. 2010, 21, 1819–1834. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.L.; Castano, A.P.; Nowlin, B.T.; Lupher, M.L.; Duffield, J.S. Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J. Immunol. 2009, 183, 6733–6743. [Google Scholar] [CrossRef] [Green Version]
- Sogawa, Y.; Nagasu, H.; Iwase, S.; Ihoriya, C.; Itano, S.; Uchida, A.; Kidokoro, K.; Taniguchi, S.; Takahashi, M.; Satoh, M.; et al. Infiltration of M1, but not M2, macrophages is impaired after unilateral ureter obstruction in Nrf2-deficient mice. Sci. Rep. 2017, 7, 8801. [Google Scholar] [CrossRef]
- Yusuf-Makagiansar, H.; Anderson, M.E.; Yakovleva, T.V.; Murray, J.S.; Siahaan, T.J. Inhibition of LFA-1/ICAM-1 and VLA-4/VCAM-1 as a therapeutic approach to inflammation and autoimmune diseases. Med. Res. Rev. 2002, 22, 146–167. [Google Scholar] [CrossRef]
- Deng, X.-S.; Wang, S.; Deng, A.; Liu, B.; Edgerton, S.M.; Lind, S.E.; Wahdan-Alasward, R.; Thor, A.D. Metformin targets Stat3 to inhibit cell growth and induce apoptosis in triple-negative breast cancers. Cell Cycle 2012, 11, 367–376. [Google Scholar] [CrossRef] [Green Version]
- Bienaimé, F.; Muorah, M.; Yammine, L.; Burtin, M.; Nguyen, C.; Baron, W.; Garbay, S.; Viau, A.; Broueilh, M.; Blanc, T.; et al. Stat3 controls tubulointerstitial communication during CKD. J. Am. Soc. Nephrol. 2016, 27, 3690–3705. [Google Scholar] [CrossRef]
- Chakraborty, D.; Šumová, B.; Mallano, T.; Chen, C.-W.; Distler, A.; Bergmann, C.; Ludolph, I.; Horch, R.E.; Gelse, K.; Ramming, A.; et al. Activation of STAT3 integrates common profibrotic pathways to promote fibroblast activation and tissue fibrosis. Nat. Commun. 2017, 8, 1130. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Moon, S.Y.; Kim, J.-S.; Baek, C.H.; Kim, M.; Min, J.Y.; Lee, S.K. Activation of AMP-activated protein kinase inhibits ER stress and renal fibrosis. Am. J. Physiol. Physiol. 2015, 308, F226–F236. [Google Scholar] [CrossRef]
- Gobe, G.; Crane, D. Mitochondria, reactive oxygen species and cadmium toxicity in the kidney. Toxicol. Lett. 2010, 198, 49–55. [Google Scholar] [CrossRef]
- Østergaard, M.; Christensen, M.; Nilsson, L.; Carlsen, I.; Frøkiær, J.; Nørregaard, R. ROS dependence of cyclooxygenase-2 induction in rats subjected to unilateral ureteral obstruction. Am. J. Physiol. Physiol. 2014, 306, F259–F270. [Google Scholar] [CrossRef]
- Graham, G.G.; Punt, J.; Arora, M.; Day, R.O.; Doogue, M.; Duong, J.; Furlong, T.J.; Greenfield, J.R.; Greenup, L.C.; Kirkpatrick, C.; et al. Clinical pharmacokinetics of metformin. Clin. Pharmacokinet. 2011, 50, 81–98. [Google Scholar] [CrossRef]
- Guo, Z.; Zhao, M.; Howard, E.W.; Zhao, Q.; Parris, A.B.; Ma, Z.; Yang, X. Phenformin inhibits growth and epithelial-mesenchymal transition of ErbB2-overexpressing breast cancer cells through targeting the IGF1R pathway. Oncotarget 2017, 8, 60342–60357. [Google Scholar] [CrossRef] [Green Version]
- Jensen, M.S.; Mutsaers, H.A.M.; Tingskov, S.J.; Christensen, M.; Madsen, M.G.; Olinga, P.; Kwon, T.; Nørregaard, R. Activation of the prostaglandin E2 EP2 receptor attenuates renal fibrosis in unilateral ureteral obstructed mice and human kidney slices. Acta Physiol. 2019, 227, e13291. [Google Scholar] [CrossRef] [Green Version]
- Bigaeva, E.; Stribos, E.G.D.; Mutsaers, H.A.M.; Piersma, B.; Leliveld, A.M.; De Jong, I.J.; Bank, R.A.; Seelen, M.A.; Van Goor, H.; Wollin, L.; et al. Inhibition of tyrosine kinase receptor signaling attenuates fibrogenesis in an ex vivo model of human renal fibrosis. Am. J. Physiol. Renal Physiol. 2020, 318, F117–F134. [Google Scholar] [CrossRef]








| Target Protein | Company | Catalog # | Dilution |
|---|---|---|---|
| Primary Antibodies | |||
| KIM-1 | R&D systems, USA | AF1817 | 1:1000 |
| STAT3 | Cell Signalling, NL | mAB9139 | 1:1000 |
| pSTAT3 | Cell Signalling, NL | mAB4113 | 1:1000 |
| HO-1 | ENZO, Denmark | ADI-SPA-896-D | 1:500 |
| 4-HNE | Abcam, USA | Ab46545 | 1:250 |
| SOD1 | ENZO, Denmark | ADI-SOD-100-D | 1:1000 |
| SOD2 | ENZO, Denmark | 06-984 | 1:1000 |
| Catalase | Abcam, USA | Ab76024 | 1:1000 |
| Secondary Antibodies | |||
| Goat anti-mouse immunoglobulin/HRP | DAKO, Denmark | P0447 | |
| Goat anti-rabbit immunoglobulin/HRP | DAKO, Denmark | P0448 | |
| Rabbit anti-goat immunoglobulin/HRP | DAKO, Denmark | P0449 | |
| Target Gene | Direction | Sequence |
|---|---|---|
| KIM-1 | Forward Reverse | 5′-CGGTACAACTTAAAGGGGCA-3′ 5′-GACGTGTGGGAATCTCTGGT-3′ |
| MCP-1 | Forward Reverse | 5′-CAAGAAGGAATGGGTCCAGA-3′ 5′-GTGCTGAAGACCTTAGGGCA-3′ |
| ICAM-1 | Forward Reverse | 5′-TCCAATTCACACTGAATGCC-3′ 5′-GTCTGCTGAGACCCCTCTTG-3′ |
| VCAM-1 | Forward Reverse | 5′-GTGGTGCTGTGACAATGACC-3′ 5′-ACGTCAGAACAACCGAATCC-3′ |
| MIP-2 | Forward Reverse | 5′-CTCTCAAGGGCGGTCAAAAAGTT-3′ 5′-TCAGACAGCGAGGCACATCAGGTA-3′ |
| KC | Forward Reverse | 5′-GCGAATTCACCATGATCCCAGCCACCCG-3′ 5′-GCTCTAGATTACTTGGGGACACCTTTTAG-3′ |
| TNF-α | Forward Reverse | 5′-AGGCTGCCCCGACTACGT-3′ 5′-GACTTTCTCCTGGTATGAGATAGCAAA-3′ |
| IL-6 | Forward Reverse | 5′-GATGCTACCAAACTGGATATAATC-3′ 5′-GGTCCTTAGCCACTCCTTCTGTG-3′ |
| RPl4 | Forward Reverse | 5′-CTTTGCCAGCTGTGTTCAA-3′ 5′-ATTTCACTGACGGCATAGGG-3′ |
| 18S | Forward Reverse | 5′-TGTGGTGTTGAGGAAAGCAG-3′ 5′-TCCCATCCTTCACATCCTTC-3′ |
| Parameter | SHAM (n = 6) | 3dUUO (n = 8) | 3dUUOmet (n = 8) | 3dUUOphen (n = 8) |
|---|---|---|---|---|
| Weight (g) | 21.7 ± 1.02 | 21.28 ± 0.36 | 21.72 ± 0.27 | 21.78 ± 0.35 |
| KW (mg/25g mouse) | 156.5 ± 6.17 | 235.35 ± 4.19 A | 279.37 ± 6 AB | 244.99 ± 6.39 AC |
| pH | 7.25 ± 0.01 | 7.25 ± 0.02 | 7.24 ± 0.01 | 7.14 ± 0.02 ABC |
| Glucose (mmol/L) | 8.42 ± 0.44 | 8.14 ± 0.33 | 8 ± 0.36 | 5.04 ± 0.0 ABC |
| Lactate (mmol/L) | 6.18 ± 0.35 | 7.10 ± 0.60 | 7.59 ± 0.17 | 10.91 ± 0.0 ABC |
| Creatinine (μmol/L) | 14.89 ± 0.83 | 13.60 ± 1.60 | 14.32 ± 0.45 | 14.16 ± 1.21 |
| Na+ (mmol/L) | 148.40 ± 0.46 | 150.50 ± 0.43 A | 150.71 ± 0.33 A | 151.63 ± 0.35 A |
| K+ (mmol/L) | 4.64 ± 0.12 | 5.00 ± 0.12 | 4.70 ± 0.05 | 5.00 ± 0.13 |
| BUN (mmol/L) | 7.32 ± 0.70 | 9.23 ± 0.44 | 9.00 ± 0.39 | 8.14 ± 0.40 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nørgård, M.Ø.; Christensen, M.; Mutsaers, H.A.M.; Nørregaard, R. Phenformin Attenuates Renal Injury in Unilateral Ureteral Obstructed Mice without Affecting Immune Cell Infiltration. Pharmaceutics 2020, 12, 301. https://doi.org/10.3390/pharmaceutics12040301
Nørgård MØ, Christensen M, Mutsaers HAM, Nørregaard R. Phenformin Attenuates Renal Injury in Unilateral Ureteral Obstructed Mice without Affecting Immune Cell Infiltration. Pharmaceutics. 2020; 12(4):301. https://doi.org/10.3390/pharmaceutics12040301
Chicago/Turabian StyleNørgård, Mikkel Ø., Michael Christensen, Henricus A.M. Mutsaers, and Rikke Nørregaard. 2020. "Phenformin Attenuates Renal Injury in Unilateral Ureteral Obstructed Mice without Affecting Immune Cell Infiltration" Pharmaceutics 12, no. 4: 301. https://doi.org/10.3390/pharmaceutics12040301