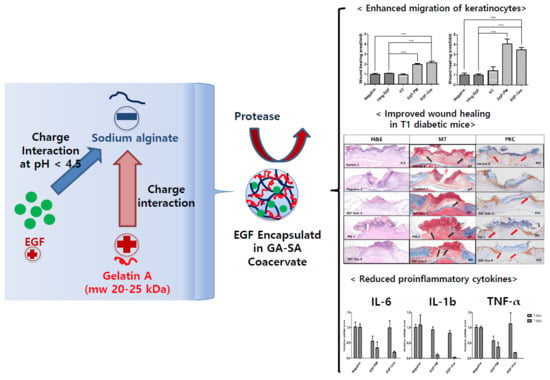
Improved Diabetic Wound Healing by EGF Encapsulation in Gelatin-Alginate Coacervates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication and Characterization of LWGA-SA Coacervates Encapsulating EGF (EGF-Coa)
2.3. In Vitro Cell Activity Assay
2.3.1. HaCaT Viability Assay
2.3.2. HaCaT Scratch Wound Assay
2.4. In Vivo Study
2.4.1. Streptozotocin (STZ)-Induced Diabetic Mouse Model
2.4.2. Wound Healing Assay in STZ-Induced Diabetic Mouse Model
2.4.3. Histological Analysis
2.5. Stability Test of Freeze-Dried EGF-Coa
3. Results
3.1. Characterization of EGF-PM and EGF-Coa
3.2. In Vitro Activity of EGF-Coacervates
3.3. Wound Healing Efficacy of EGF-Coa in Diabetic Wound Model in Mice
3.4. Histological Analysis
3.5. Inflammatory Cytokine Levels
3.6. Stability of Freeze-Dried EGF-Coa
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- He, J.; Liang, Y.; Shi, M.; Guo, B. Anti-oxidant electroactive and antibacterial nanofibrous wound dressings based on poly (ε-caprolactone)/quaternized chitosan-graft-polyaniline for full-thickness skin wound healing. Chem. Eng. J. 2020, 385, 123464. [Google Scholar] [CrossRef]
- Xue, H.; Hu, L.; Xiong, Y.; Zhu, X.; Wei, C.; Cao, F.; Zhou, W.; Sun, Y.; Endo, Y.; Liu, M.; et al. Quaternized chitosan-Matrigel-polyacrylamide hydrogels as wound dressing for wound repair and regeneration. Carbohydr. Polym. 2019, 226, 115302. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Aoki, H.; Nakamura, S.; Nakamura, S.-I.; Takikawa, M.; Hanzawa, M.; Kishimoto, S.; Hattori, H.; Tanaka, Y.; Kiyosawa, T.; et al. Hydrogel blends of chitin/chitosan, fucoidan and alginate as healing-impaired wound dressings. Biomaterials 2010, 31, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Lobmann, R.; Schultz, G.; Lehnert, H. Proteases and the diabetic foot syndrome: Mechanisms and therapeutic implications. Diabetes Care 2005, 28, 461–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Traversa, B. The role of growth factors, cytokines and proteases in wound management. Aust. J. Wound Manag. 2001, 9, 161–167. [Google Scholar]
- Devalliere, J.; Dooley, K.; Hu, Y.; Kelangi, S.S.; Uygun, B.; Yarmush, M.L.; Yu, Y. Co-delivery of a growth factor and a tissue-protective molecule using elastin biopolymers accelerates wound healing in diabetic mice. Biomaterials 2017, 141, 149–160. [Google Scholar] [CrossRef]
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Acute and impaired wound healing: Pathophysiology and current methods for drug delivery, part 1: Normal and chronic wounds: Biology, causes, and approaches to care. Adv. Skin Wound Care 2012, 25, 304–314. [Google Scholar] [CrossRef] [Green Version]
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Acute and impaired wound healing: Pathophysiology and current methods for drug delivery, part 2: Role of growth factors in normal and pathological wound healing: Therapeutic potential and methods of delivery. Adv. Skin Wound Care 2012, 25, 349–370. [Google Scholar] [CrossRef] [Green Version]
- Gokce, E.H.; Tanrıverdi, S.T.; Eroglu, I.; Tsapis, N.; Gokce, G.; Tekmen, I.; Fattal, E.; Ozer, O. Wound healing effects of collagen-laminin dermal matrix impregnated with resveratrol loaded hyaluronic acid-DPPC microparticles in diabetic rats. Eur. J. Pharm. Biopharm. 2017, 119, 17–27. [Google Scholar] [CrossRef]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in Wound Healing: A Comprehensive Review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef] [Green Version]
- Berlanga-Acosta, J.; Schultz, G.S.; López-Mola, E.; Guillen-Nieto, G.; García-Siverio, M.; Herrera-Martínez, L. Glucose Toxic Effects on Granulation Tissue Productive Cells: The Diabetics’ Impaired Healing. BioMed Res. Int. 2012, 2013, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, K.H.; Park, S.H.; Kim, M.K.; Park, H.D.; Son, T.I. Stabilization of epidermal growth factor on thermal and proteolytic degradation by conjugating with low molecular weight chitosan. J. Appl. Polym. Sci. 2006, 102, 5072–5082. [Google Scholar] [CrossRef]
- Okumura, K.; Kiyohara, Y.; Komada, F.; Iwakawa, S.; Hirai, M.; Fuwa, T. Improvement in wound healing by epidermal growth factor (EGF) ointment. I. Effect of nafamostat, gabexate, or gelatin on stabilization and efficacy of EGF. Pharm. Res. 1990, 7, 1289–1293. [Google Scholar] [CrossRef] [PubMed]
- Lau, H.-C.; Kim, A. Pharmaceutical perspectives of impaired wound healing in diabetic foot ulcer. J. Pharm. Investig. 2016, 46, 403–423. [Google Scholar] [CrossRef]
- Kim, H.; Kong, W.H.; Seong, K.-Y.; Sung, D.K.; Jeong, H.; Kim, J.K.; Yang, S.Y.; Hahn, S.K. Hyaluronate—Epidermal Growth Factor Conjugate for Skin Wound Healing and Regeneration. Biomacromolecules 2016, 17, 3694–3705. [Google Scholar] [CrossRef]
- Lau, H.C.; Jeong, S.; Kim, A. Gelatin-alginate coacervates for circumventing proteolysis and probing intermolecular interactions by SPR. Int. J. Biol. Macromol. 2018, 117, 427–434. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, B.; Lau, H.C.; Kim, A. Gelatin-Alginate Complexes for EGF Encapsulation: Effects of H-Bonding and Electrostatic Interactions. Pharmaceutics 2019, 11, 530. [Google Scholar] [CrossRef] [Green Version]
- Johnson, N.R.; Ambe, T.; Wang, Y. Lysine-based polycation:heparin coacervate for controlled protein delivery. Acta Biomater. 2013, 10, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Chu, H.; Gao, J.; Chen, W.C.; Huard, J.; Wang, Y. Injectable fibroblast growth factor-2 coacervate for persistent angiogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 13444–13449. [Google Scholar] [CrossRef] [Green Version]
- Jeon, O.; Wolfson, D.W.; Alsberg, E. In situ forming growth factor-loaded coacervate microparticle-embedded hydrogel for directing encapsulated stem cell fate. Adv. Mater. 2015, 27, 2216–2223. [Google Scholar] [CrossRef]
- Jeong, S.; Jeong, S.; Chung, S.; Kim, A. Revisiting in vitro release test for topical gel formulations: The effect of osmotic pressure explored for better bio-relevance. Eur. J. Pharm. Sci. 2018, 112, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.-J.; Lim, H.-S.; Seo, C.-S.; Jin, S.-E.; Yoo, S.-R.; Lee, N.; Shin, H.-K. Anti-inflammatory Actions of Herbal Formula Gyejibokryeong-Hwan Regulated by Inhibiting Chemokine Production and STAT1 Activation in HaCaT Cells. Boil. Pharm. Bull. 2015, 38, 425–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruse, C.R.; Singh, M.; Sørensen, J.A.; Eriksson, E.; Nuutila, K. The effect of local hyperglycemia on skin cells in vitro and on wound healing in euglycemic rats. J. Surg. Res. 2016, 206, 418–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, C.C.; Liu, I.H.; Fang, A.H.; Wen, C.H.; Wu, C.S. Hyperglycaemic conditions decrease cultured keratinocyte mobility: Implications for impaired wound healing in patients with diabetes. Br. J. Dermatol. 2008, 159, 1103–1115. [Google Scholar] [CrossRef]
- Leguina-Ruzzi, A.; Valderas, J.P. BLT2 expression improves skin integrity and protects from alterations caused by hyperglycemia in type 2 diabetes. Dermato-Endocrinology 2016, 9, e1267078. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Zhang, J.; Zhang, Q.; Zhang, D.; Xiang, F.; Jia, J.; Wei, P.; Zhang, J.; Hu, J.; Huang, Y. High Glucose Suppresses Keratinocyte Migration Through the Inhibition of p38 MAPK/Autophagy Pathway. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Burns, N.; Gold, B. The effect of 3-methyladenine DNA glycosylase-mediated DNA repair on the induction of toxicity and diabetes by the beta-cell toxicant streptozotocin. Toxicol. Sci. 2007, 95, 391–400. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.-H.; Bae, J.-S.; Hahm, K.B.; Cha, J.-Y. Endogenously synthesized n-3 polyunsaturated fatty acids in fat-1 mice ameliorate high-fat diet-induced non-alcoholic fatty liver disease. Biochem. Pharmacol. 2012, 84, 1359–1365. [Google Scholar] [CrossRef]
- Handbook of Chemistry and Physics, 55th ed.; CRC Press: Boca Raton, FL, USA, 1974.
- Kang, S.-W.; Choi, J.-J.; Kim, H.-N.; Seo, J.; Park, S.-J.; Kim, E.-Y.; Park, S.-P.; Huh, K.M.; Chung, H.-M.; Chung, H.Y.; et al. EGF-Loaded Hyaluronic Acid Based Microparticles as Effective Carriers in a Wound Model. Part. Part. Syst. Charact. 2017, 34, 1600320. [Google Scholar] [CrossRef]
- Schreier, T.; Degen, E.; Baschong, W. Fibroblast migration and proliferation during in vitro wound healing. A quantitative comparison between various growth factors and a low molecular weight blood dialysate used in the clinic to normalize impaired wound healing. Res. Exp. Med. 1993, 193, 195. [Google Scholar] [CrossRef]
- Qing, C. The molecular biology in wound healing & non-healing wound. Chin. J. Traumatol. 2017, 20, 189–193. [Google Scholar]
- Rowe, D.W.; Starman, B.J.; Fujimoto, W.Y.; Williams, R.H. Abnormalities in proliferation and protein synthesis in skin fibroblast cultures from patients with diabetes mellitus. Diabetes 1977, 26, 284. [Google Scholar] [CrossRef]
- Hehenberger, K.; Hansson, A. High glucose-induced growth factor resistance in human fibroblasts can be reversed by antioxidants and protein kinase C-inhibitors. Cell Biochem. Funct. 1997, 15, 197–201. [Google Scholar] [CrossRef]
- Choi, S.M.; Lee, K.-M.; Kim, H.J.; Park, I.K.; Kang, H.J.; Shin, H.-C.; Baek, D.; Choi, Y.; Park, K.H.; Lee, J.W. Effects of structurally stabilized EGF and bFGF on wound healing in type I and type II diabetic mice. Acta Biomater. 2018, 66, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Gainza, G.; Pastor, M.; Aguirre, J.J.; Villullas, S.; Pedraz, J.L.; Bayat, A.; Igartua, M. A novel strategy for the treatment of chronic wounds based on the topical administration of rhEGF-loaded lipid nanoparticles: In vitro bioactivity and in vivo effectiveness in healing-impaired db/db mice. J. Control. Release 2014, 185, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, M.F.; Li, J.; Chehab, L.; Desta, T.; Chino, T.; Krothpali, N.; Behl, Y.; Alikhani, M.; Yang, J.; Braasch, C.; et al. Impaired wound healing in mouse models of diabetes is mediated by TNF-α dysregulation and associated with enhanced activation of forkhead box O1 (FOXO1). Diabetology 2009, 53, 378–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Elution Time (min) | Solution A (%) | Solution B (%) |
|---|---|---|
| 4 | 95 | 5 |
| 12 | 80 | 20 |
| 14 | 80 | 20 |
| 22 | 65 | 35 |
| 25 | 65 | 35 |
| 30 | 80 | 20 |
| 35 | 95 | 5 |
| 55 | End of the run | |
| Gene | Forward | Reverse | Size (bp) |
|---|---|---|---|
| IL-6 | TGGTGACAACCACGGCCTTC | GCCTCCGACTTGTGAAGTGGT | 104 |
| IL-1b | GCTGTGGAGAAGCTGTGGCA | GGGAACGTCACACACCAGCA | 153 |
| TNF-α | GACAAGGCTGCCCCGACTACG | CTTGGGGCAGGGGCTCTTGAC | 110 |
| 18s rRNA | GCAATTATTCCCCATGAACG | GGCCTCACTAAACCATCCAA | 111 |
| Sample | MT | PCK |
|---|---|---|
| Normal | 8/10 | 8/10 |
| Negative | 4/10 | 4/10 |
| EGF solution | 5/10 | 5/10 |
| PM | 6/10 | 6/10 |
| EGF-Coa | 8/10 | 8/10 |
| Sample | Average Area % (n = 2) | Range |
|---|---|---|
| −20 °C (control) | 100.0 | 98.8, 101.2 |
| 11.1% RH | 99.6 | 97.7, 101.5 |
| 33.3% RH | 92.8 | 93.3, 92.2 |
| 54% RH | 93.0 | 93.4, 92.6 |
| 75% RH | 88.2 | 89.2, 87.1 |
| 94% RH | 67.4 | 69.4, 65.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.; Kim, B.; Park, M.; Ban, E.; Lee, S.-H.; Kim, A. Improved Diabetic Wound Healing by EGF Encapsulation in Gelatin-Alginate Coacervates. Pharmaceutics 2020, 12, 334. https://doi.org/10.3390/pharmaceutics12040334
Jeong S, Kim B, Park M, Ban E, Lee S-H, Kim A. Improved Diabetic Wound Healing by EGF Encapsulation in Gelatin-Alginate Coacervates. Pharmaceutics. 2020; 12(4):334. https://doi.org/10.3390/pharmaceutics12040334
Chicago/Turabian StyleJeong, Seonghee, ByungWook Kim, Minwoo Park, Eunmi Ban, Soo-Hyeon Lee, and Aeri Kim. 2020. "Improved Diabetic Wound Healing by EGF Encapsulation in Gelatin-Alginate Coacervates" Pharmaceutics 12, no. 4: 334. https://doi.org/10.3390/pharmaceutics12040334
APA StyleJeong, S., Kim, B., Park, M., Ban, E., Lee, S.-H., & Kim, A. (2020). Improved Diabetic Wound Healing by EGF Encapsulation in Gelatin-Alginate Coacervates. Pharmaceutics, 12(4), 334. https://doi.org/10.3390/pharmaceutics12040334