Oral Administration System Based on Meloxicam Nanocrystals: Decreased Dose Due to High Bioavailability Attenuates Risk of Gastrointestinal Side Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Animals
2.2. Preparation of MLX Solid Nanoparticle-based Oral Formulations
2.3. General Characteristics of MLX Formulations
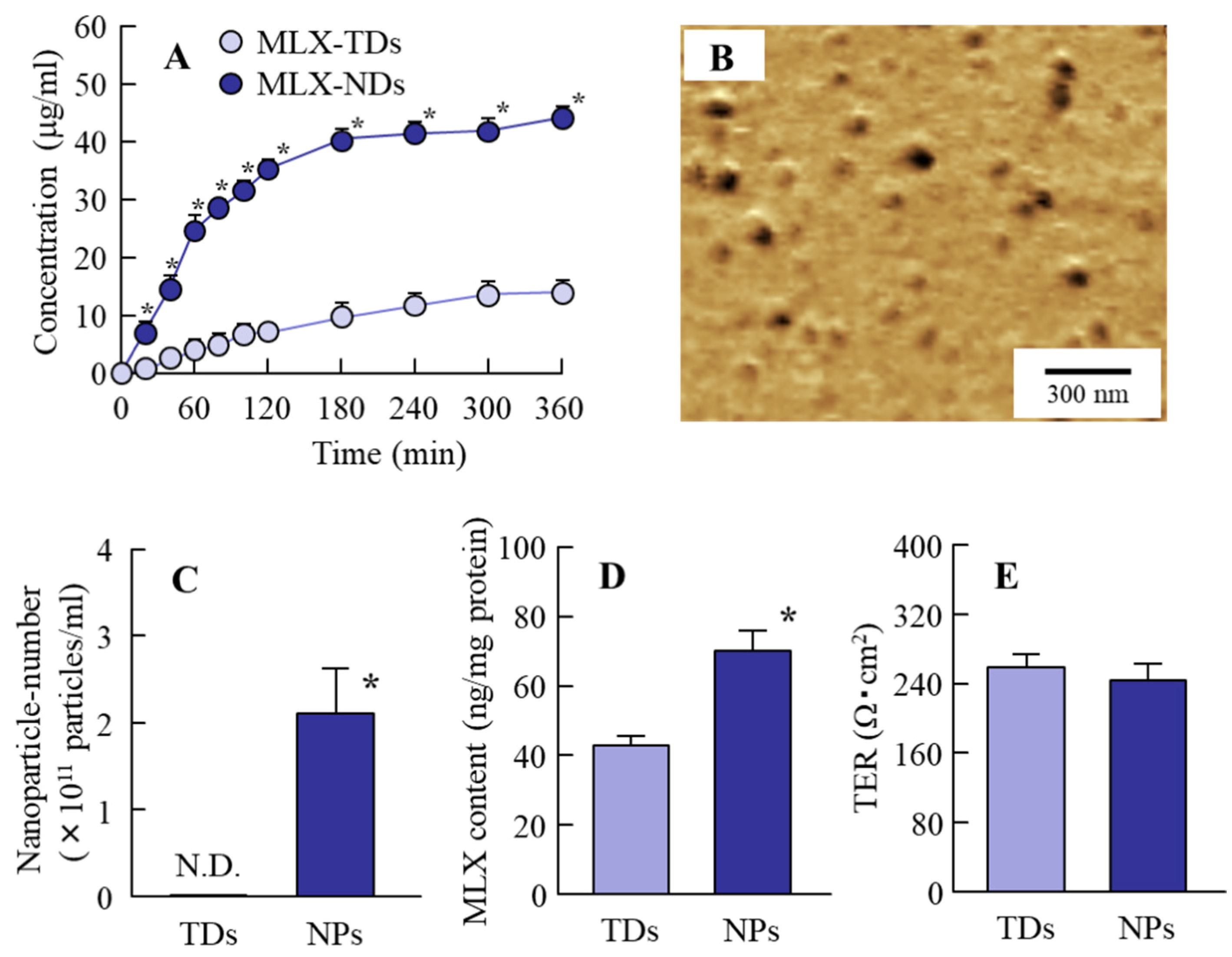
2.4. Transepithelial Penetration of MLX using Caco-2 Cell Monolayers
2.5. Intestinal Penetration of MLX using Rat Jejunum and Ileum
2.6. Absorption of Orally Administered MLX
2.7. Content of MLX in Gastrointestinal Mucosa after the Oral Administration of MLX
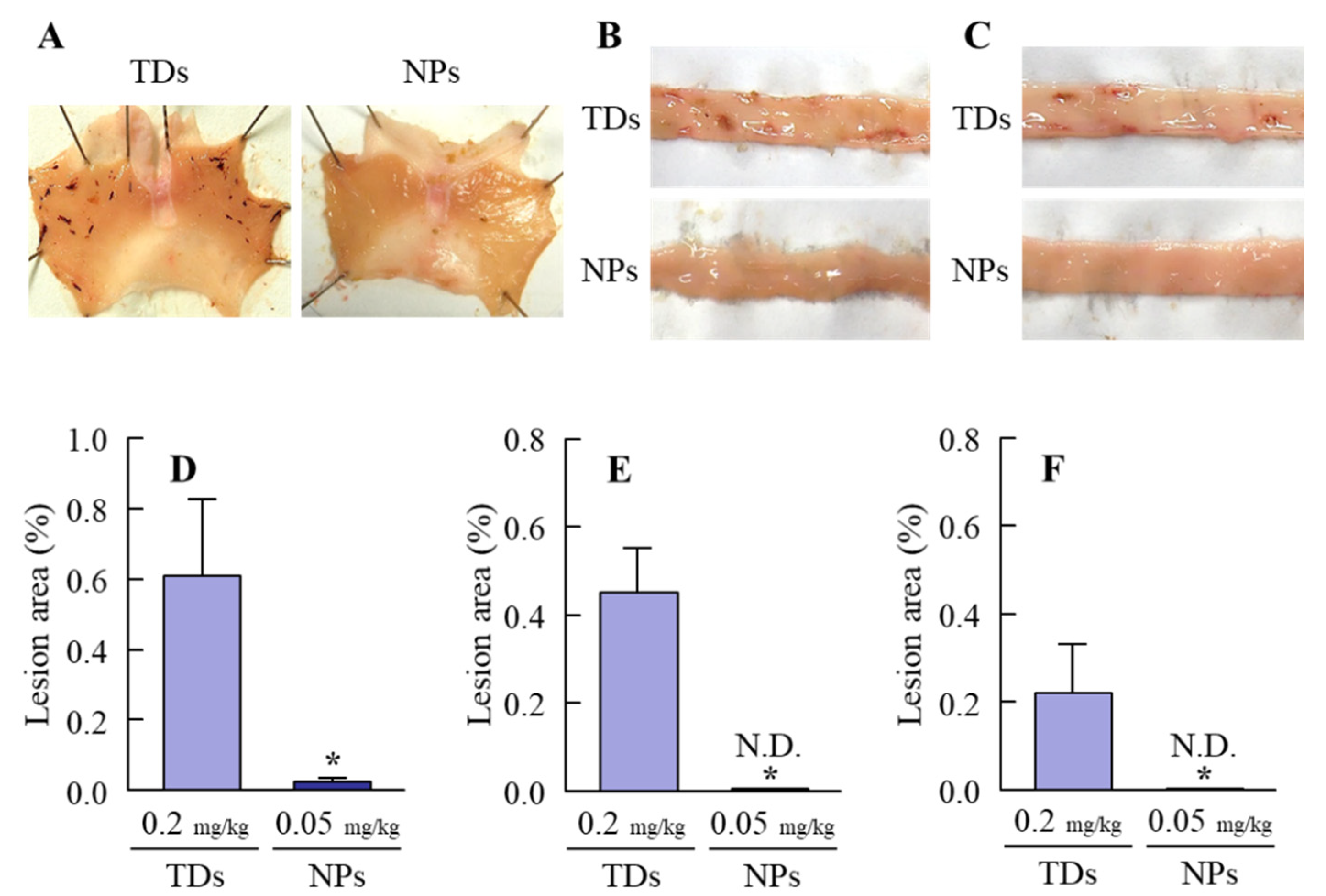
2.8. Lesions in the Gastrointestinal Mucosa after the Oral Administration of MLX
2.9. Anti-Inflammatory Effect of MLX Formulations in AA Rats
2.10. Statistical Analysis
3. Results and Discussion
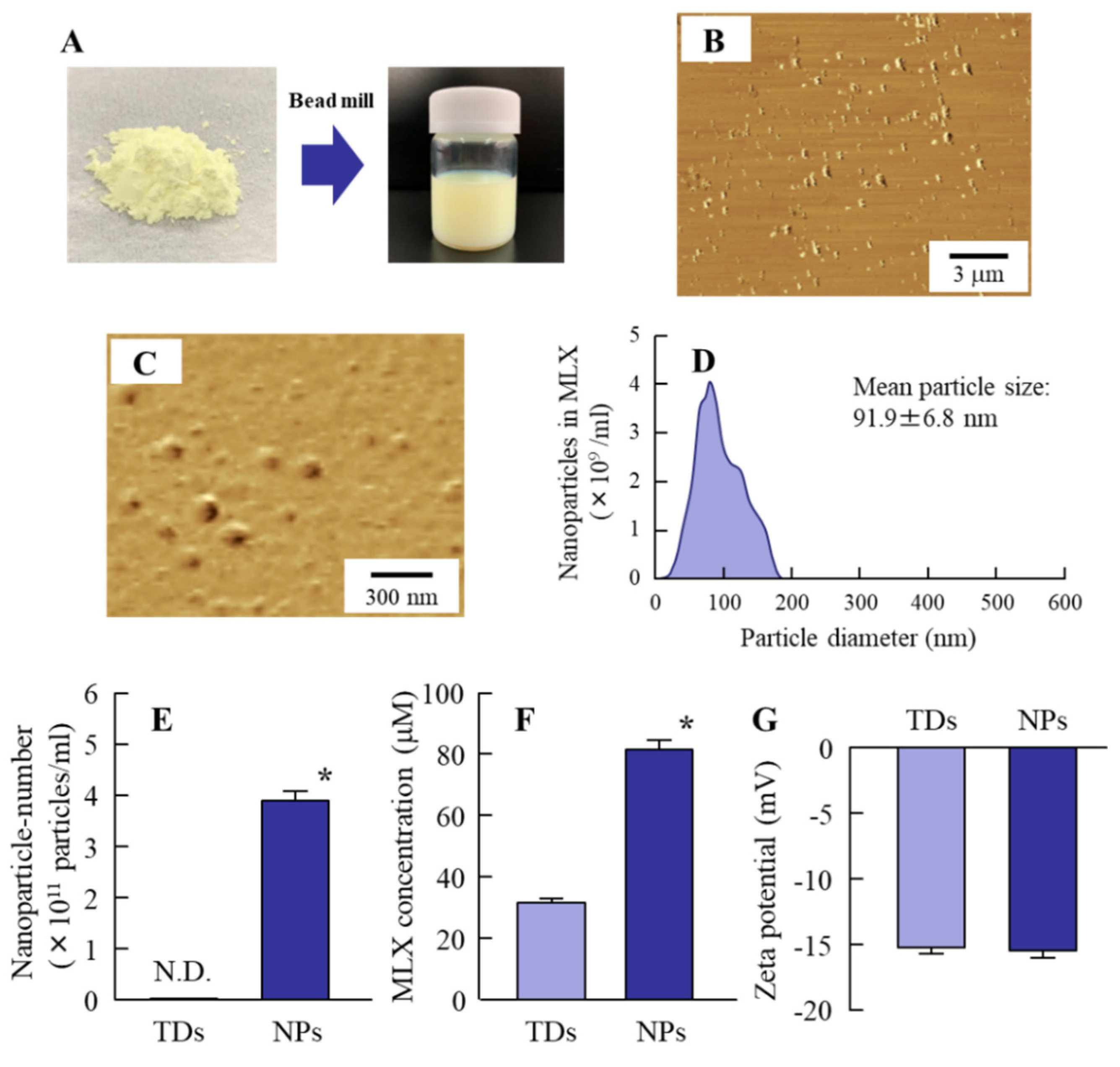
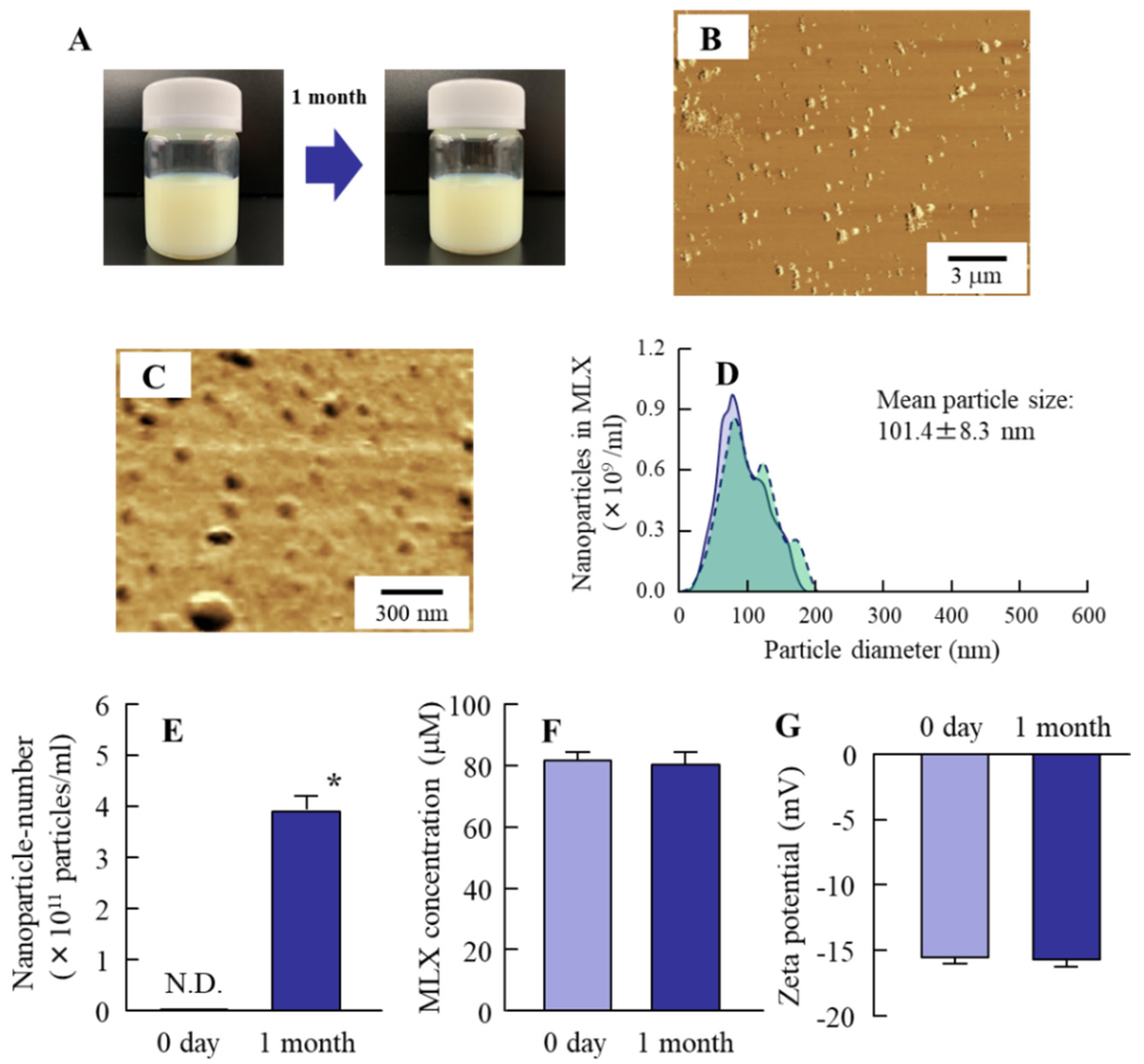
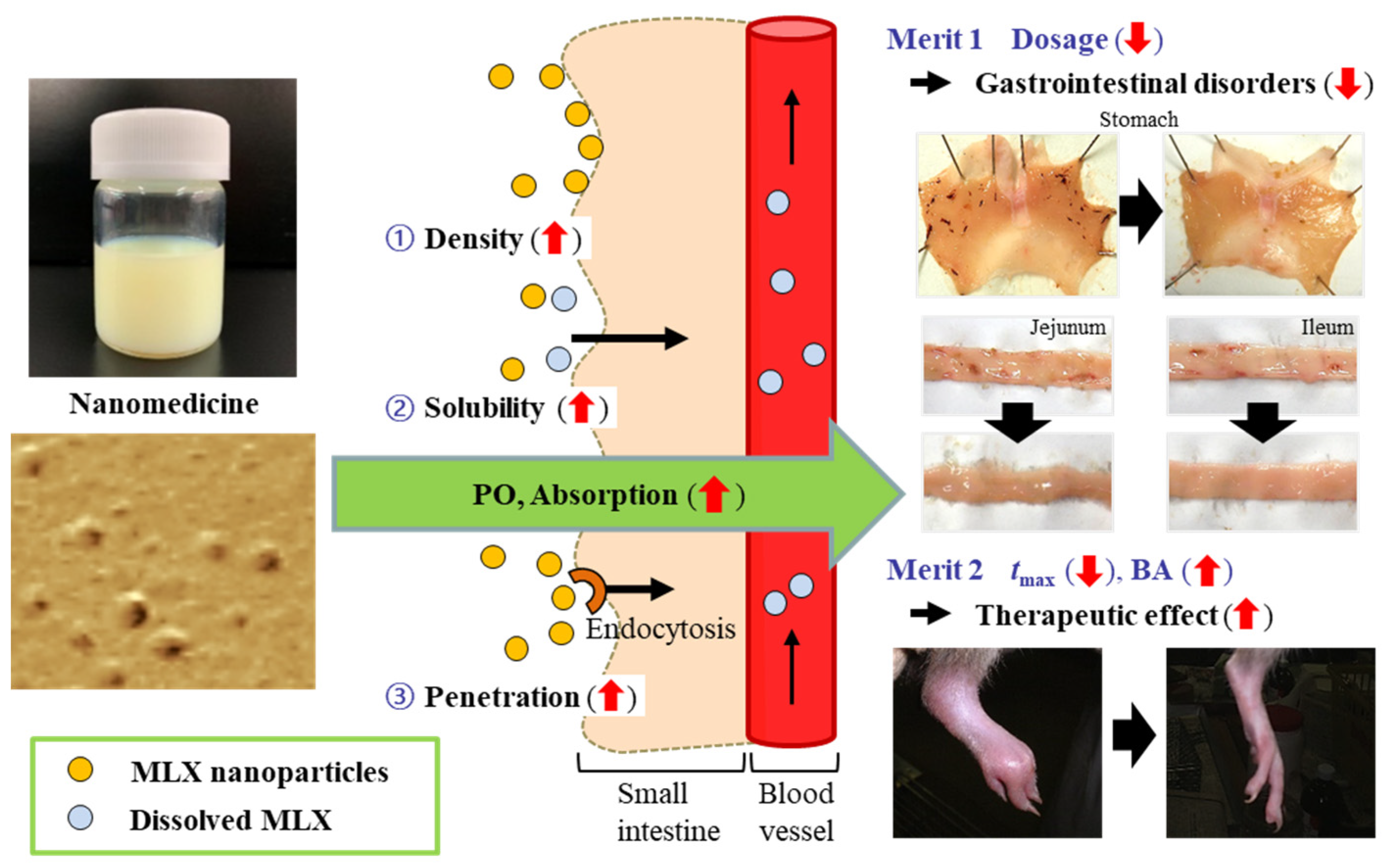
3.1. Preparation of MLX Solid Nanoparticles for Oral Formulations
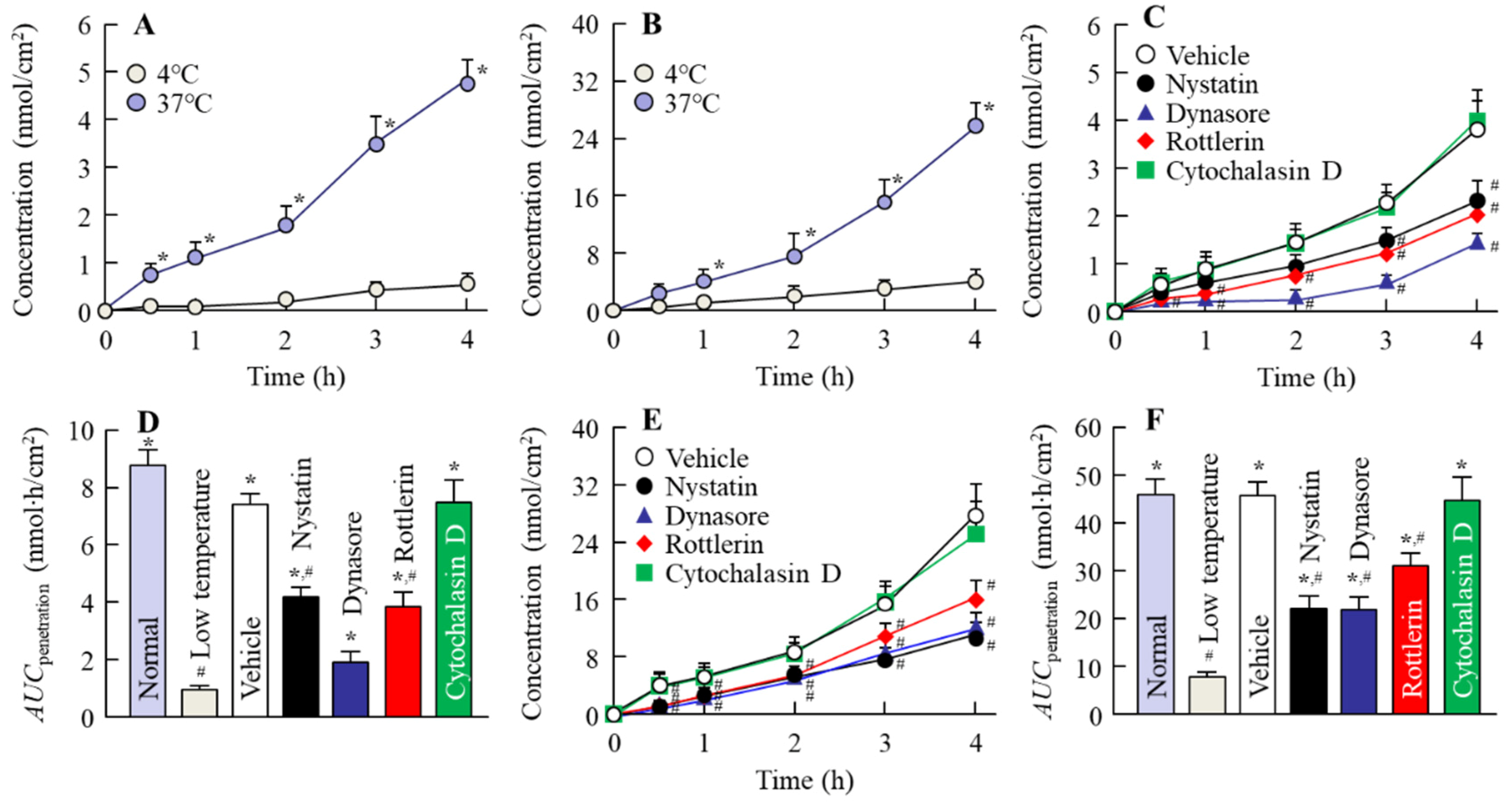
3.2. Relationships between Energy-Dependent Endocytosis and the Intestinal Penetration of MLX Solid Nanoparticles from Orally Administered Formulations
3.3. Usefulness of Oral Formulations of MLX Solid Nanoparticles as Therapy for RA Patients
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ambrus, R.; Kocbek, P.; Kristl, J.; Sibanc, R.; Rajko, R.; Szabo-Revesz, P. Investigation of preparation parameters to improve the dissolution of poorly water-soluble meloxicam. Int. J. Pharm. 2009, 381, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, B.P.; Klinck, M.P.; Moreau, M.; Guillot, M.; Steagall, P.V.M.; Edge, D.K.; Pelletier, J.-P.; Martel-Pelletier, J.; Gauvin, D.; del Castillo, J.R.E.; et al. Analgesic efficacy of an oral transmucosal spray formulation of meloxicam alone or in combination with tramadol in cats with naturally occurring osteoarthritis. Vet. Anaesth. Analg. 2016, 43, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Khachane, P.; Date, A.A.; Nagarsenker, M.S. Positively charged polymeric nanoparticles: Application in improving therapeutic efficacy of meloxicam after oral administration. Die Pharm. 2011, 66, 334–338. [Google Scholar]
- Goldman, A.P.; Williams, C.S.; Sheng, H.; Lamps, L.W.; Williams, V.P.; Pairet, M.; Morrow, J.D.; DuBois, R.N. Meloxicam inhibits the growth of colorectal cancer cells. Carcinogenesis 1998, 19, 2195–2199. [Google Scholar] [CrossRef]
- Hanft, G.; Türck, D.; Scheuerer, S.; Sigmund, R. Meloxicam oral suspension: A treatment alternative to solid meloxicam formulations. Inflamm. Res. 2001, 50, S35–S37. [Google Scholar] [CrossRef] [PubMed]
- Dellgado, D.R.; Jouyban, A.; Martinez, F. Solubility and preferential solvation of meloxicam in methanol + water mixtures at 298.15 K. J. Mol. Liq. 2014, 197, 368–373. [Google Scholar] [CrossRef]
- Del Tacca, M.; Colucci, R.; Fornai, M.; Blandizzi, C. Efficacy and tolerability of meloxicam, a COX-2 preferential nonsteroidal antiinflamatory drug: A review. Clin. Drug Investig. 2002, 22, 799–818. [Google Scholar] [CrossRef]
- Turck, D.; Busch, U.; Heinzel, G.; Narjes, H. Clinical pharmacokinetics of meloxicam. Arzneimittelforschung 1997, 47, 253–258. [Google Scholar]
- Hobbs, D.C. Piroxicam pharmacokinetics: Recent clinical results relating kinetics and plasma levels to age, sex, and adverse effects. Am. J. Med. 1986, 81, 22–28. [Google Scholar]
- Nilsen, O.G. Clinical pharmacokinetics of tenoxicam. Clin. Pharmacokinet. 1994, 26, 16–43. [Google Scholar] [CrossRef]
- Leuner, C.; Dressman, J. Improving drug solubility for oral delivery using solid dispersions. Eur. J. Pharm. Biopharm. 2000, 50, 47–60. [Google Scholar] [CrossRef]
- He, C.X.; He, Z.G.; Gao, J.Q. Microemulsions as drug delivery systems to improve the solubility and the bioavailability of poorly water-soluble drugs. Expert Opin. Drug Deliv. 2010, 7, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Merisko-Liversidge, E.; Liversidge, G.G.; Cooper, E.R. Nanosizing: A formulation approach for poorly-water-soluble compounds. Eur. J. Pharm. Sci. 2003, 18, 113–120. [Google Scholar] [CrossRef]
- Serajuddin, A.T.M. Salt formation to improve drug solubility. Adv. Drug Deliv. Rev. 2007, 59, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Rabinow, B.E. Nanosuspension in drug delivery. Nat. Rev Drug Discov. 2004, 3, 785–796. [Google Scholar] [CrossRef]
- Patravale, V.B.; Date, A.A.; Kulkarni, R.M. Nanosuspensions: A promising drug delivery strategy. J. Pharm. Pharmacol. 2004, 56, 827–840. [Google Scholar] [CrossRef] [Green Version]
- Kesisoglon, F.; Panmai, S.; Wu, Y. Nanosizing oral formulation development and biopharmaceutical evaluation. Adv. Drug. Dev. Rev. 2007, 59, 631–644. [Google Scholar] [CrossRef]
- Nagai, N.; Ogata, F.; Otake, H.; Nakazawa, Y.; Kawasaki, N. Energy-dependent endocytosis is responsible for drug transcorneal penetration following the instillation of ophthalmic formulations containing indomethacin nanoparticles. Int. J. Nanomed. 2019, 14, 1213–1227. [Google Scholar] [CrossRef] [Green Version]
- Nagai, N.; Ogata, F.; Yamaguchi, M.; Fukuoka, Y.; Otake, H.; Nakazawa, Y.; Kawasaki, N. Combination with l-Menthol Enhances Transdermal Penetration of Indomethacin Solid Nanoparticles. Int. J. Mol. Sci. 2019, 20, 3644. [Google Scholar] [CrossRef] [Green Version]
- Nagai, N.; Iwamae, A.; Tanimoto, S.; Yoshioka, C.; Ito, Y. Pharmacokinetics and antiinflammatory effect of a novel gel system containing ketoprofen solid nanoparticles. Biol. Pharm. Bull. 2015, 38, 1918–1924. [Google Scholar] [CrossRef] [Green Version]
- Ishii, M.; Fukuoka, Y.; Deguchi, S.; Otake, H.; Tanino, T.; Nagai, N. Energy-dependent endocytosis is involved in the absorption of indomethacin nanoparticles in the small intestine. Int. J. Mol. Sci. 2019, 20, 476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mäger, I.; Langel, K.; Lehto, T.; Eiríksdóttir, E.; Langel, U. The role of endocytosis on the uptake kinetics of luciferin-conjugated cell-penetrating peptides. Biochim. Biophys. Acta 2012, 1818, 502–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malomouzh, A.I.; Mukhitov, A.R.; Proskurina, S.E.; Vyskocil, F.; Nikolsky, E.E. The effect of dynasore, a blocker of dynamin-dependent endocytosis, on spontaneous quantal and non-quantal release of acetylcholine in murine neuromuscular junctions. Dokl. Biol. Sci. 2014, 459, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Hufnagel, H.; Hakim, P.; Lima, A.; Hollfelder, F. Fluid phase endocytosis contributes to transfection of DNA by PEI-25. Mol. Ther. 2009, 17, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Gawde, K.A.; Kesharwani, P.; Sau, S.; Sarkar, F.H.; Padhye, S.; Kashaw, S.K.; Iyer, A.K. Synthesis and characterization of folate decorated albumin bio-conjugate nanoparticles loaded with a synthetic curcumin difluorinated analogue. J. Colloid Interface Sci. 2017, 496, 290–299. [Google Scholar] [CrossRef]
- Kocbek, P.; Baumgartner, S.; Kristl, J. Preparation and evaluation of nanosuspensions for enhancing the dissolution of poorly soluble drugs. Int. J. Pharm. 2006, 312, 179–186. [Google Scholar] [CrossRef]
- Müller, R.H.; Akkar, A. Encyclopedia of Nanoscience and Nanotechnology; American Scientific Publishers: California, CA, USA, 2004; pp. 624–638. [Google Scholar]
- Moschwitzer, J.P. Drug nanocrystals in the commercial pharmaceutical development process. Int. J. Pharm. 2013, 453, 142–156. [Google Scholar] [CrossRef]
- Quan, P.; Shi, K.; Piao, H.; Liang, N.; Xia, D.; Cui, F. A novel surface modified nitrendipine nanocrystals with enhancement of bioavailability and stability. Int. J. Pharm. 2012, 430, 366–371. [Google Scholar] [CrossRef]
- Abdelwahed, W.; Degobert, G.; Stainmesse, S.; Fessi, H. Freeze-drying of nanoparticles: Formulation, process and storage considerations. Adv. Drug Deliv. Rev. 2006, 58, 1688–1713. [Google Scholar] [CrossRef]
- Gallardo, E.; Sarria, B.; Espartero, J.L.; Correa, J.A.G.; Bravo-Clemente, L.; Mateos, R. Evaluation of the bioavailability and metabolism of nitroderivatives of hydroxytyrosol using Caco-2 and HepG2 human cell models. J. Agric. Food Chem. 2016, 64, 2289–2297. [Google Scholar] [CrossRef]
- Qiu, J.; Kitamura, Y.; Miyata, Y.; Tamaru, S.; Tanaka, K.; Tanaka, T.; Matsui, T. Transepithelial transport of theasinensins through Caco-2 cell monolayers and their absorption in Sprague-Dawley rats after oral administration. J. Agric. Food Chem. 2012, 60, 8036–8043. [Google Scholar] [CrossRef]
- Hughson, E.J.; Cutler, D.F.; Hopkins, C.R. Basolateral secretion of kappa light chain in the polarized epithelial cell line, Caco-2. J. Cell Sci. 1989, 94, 327–332. [Google Scholar] [PubMed]
- Grasset, E.; Pinto, M.; Dussaulx, E.; Zweibaum, A.; Desjeux, J.F. Epithelial properties of human colonic carcinoma cell line Caco-2: Electrical parameters. Am. J. Physiol. 1984, 247, 260–267. [Google Scholar] [CrossRef]
- Finlay, B.B.; Falkow, S. Salmonella interactions with polarized human intestinal Caco-2 epithelial cells. J. Infect. Dis. 1990, 162, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Liu, K.; Manaloto, E.; Casey, A.; Cribaro, G.P.; Byrne, H.J.; Tian, F.; Barcia, C.; Conway, G.E.; Cullen, P.J.; et al. Cold atmospheric plasma induces ATP-dependent endocytosis of nanoparticles and synergistic U373MG cancer cell death. Sci. Rep. 2018, 8, 5298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smalley, W.E.; Ray, W.A.; Daugherty, J.R.; Grin, M.R. Nonsteroidal anti-inflammatory drugs and the incidence of hospitalizations for peptic ulcer disease in elderly persons. Am. J. Epidemiol. 1995, 141, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Fries, J.F.; Williams, C.A.; Bloch, D.A.; Michel, B.A. Non-steroidal anti-inflammatory drug-associated gastropathy: Incidence and risk factor models. Am. J. Med. 1991, 91, 213–222. [Google Scholar] [CrossRef]
- Kato, S.; Ohkawa, F.; Ito, Y.; Amagase, K.; Takeuchi, K. Role of endothelial nitric oxide synthase in aggravation of indomethacin-induced gastric damage in adjuvant arthritic rats. J. Physiol. Pharmacol. 2009, 60, 147–155. [Google Scholar]
- Nagai, N.; Sakamoto, R.; Yamamoto, S.; Deguchi, S.; Otake, H.; Tanino, T. Solid nanocrystals of rebamipide promote recovery from indomethacin-induced gastrointestinal bleeding. Int. J. Mol. Sci. 2019, 20, 4990. [Google Scholar] [CrossRef] [Green Version]
- Nagai, N.; Ito, Y. Effect of solid nanoparticle of indomethacin on therapy for rheumatoid arthritis in adjuvant-induced arthritis rat. Biol. Pharm. Bull. 2014, 37, 1109–1118. [Google Scholar] [CrossRef] [Green Version]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagai, N.; Ogata, F.; Otake, H.; Kawasaki, N. Oral Administration System Based on Meloxicam Nanocrystals: Decreased Dose Due to High Bioavailability Attenuates Risk of Gastrointestinal Side Effects. Pharmaceutics 2020, 12, 313. https://doi.org/10.3390/pharmaceutics12040313
Nagai N, Ogata F, Otake H, Kawasaki N. Oral Administration System Based on Meloxicam Nanocrystals: Decreased Dose Due to High Bioavailability Attenuates Risk of Gastrointestinal Side Effects. Pharmaceutics. 2020; 12(4):313. https://doi.org/10.3390/pharmaceutics12040313
Chicago/Turabian StyleNagai, Noriaki, Fumihiko Ogata, Hiroko Otake, and Naohito Kawasaki. 2020. "Oral Administration System Based on Meloxicam Nanocrystals: Decreased Dose Due to High Bioavailability Attenuates Risk of Gastrointestinal Side Effects" Pharmaceutics 12, no. 4: 313. https://doi.org/10.3390/pharmaceutics12040313
APA StyleNagai, N., Ogata, F., Otake, H., & Kawasaki, N. (2020). Oral Administration System Based on Meloxicam Nanocrystals: Decreased Dose Due to High Bioavailability Attenuates Risk of Gastrointestinal Side Effects. Pharmaceutics, 12(4), 313. https://doi.org/10.3390/pharmaceutics12040313





