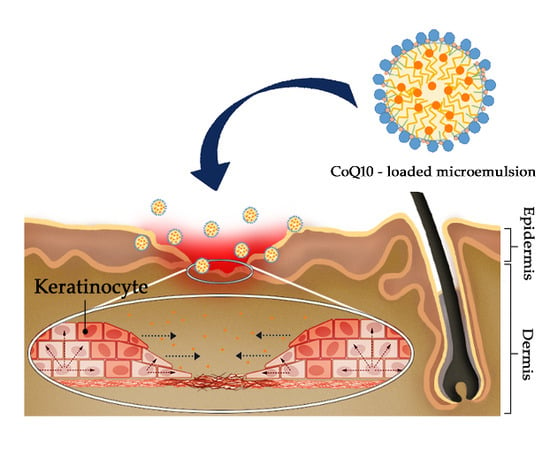
Topical Delivery of Coenzyme Q10-Loaded Microemulsion for Skin Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Selection of Oils, Surfactants, and Co-Surfactant Mixtures for Microemulsions
2.3. Quantitative Analysis of CoQ10
2.4. Construction of Pseudo-Ternary Phase Diagram
2.5. Preparation of CoQ10-Loaded o/w Microemulsion
2.6. Characterization of CoQ10-Loaded o/w Microemulsion
2.6.1. Stability Assessment
2.6.2. Entrapment Efficacy of CoQ10 in o/w Microemulsion
2.6.3. Measurement of Droplet Size and Zeta Potential Value
2.7. In Vitro Cell Experiment
2.7.1. Preparation of Cells
2.7.2. Cell Proliferation Assay
2.7.3. Wound Healing Scratch Assay
2.8. Permeation Experiment
2.8.1. In Vitro Membrane Permeation Experiment
2.8.2. In Vitro Skin Permeation Experiment
2.9. Statistics
3. Results
3.1. Screening of CoQ10 in Oils, Surfactants, and Co-Surfactants
3.2. Phase Behavior
3.3. Optimization of CoQ10-Loaded o/w Microemulsion
3.4. Physicochemical characterization of CoQ10-loaded o/w microemulsion
3.5. Permeation Experiments
3.6. Effect of CoQ10 on Skin Regeneration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nestle, F.O.; Di Meglio, P.; Qin, J.Z.; Nickoloff, B.J. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 2009, 9, 679–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baroni, A.; Buommino, E.; De Gregorio, V.; Ruocco, E.; Ruocco, V.; Wolf, R. Structure and function of the epidermis related to barrier properties. Clin. Dermatol. 2012, 30, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Proksch, E.; Brandner, J.M.; Jensen, J.M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Bolzinger, M.A.; Briançon, S.; Pelletier, J.; Chevalier, Y. Penetration of drugs through skin, a complex rate-controlling membrane. Curr. Opin. Colloid Interface Sci. 2012, 17, 156–165. [Google Scholar] [CrossRef]
- Kalinin, A.E.; Kajava, A.V.; Steinert, P.M. Epithelial barrier function: Assembly and structural features of the cornified cell envelope. Bioessays 2002, 24, 789–800. [Google Scholar] [CrossRef]
- Harding, C.R. The stratum corneum: Structure and function in health and disease. Dermatol. Ther. 2004, 17, 6–15. [Google Scholar] [CrossRef]
- Touitou, E.; Godin, B.; Weiss, C. Enhanced delivery of drugs into and across the skin by ethosomal carriers. Drug Dev. Res. 2000, 50, 406–415. [Google Scholar] [CrossRef]
- Rawlings, A.; Harding, C. Moisturization and skin barrier function. Dermatol. Ther. 2004, 17, 43–48. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Mitragotri, S.; Langer, R. Current status and future potential of transdermal drug delivery. Nat. Rev. Drug Discov. 2004, 3, 115–124. [Google Scholar] [CrossRef]
- Alkilani, A.Z.; McCrudden, M.T.; Donnelly, R.F. Transdermal drug delivery: Innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [Green Version]
- Bos, J.D.; Meinardi, M.M. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp. Dermatol. 2000, 9, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Schoellhammer, C.M.; Blankschtein, D.; Langer, R. Skin permeabilization for transdermal drug delivery: Recent advances and future prospects. Expert Opin. Drug Deliv. 2014, 11, 393–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moniruzzaman, M.; Tahara, Y.; Tamura, M.; Kamiya, N.; Goto, M. Ionic liquid-assisted transdermal delivery of sparingly soluble drugs. Chem. Commun. 2010, 46, 1452–1454. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Agrawal, U.; Vyas, S.P. Nanocarrier-based topical drug delivery for the treatment of skin diseases. Expert Opin. Drug Deliv. 2012, 9, 783–804. [Google Scholar] [CrossRef] [PubMed]
- Das Kurmi, B.; Tekchandani, P.; Paliwal, R.; Rai Paliwal, S. Transdermal drug delivery: Opportunities and challenges for controlled delivery of therapeutic agents using nanocarriers. Curr. Drug Metab. 2017, 18, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.; Watkinson, A.; Hadgraft, J.; Lane, M. Application of microemulsions in dermal and transdermal drug delivery. Skin Pharmacol. Physiol. 2008, 21, 246–259. [Google Scholar] [CrossRef]
- Lawrence, M.J.; Rees, G.D. Microemulsion-based media as novel drug delivery systems. Adv. Drug Delivery Rev. 2000, 45, 89–121. [Google Scholar] [CrossRef]
- Schulman, J.H.; Stoeckenius, W.; Prince, L.M. Mechanisms of formation and structure of micro emulsions by electron microscopy. J. Phys. Chem. 1959, 63, 1677–1680. [Google Scholar] [CrossRef]
- Kogan, A.; Garti, N. Microemulsions as transdermal drug delivery vehicles. Adv. Colloid Interface Sci. 2006, 123, 369–385. [Google Scholar] [CrossRef]
- Heuschkel, S.; Goebel, A.; Neubert, R.H. Microemulsions—Modern colloidal carrier for dermal and transdermal drug delivery. J. Pharm. Sci. 2008, 97, 603–631. [Google Scholar] [CrossRef]
- Date, A.A.; Nagarsenker, M. Parenteral microemulsions: An overview. Int. J. Pharm. 2008, 355, 19–30. [Google Scholar] [CrossRef]
- Choi, J.H.; Ryu, Y.W.; Seo, J.H. Biotechnological production and applications of coenzyme Q 10. Appl. Microbiol. Biotechnol. 2005, 68, 9–15. [Google Scholar] [CrossRef]
- Federico, A.; Cardaioli, E.; Da Pozzo, P.; Formichi, P.; Gallus, G.N.; Radi, E. Mitochondria, oxidative stress and neurodegeneration. J. Neurol. Sci. 2012, 322, 254–262. [Google Scholar] [CrossRef]
- Villalba, J.M.; Parrado, C.; Santos-Gonzalez, M.; Alcain, F.J. Therapeutic use of coenzyme Q10 and coenzyme Q10-related compounds and formulations. Expert Opin. Invest. Drugs 2010, 19, 535–554. [Google Scholar] [CrossRef]
- Singh, R.B.; Wander, G.S.; Rastogi, A.; Shukla, P.K.; Mittal, A.; Sharma, J.P.; Mehrotra, S.K.; Kapoor, R.; Chopra, R.K. Randomized, double-blind placebo-controlled trial of coenzyme Q10 in patients with acute myocardial infarction. Cardiovasc. Drugs Ther. 1998, 12, 347–353. [Google Scholar] [CrossRef]
- Overvad, K.; Diamant, B.; Holm, L.; Hølmer, G.; Mortensen, S.; Stender, S. Coenzyme Q 10 in health and disease. Eur. J. Clin. Nutr. 1999, 53, 764–770. [Google Scholar] [CrossRef] [Green Version]
- Baggio, E.; Gandini, R.; Plancher, A.C.; Passeri, M.; Carmosino, G. Italian multicenter study on the safety and efficacy of coenzyme Q10 as adjunctive therapy in heart failure. Mol. Aspects Med. 1994, 15, s287–s294. [Google Scholar] [CrossRef]
- Lee, B.J.; Tseng, Y.F.; Yen, C.H.; Lin, P.T. Effects of coenzyme Q10 supplementation (300 mg/day) on antioxidation and anti-inflammation in coronary artery disease patients during statins therapy: A randomized, placebo-controlled trial. Nutr. J. 2013, 12, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, B.S.; Song, H.S.; Kim, H.R.; Park, T.W.; Kim, T.D.; Cho, B.J.; Kim, C.J.; Sim, S.S. Effect of coenzyme Q10 on cutaneous healing in skin-incised mice. Arch. Pharmacal Res. 2009, 32, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Martinotti, S.; Ranzato, E. Scratch wound healing assay. Methods Mol. Biol. 2020, 2019, 225–229. [Google Scholar]
- Parrado, C.; Mercado-Saenz, S.; Perez-Davo, A.; Gilaberte, Y.; Gonzalez, S.; Juarranz, A. Environmental stressors on skin aging. Mechanistic Insights. Front. Pharmacol. 2019, 10, 759. [Google Scholar] [CrossRef]
- Paul, M. Wound healing—Aiming for perfect skin regeneration. Science 1997, 276, 75–81. [Google Scholar]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug delivery systems and materials for wound healing applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef] [PubMed]
- Funke, A.P.; Schiller, R.; Motzkus, H.W.; Günther, C.; Müller, R.H.; Lipp, R. Transdermal delivery of highly lipophilic drugs: In vitro fluxes of antiestrogens, permeation enhancers, and solvents from liquid formulations. Pharm. Res. 2000, 19, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Agatonovic-Kustrin, S.; Glass, B.D.; Wisch, M.H. Strategy for the development of a thermodynamically stable oral microemulsion. Curr. Drug Discov. Technol. 2004, 1, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Spernath, A.; Aserin, A. Microemulsions as carriers for drugs and nutraceuticals. Adv. Colloid Interface Sci. 2006, 128, 47–64. [Google Scholar] [CrossRef]
- Shah, A.V.; Desai, H.H.; Thool, P.; Dalrymple, D.; Serajuddin, A.T. Development of self-microemulsifying drug delivery system for oral delivery of poorly water-soluble nutraceuticals. Drug Dev. Ind. Pharm. 2018, 44, 895–901. [Google Scholar] [CrossRef]
- El-Leithy, E.S.; Makky, A.M.; Khattab, A.M.; Hussein, D.G. Optimization of nutraceutical coenzyme Q10 nanoemulsion with improved skin permeability and anti-wrinkle efficiency. Drug Dev. Ind. Pharm. 2018, 44, 316–328. [Google Scholar] [CrossRef]
- Uchiyama, H.; Chae, J.; Kadota, K.; Tozuka, Y. Formation of food grade microemulsion with rice glycosphingolipids to enhance the oral absorption of coenzyme Q10. Foods 2019, 8, 502. [Google Scholar] [CrossRef] [Green Version]
- Karande, P.; Mitragotri, S. Enhancement of transdermal drug delivery via synergistic action of chemicals. Biochim. Biophys. Acta 2009, 1788, 2362–2373. [Google Scholar] [CrossRef] [Green Version]
- Nastiti, C.M.; Ponto, T.; Abd, E.; Grice, J.E.; Benson, H.A.; Roberts, M.S. Topical nano and microemulsions for skin delivery. Pharmaceutics 2017, 9, 37. [Google Scholar] [CrossRef]
- Gupta, S.; Moulik, S.P. Biocompatible microemulsions and their prospective uses in drug delivery. J. Pharm. Sci. 2008, 97, 22–45. [Google Scholar] [CrossRef] [PubMed]
- Raval, N.; Khunt, D.; Misra, M. Microemulsion-based delivery of triamcinolone acetonide to posterior segment of eye using chitosan and butter oil as permeation enhancer: An in vitro and in vivo investigation. J. Microencapsul. 2018, 35, 62–77. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Xin, X.; Zhang, Y. Development and characterization of promising Cremophor EL-stabilized o/w nanoemulsions containing short-chain alcohols as a cosurfactant. RSC Adv. 2017, 7, 19815–19827. [Google Scholar] [CrossRef] [Green Version]
- Bhakay, A.; Rahman, M.; Dave, R.N.; Bilgili, E. Bioavailability enhancement of poorly water-soluble drugs via nanocomposites: Formulation–processing aspects and challenges. Pharmaceutics 2018, 10, 86. [Google Scholar] [CrossRef] [Green Version]
- Osborne, D.W.; Musakhanian, J. Skin penetration and permeation properties of Transcutol®—neat or diluted mixtures. AAPS PharmSciTech. 2018, 19, 3512–3533. [Google Scholar] [CrossRef]
- Leung, R.; Shah, D.O. Solubilization and phase equilibria of water-in-oil microemulsions: I. Effects of spontaneous curvature and elasticity of interfacial films. J. Colloid Interface Sci. 1987, 120, 320–329. [Google Scholar] [CrossRef]
- Djekic, L.; Primorac, M.; Filipic, S.; Agbaba, D. Investigation of surfactant/cosurfactant synergism impact on ibuprofen solubilization capacity and drug release characteristics of nonionic microemulsions. Int. J. Pharm. 2012, 433, 25–33. [Google Scholar] [CrossRef]
- Wojtowicz, A.M.; Oliveira, S.; Carlson, M.W.; Zawadzka, A.; Rousseau, C.F.; Baksh, D. The importance of both fibroblasts and keratinocytes in a bilayered living cellular construct used in wound healing. Wound Repair Regen. 2014, 22, 246–255. [Google Scholar] [CrossRef] [Green Version]





| Substance | Solubility (mg/mL) | Substance | Solubility (mg/mL) |
|---|---|---|---|
| Isopropyl myristate (IPM) | 209.88 ± 7.42 | Tween 80 | 21.88 ± 12.06 |
| Oleic acid (OA) | 87.36 ± 34.59 | Cremophor EL® | 33.10 ± 11.71 |
| IPM:OA (3:1) | 143.50 ± 18.97 | Labarasol | 26.53 ± 12.88 |
| IPM:OA (2:1) | 137.53 ± 14.17 | PG | 2.13 ± 2.13 |
| Labrafil M1944 | 86.80 ± 12.98 | Transcutol® HP | 9.35 ± 1.96 |
| Cottonseed oil | 107.12 ± 23.16 | PEG-400 | 0.24 ± 0.21 |
| Formulation | S/CoS ratio | Composition of ME w/w % * | Drug (%) | Dilution Test | Thermodynamic Stability Test | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Oil | S/CoS | Water | Centrifugation | Heating-Cooling | Freeze-Thaw | Decision | ||||
| F1 | 2:1 | 5.06 | 35.44 | 59.5 | 0.5 | Clear | √ | √ | √ | Pass |
| F2 | 5.06 | 35.44 | 59.5 | 1 | Clear | √ | √ | √ | Pass | |
| F3 | 6.56 | 32.79 | 60.65 | 0.5 | Clear | √ | × | - | Failed | |
| F4 | 6.56 | 32.79 | 60.65 | 1 | Clear | √ | × | - | Failed | |
| F5 | 5.8 | 40.58 | 53.62 | 0.5 | Clear | √ | √ | △ | Failed | |
| F6 | 5.8 | 40.58 | 53.62 | 1 | Clear | √ | √ | △ | Failed | |
| F7 | 5 | 45 | 50 | 0.5 | Clear | √ | √ | × | Failed | |
| F8 | 5 | 45 | 50 | 1 | Clear | √ | √ | × | Failed | |
| F9 | 3:1 | 5.06 | 35.44 | 59.5 | 0.5 | Clear | √ | √ | √ | Pass |
| F10 | 5.06 | 35.44 | 59.5 | 1 | Clear | √ | √ | √ | Pass | |
| F11 | 6.56 | 32.79 | 60.65 | 0.5 | Clear | √ | △ | × | Failed | |
| F12 | 6.56 | 32.79 | 60.65 | 1 | Clear | √ | △ | × | Failed | |
| F13 | 5.8 | 40.58 | 53.62 | 0.5 | Clear | √ | △ | △ | Failed | |
| F14 | 5.8 | 40.58 | 53.62 | 1 | Clear | √ | △ | △ | Failed | |
| F15 | 5 | 45 | 50 | 0.5 | Clear | √ | × | - | Failed | |
| F16 | 5 | 45 | 50 | 1 | Clear | √ | × | - | Failed | |
| F17 | 4:1 | 5.06 | 35.44 | 59.5 | 0.5 | Clear | √ | √ | √ | Pass |
| F18 | 5.06 | 35.44 | 59.5 | 1 | Clear | √ | √ | √ | Pass | |
| F19 | 6.56 | 32.79 | 60.65 | 0.5 | Clear | √ | △ | × | Failed | |
| F20 | 6.56 | 32.79 | 60.65 | 1 | Clear | √ | △ | × | Failed | |
| F21 | 5.8 | 40.58 | 53.62 | 0.5 | Turbid | - | - | - | Failed | |
| F22 | 5.8 | 40.58 | 53.62 | 1 | Turbid | - | - | - | Failed | |
| F23 | 5 | 45 | 50 | 0.5 | Turbid | - | - | - | Failed | |
| F24 | 5 | 45 | 50 | 1 | Turbid | - | - | - | Failed | |
| Formulation | S/CoS Ratio | Composition of ME w/w % * | Drug (%) | Physicochemical Properties | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Oil | S/CoS | Water | Droplet Size (nm) | PDI | Zeta Potential (mV) | Drug Content(%) | pH | |||
| F2 | 2:1 | 5.06 | 35.44 | 59.5 | 1 | 16.89 ± 0.050 | 0.040 ± 0.012 | −13.1 ± 1.32 | 102.01 ± 0.128 | 7.12 ± 0.064 |
| F10 | 3:1 | 5.06 | 35.44 | 59.5 | 1 | 16.72 ± 0.055 | 0.058 ± 0.007 | −14.7 ± 1.23 | 101.7 ± 0.41 | 7.01 ± 0.021 |
| F18 | 4:1 | 5.06 | 35.44 | 59.5 | 1 | 16.65 ± 0.172 | 0.080 ± 0.006 | −11.8 ± 1.01 | 101.62 ± 0.4 | 7.00 ± 0.042 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, K.-A.; Park, P.J.; Kim, S.-B.; Bin, B.-H.; Jang, D.-J.; Kim, S.T. Topical Delivery of Coenzyme Q10-Loaded Microemulsion for Skin Regeneration. Pharmaceutics 2020, 12, 332. https://doi.org/10.3390/pharmaceutics12040332
Ryu K-A, Park PJ, Kim S-B, Bin B-H, Jang D-J, Kim ST. Topical Delivery of Coenzyme Q10-Loaded Microemulsion for Skin Regeneration. Pharmaceutics. 2020; 12(4):332. https://doi.org/10.3390/pharmaceutics12040332
Chicago/Turabian StyleRyu, Kyeong-A, Phil June Park, Seong-Bo Kim, Bum-Ho Bin, Dong-Jin Jang, and Sung Tae Kim. 2020. "Topical Delivery of Coenzyme Q10-Loaded Microemulsion for Skin Regeneration" Pharmaceutics 12, no. 4: 332. https://doi.org/10.3390/pharmaceutics12040332
APA StyleRyu, K.-A., Park, P. J., Kim, S.-B., Bin, B.-H., Jang, D.-J., & Kim, S. T. (2020). Topical Delivery of Coenzyme Q10-Loaded Microemulsion for Skin Regeneration. Pharmaceutics, 12(4), 332. https://doi.org/10.3390/pharmaceutics12040332