Recent Advances in Manufacturing Innovative Stents
Abstract
:1. Introduction
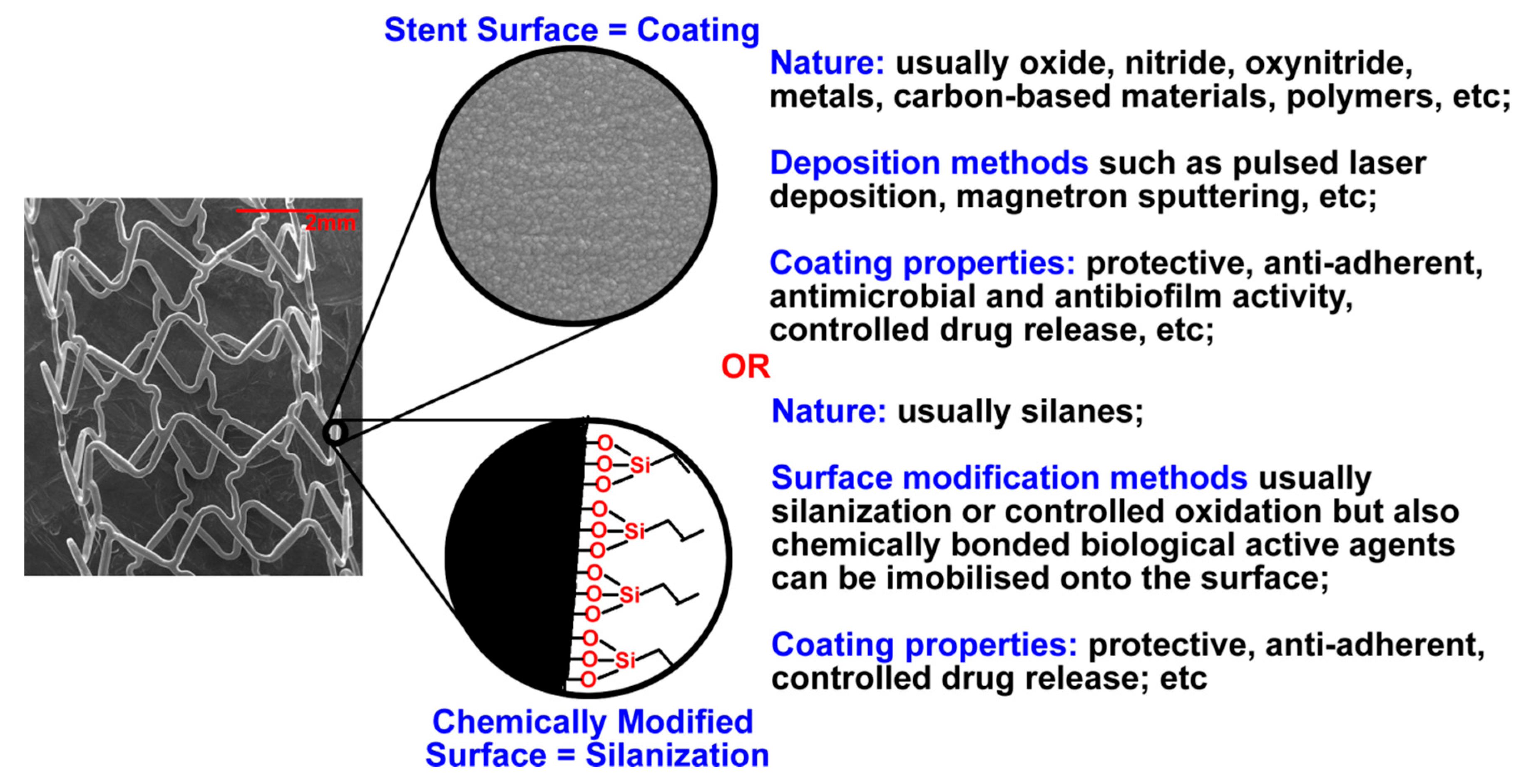
2. Coated Stents
2.1. Coating Types and Materials
2.1.1. Organic Coatings
2.1.2. Bio-Based Coatings
2.1.3. Inorganic Coatings
Titanium Oxide and Titanium Oxynitride Coatings
Diamond-Like Carbon (DLC) Coatings
Other Inorganic Coatings
3. Bioresorbable Stents
3.1. Metallic Bioresorbable Stents
3.1.1. Mg Stents
3.1.2. Zn Stents
3.1.3. Fe Stents
3.2. Polymeric Bioresorbable Stents
3.3. Comparison of Bioresorbable Metal and Polymer Stents
4. Drug, Nanoparticle, and Gene-Eluting Stents
4.1. General Aspects
4.2. Drug-Related Surface Modification
4.3. Drugs Used in DES
4.4. Drug Delivery Mechanisms
5. Mechanical Aspects of Stents
- −
- reducing the number of crowns;
- −
- curving of the linear parts of the struts with maximally high curvature radiuses permitting further stent deformation and deployment;
- −
- design improvement of the crowns.
6. Conclusions
Funding
Conflicts of Interest
References
- Wu, T.; McCarthy, S. Coronary Arterial Drug-Eluting Stent: From Structure to Clinical Coronary Artery Diseases; Chaikovsky, I., Ed.; InTech: Rijeka, Croatia, 2012; pp. 197–224. [Google Scholar]
- Koo, Y.; Tiasha, T.; Shanov, V.N.; Yun, Y. Expandable Mg-based Helical Stent Assessment using Static, Dynamic, and Porcine Ex Vivo Models. Sci Rep.-Uk 2017, 7, 1–10. [Google Scholar]
- Zhu, Y.Q.; Yang, K.; Cheng, R.Y.; Xiang, Y.; Yuan, T.W.; Sarmento, B.N.; Chen, Y.S.; Cui, W.G. The current status of biodegradable stent to treat benign luminal disease. Mater. Today 2017, 20, 516–529. [Google Scholar]
- Yoon, N.K.; Awad, A.W.; Yashar, M.; Kalani, S.; Taussky, P.; Park, M.S. Stent technology in ischemic stroke. Neurosurg Focus 2017, 42, E11. [Google Scholar] [CrossRef] [Green Version]
- Khan, W.; Farah, S.; Domb, A.J. Drug eluting stents: Developments and current status. J. Control. Release 2012, 161, 703–712. [Google Scholar]
- Camici, G.G. What is an optimal stent? Biological requirements of drug eluting stents. Cardiovasc Med. 2008, 11, 2–25. [Google Scholar]
- Mani, G.; Feldman, M.D.; Patel, D.; Agrawal, C.M. Coronary stents: A materials perspective. Biomaterials 2007, 28, 1689–1710. [Google Scholar]
- Kommineni, N.; Saka, R.; Khan, W.; Domb, A. Non-polymer drug-eluting coronary stents. Drug Deliv. Transl. Res. 2018, 8, 903–917. [Google Scholar]
- Vo, T.T.N.; Morgan, S.; McCormick, C.; McGinty, S.; McKee, S.; Meere, M. Modelling drug release from polymer-free coronary stents with microporous surfaces. Int. J. Pharm. 2018, 544, 392–401. [Google Scholar] [CrossRef] [Green Version]
- Hossainy, S.F.A.; Pacetti, S.D.; Fong, K.E.; Bhat, V.; Millare, D.S.; Guruwaiya, J.A.; Mirzaee, D.; Mandrusov, E. Primer coatings for stents with oxide, anionic, or hydroxyl surface moieties. U.S. Patent 9,101,689 B2, 11 August 2015. [Google Scholar]
- Khlusov, I.A.; Dekhtyar, Y.; Sharkeev, Y.P.; Pichugin, V.F.; Khlusova, M.Y.; Polyaka, N.; Tyulkin, F.; Vendinya, V.; Legostaeva, E.V.; Litvinova, L.S.; et al. Nanoscale electrical potential and roughness of a calcium phosphate surface promotes the osteogenic phenotype of stromal cells. Materials 2018, 11, 978. [Google Scholar] [CrossRef] [Green Version]
- Nanci, A.; Wuest, J.D.; Peru, L.; Brunet, P.; Sharma, V.; Zalzal, S.; McKee, M.D. Chemical modification of titanium surfaces for covalent attachment of biological molecules. J. Biomed. Mater. Res. 1998, 40, 324–335. [Google Scholar] [CrossRef]
- Hiob, M.A.; Wise, S.G.; Kondyurin, A.; Waterhouse, A.; Bilek, M.M.; Ng, M.K.C.; Weiss, A.S. The use of plasma-activated covalent attachment of early domains of tropoelastin to enhance vascular compatibility of surfaces. Biomaterials 2013, 34, 7584–7591. [Google Scholar] [CrossRef] [Green Version]
- Ravenscroft-Chang, M.S.; Stohlman, J.M.; Molnar, P.; Natarajan, A.; Canavan, H.E.; Teliska, M.; Stancescu, M.; Krauthamer, V.; Hickman, J.J. Altered calcium dynamics in cardiac cells grown on silane-modified surfaces. Biomaterials 2010, 31, 602–607. [Google Scholar] [CrossRef] [Green Version]
- Hauser, J.; Kruger, C.D.; Halfmann, H.; Awakowicz, P.; Koller, M.; Esenwein, S.A. Surface modification of metal implant materials by low-pressure plasma treatment. Biomed. Tech. 2009, 54, 98–106. [Google Scholar] [CrossRef]
- Paredes, V.; Salvagni, E.; Rodriguez-Castellon, E.; Manero, J.M. Comparative Study of Surface Chemical Composition and Oxide Layer Modification upon Oxygen Plasma Cleaning and Piranha Etching on a Novel Low Elastic Modulus Ti25Nb21Hf Alloy. Met. Mater. Trans. A 2017, 48A, 3770–3776. [Google Scholar] [CrossRef]
- Wise, S.G.; Waterhouse, A.; Kondyurin, A.; Bilek, M.M.; Weiss, A.S. Plasma-based biofunctionalization of vascular implants. Nanomed. -Uk 2012, 7, 1907–1916. [Google Scholar] [CrossRef]
- Bognar, E.; Ring, G.; Marton, H.Z.; Dobranszky, J.; Ginsztler, J. Development and Examination of Coated Coronary Stents. Anyagok Vilaga 2007, 7, 1–7. [Google Scholar]
- Bognar, E.; Ring, G.; Marton, H.Z.; Dobranszky, J.; Ginsztler, J. Polyurethane Coating on Coronary Stents. Key Eng. Mater. 2007, 345–346, 1269–1272. [Google Scholar] [CrossRef]
- Bourantas, C.V.; Papafaklis, M.I.; Kotsia, A.; Farooq, V.; Muramatsu, T.; Gomez-Lara, J.; Zhang, Y.J.; Iqbal, J.; Kalatzis, F.G.; Naka, K.K.; et al. Effect of the Endothelial Shear Stress Patterns on Neointimal Proliferation Following Drug-Eluting Bioresorbable Vascular Scaffold Implantation An Optical Coherence Tomography Study. JACC-Cardiovasc Int. 2014, 7, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Neamtu, I.; Chiriac, A.P.; Diaconu, A.; Nita, L.E.; Balan, V.; Nistor, M.T. Current Concepts on Cardiovascular Stent Devices. Mini-Rev. Med. Chem. 2014, 14, 505–536. [Google Scholar] [CrossRef]
- Strohbach, A.; Busch, R. Polymers for Cardiovascular Stent Coatings. Int. J. Polym. Sci. 2015, 2015, 11. [Google Scholar] [CrossRef] [Green Version]
- Charpentier, E.; Barna, A.; Guillevin, L.; Juliard, J.M. Fully bioresorbable drug-eluting coronary scaffolds: A review. Arch. Cardiovasc Dis. 2015, 108, 385–397. [Google Scholar] [CrossRef] [Green Version]
- Heublein, B.; Rohde, R.; Kaese, V.; Niemeyer, M.; Hartung, W.; Haverich, A. Biocorrosion of magnesium alloys: A new principle in cardiovascular implant technology? Heart 2003, 89, 651–656. [Google Scholar] [CrossRef] [Green Version]
- Peuster, M.; Wohlsein, P.; Brugmann, M.; Ehlerding, M.; Seidler, K.; Fink, C.; Brauer, H.; Fischer, A.; Hausdorf, G. A novel approach to temporary stenting: Degradable cardiovascular stents produced from corrodible metal-results 6–18 months after implantation into New Zealand white rabbits. Heart 2001, 86, 563–569. [Google Scholar] [CrossRef] [Green Version]
- Tamai, H.; Igaki, K.; Kyo, E.; Kosuga, K.; Kawashima, A.; Matsui, S.; Komori, H.; Tsuji, T.; Motohara, S.; Uehata, H. Initial and 6-month results of biodegradable poly-l-lactic acid coronary stents in humans. Circulation 2000, 102, 399–404. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.F.; Huang, N.; Xu, L.; Zhang, Y.; Liu, H.Q.; Sun, H.; Leng, Y.X. Biocompatibility of pure iron: In vitro assessment of degradation kinetics and cytotoxicity on endothelial cells. Mat. Sci. Eng. C 2009, 29, 1589–1592. [Google Scholar] [CrossRef]
- Gu, X.N.; Zheng, Y.F.; Cheng, Y.; Zhong, S.P.; Xi, T.F. In vitro corrosion and biocompatibility of binary magnesium alloys. Biomaterials 2009, 30, 484–498. [Google Scholar] [CrossRef]
- Jurgeleit, T.; Quandt, E.; Zamponi, C. Magnetron Sputtering as a Fabrication Method for a Biodegradable Fe32Mn Alloy. Materials 2017, 10, 1196. [Google Scholar] [CrossRef] [Green Version]
- Mueller, P.P.; May, T.; Perz, A.; Hauser, H.; Peuster, M. Control of smooth muscle cell proliferation by ferrous iron. Biomaterials 2006, 27, 2193–2200. [Google Scholar] [CrossRef]
- Peuster, M.; Hesse, C.; Schloo, T.; Fink, C.; Beerbaum, P.; von Schnakenburg, C. Long-term biocompatibility of a corrodible peripheral iron stent in the porcine descending aorta. Biomaterials 2006, 27, 4955–4962. [Google Scholar] [CrossRef]
- Fischman, D.L.; Leon, M.B.; Baim, D.S.; Schatz, R.A.; Savage, M.P.; Penn, I.; Detre, K.; Veltri, L.; Ricci, D.; Nobuyoshi, M.; et al. A Randomized Comparison of Coronary-Stent Placement and Balloon Angioplasty in the Treatment of Coronary-Artery Disease. N. Engl J. Med. 1994, 331, 496–501. [Google Scholar] [CrossRef]
- Lyakishev, A.A. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. Results of TAXUS IV. Kardiologiya 2004, 44, 77. [Google Scholar]
- Stone, G.W.; Ellis, S.G.; Cox, D.A.; Hermiller, J.; O’Shaughnessy, C.; Mann, J.T.; Turco, M.; Caputo, R.; Bergin, P.; Greenberg, J.; et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N. Engl. J. Med. 2004, 350, 221–231. [Google Scholar] [CrossRef]
- Rechavia, E.; Litvack, F.; Fishbien, M.C.; Nakamura, M.; Eigler, N. Biocompatibility of polyurethane-coated stents: Tissue and vascular aspects. Catheter Cardio. Diag. 1998, 45, 202–207. [Google Scholar] [CrossRef]
- Bloch, R.; Pavcnik, D.; Uchida, B.T.; Krajina, A.; Kamino, T.; Timmermans, H.; Loriaux, M.; Hulek, P. Polyurethane-coated Dacron-covered stent-grafts for TIPS: Results in swine. Cardiovasc Inter. Rad. 1998, 21, 497–500. [Google Scholar] [CrossRef]
- Hernandez-Enriquez, M.; Lairez, O.; Campelo-Parada, F.; Lhermusier, T.; Bouisset, F.; Roncalli, J.; Elbaz, M.; Carrie, D.; Boudou, N. Outcomes after use of covered stents to treat coronary artery perforations. Comparison of old and new-generation covered stents. J. Interv. Cardiol. 2018, 31, 617–623. [Google Scholar] [CrossRef]
- Dumonceau, J.M.; Deviere, J.; Cremer, M. Self expandable, polyurethane-covered, stent for esophagorespiratory fistulas. Gastrointest. Endosc. 1996, 43, 173. [Google Scholar] [CrossRef]
- Bethge, N.; Sommer, A.; Vakil, N. Treatment of Esophageal Fistulas with a New Polyurethane-Covered, Self-Expanding Mesh Stent-a Prospective-Study. Am. J. Gastroenterol. 1995, 90, 2143–2146. [Google Scholar]
- Kim, M.T.; Kim, K.Y.; Song, H.Y.; Park, J.H.; Tsauo, J.; Wang, Z.; Kim, P.H. Recurrent Benign Urethral Strictures Treated with Covered Retrievable Self-Expandable Metallic Stents: Long-Term Outcomes over an 18-Year Period. J. Vasc. Interv. Radiol. 2017, 28, 1584–1591. [Google Scholar] [CrossRef]
- Hausegger, K.A.; Thurnher, S.; Bodendorfer, G.; Zollikofer, C.L.; Uggowitzer, M.; Kugler, C.; Lammer, J. Treatment of malignant biliary obstruction with polyurethane-covered wallstents. Am. J. Roentgenol. 1998, 170, 403–408. [Google Scholar] [CrossRef] [Green Version]
- Shim, C.S.; Lee, Y.H.; Cho, Y.D.; Bong, H.K.; Kim, J.O.; Cho, J.Y.; Kim, Y.S.; Lee, J.S.; Lee, M.S.; Hwang, S.G.; et al. Preliminary results of a new covered biliary metal stent for malignant biliary obstruction. Endoscopy 1998, 30, 345–350. [Google Scholar] [CrossRef]
- Hussain, Z.; Diamantopoulos, A.; Krokidis, M.; Katsanos, K. Double-layered covered stent for the treatment of malignant oesophageal obstructions: Systematic review and meta-analysis. World J. Gastroentero. 2016, 22, 7841–7850. [Google Scholar] [CrossRef]
- Shaikh, M.; Choudhury, N.R.; Knott, R.; Garg, S. Engineering Stent Based Delivery System for Esophageal Cancer Using Docetaxel. Mol. Pharm. 2015, 12, 2305–2317. [Google Scholar] [CrossRef]
- Park, S.; Son, H.; Park, M.; Jung, G.; Koo, J. Use of a polyurethane covered nitinol stent for malignant gastroduodenal obstruction. Gastroenterology 2000, 118, A524. [Google Scholar] [CrossRef]
- Watkinson, A.F.; Ellul, J.; Entwisle, K.; Mason, R.C.; Adam, A. Esophageal-Carcinoma-Initial Results of Palliative Treatment with Covered Self-Expanding Endoprostheses. Radiology 1995, 195, 821–827. [Google Scholar] [CrossRef]
- He, W.; Hu, Z.J.; Xu, A.W.; Liu, R.M.; Yin, H.H.; Wang, J.S.; Wang, S.M. The Preparation and Performance of a New Polyurethane Vascular Prosthesis. Cell Biochem. Biophys. 2013, 66, 855–866. [Google Scholar] [CrossRef]
- Han, J.J.; Farah, S.; Domb, A.J.; Lelkes, P.I. Electrospun Rapamycin-Eluting Polyurethane Fibers for Vascular Grafts. Pharm. Res.-Dordr. 2013, 30, 1735–1748. [Google Scholar] [CrossRef]
- Amjadi, I.; Rabiee, M.; Hosseini, M.S.; Mozafari, M. Synthesis and Characterization of Doxorubicin-Loaded Poly(Lactide-co-glycolide) Nanoparticles as a Sustained-Release Anticancer Drug Delivery System. Appl. Biochem. Biotech. 2012, 168, 1434–1447. [Google Scholar] [CrossRef]
- Zhilu, Y.; Nan, H.; Jin, W.; Yajun, W. A method for constructing nitric oxide-generating adherent coating. U.S. Patent 2017246353, 31 August 2017. [Google Scholar]
- Giessen, W.; Serruys, P.; Visser, W. Endothelialization of intravascular stents. J. Interv. Cardiol. 1988, 1, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Consigny, P.M. Endothelial cell seeding on prosthetic surfaces. J. Long-Term. Eff. Med. 2000, 10, 79–95. [Google Scholar]
- Cui, S.; Liu, J.H.; Song, X.T.; Ma, G.L.; Du, B.J.; Lv, S.Z.; Meng, L.J.; Gao, Q.S.; Li, K.F. A Novel Stent Coated with Antibodies to Endoglin Inhibits Neointimal Formation of Porcine Coronary Arteries. Biomed. Res. Int. 2014, 2014, 7. [Google Scholar] [CrossRef]
- Garg, S.; Serruys, P.W. Coronary Stents Looking Forward. J. Am. Coll. Cardiol. 2010, 56, S43–S78. [Google Scholar] [CrossRef] [Green Version]
- Song, C.L.; Li, Q.; Yu, Y.P.; Wang, G.; Wang, J.P.; Lu, Y.; Zhang, J.C.; Diao, H.Y.; Liu, J.G.; Liu, Y.H.; et al. Study of novel coating strategy for coronary stents: Simutaneous coating of VEGF and anti-CD34 antibody. Rev. Bras. Cir. Cardiov. 2015, 30, 159–163. [Google Scholar]
- Ping, Y.; Pan, X.; Jiang, C.; Sheng, D.; Shuang, H.; Yuzhen, L.; Luying, L.; Ting, J.; Nan, H. Preparation method and application of amino-rich stent material modified by copper 4-carboxyphenyl porphyrin, blood vessel stent material and application. CN Patent 109663151, 23 April 2019. [Google Scholar]
- Salunkhe, P.; Chakrabarty, A.; Salunkhe, P.; Chakrabarty, A. A novel biomedical device for cancer therapy. WO Patent 2014049604, 3 April 2014. [Google Scholar]
- Sych, O.; Iatsenko, A.; Tomila, T.; Otychenko, O.; Bykov, O.; Yevych, Y. Si-modified highly-porous ceramics based on nanostructured biogenic hydroxyapatite for medical use. Adv. Nano-Biol. MD 2018, 2, 223–229. [Google Scholar]
- Yadav, S.; Singh, M.K.; Verma, D.K.; Jaiswar, G. X- ray diffraction study of the effects of dopant on the Lattice strain of ZnO nanoparticles. Adv. Nanomater. Technol. Energy Sect. 2017, 1, 73–89. [Google Scholar]
- Beshchasna, N.; Ho, A.Y.K.; Saqib, M.; Kraskiewicz, H.; Wasyluk, L.; Kuzmin, O.; Duta, O.C.; Ficai, D.; Trusca, R.D.; Ficai, A.; et al. Surface evaluation of titanium oxynitride coatings used for developing layered cardiovascular stents. Mat. Sci. Eng. C-Mater. 2019, 99, 405–416. [Google Scholar] [CrossRef]
- Yang, P.; Leng, Y.X.; Zhao, A.S.; Zhou, H.F.; Xu, L.; Hong, S.; Huang, N. Bloodcompatibility improvement of titanium oxide film modified by phosphorus ion implantation. Nucl Instrum Meth. B 2006, 242, 15–17. [Google Scholar] [CrossRef]
- Castellino, M.; Stolojan, V.; Virga, A.; Rovere, M.; Cabiale, K.; Galloni, M.R.; Tagliaferro, A. Chemico-physical characterisation and in vivo biocompatibility assessment of DLC-coated coronary stents. Anal. Bioanal. Chem. 2013, 405, 321–329. [Google Scholar] [CrossRef] [Green Version]
- Love, C.A.; Cook, R.B.; Harvey, T.J.; Dearnley, P.A.; Wood, R.J.K. Diamond like carbon coatings for potential application in biological implants-a review. Tribol. Int. 2013, 63, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Schuler, P.; Assefa, D.; Ylanne, J.; Basler, N.; Olschewski, M.; Ahrens, I.; Nordt, T.; Bode, C.; Peter, K. Adhesion of monocytes to medical steel as used for vascular stents is mediated by the integrin receptor Mac-1 (CD11b/CD18; alpha(M) beta(2)) and can be inhibited by semiconductor coating. Cell Commun. Adhes. 2003, 10, 17–26. [Google Scholar] [CrossRef]
- Kalnins, U.; Erglis, A.; Dinne, I.; Kumsars, I.; Jegere, S. Clinical outcomes of silicon carbide coated stents in patients with coronary artery disease. Med. Sci. Monit. 2002, 8, 116–120. [Google Scholar]
- Unverdorben, M.; Sattler, K.; Degenhardt, R.; Fries, R.; Abt, B.; Wagner, E.; Koelhler, H.; Scholtz, M.; Ibrahim, H.; Tews, K.; et al. Comparison of silicon carbide coated stent versus a noncoated stent in humans: The Tenax-versus Nir-stent Study. J. Interv. Cardiol. 2003, 16, 325–333. [Google Scholar] [CrossRef]
- Lehtinen, T.; Airaksinen, K.E.J.; Ylitalo, A.; Karjalainen, P.P. Stent strut coverage of titanium-nitride-oxide coated stent compared to paclitaxel-eluting stent in acute myocardial infarction: TITAX-OCT study. Int. J. Cardiovas. Imag. 2012, 28, 1859–1866. [Google Scholar] [CrossRef]
- Karjalainen, P.P.; Ylitalo, A.; Niemela, M.; Kervinen, K.; Makikallio, T.; Pietila, M.; Sia, J.; Tuomainen, P.; Nyman, K.; Airaksinen, K.E.J. Two-year follow-up after percutaneous coronary intervention with titanium-nitride-oxide-coated stents versus paclitaxel-eluting stents in acute myocardial infarction. Ann. Med. 2009, 41, 599–607. [Google Scholar] [CrossRef]
- Karjalainen, P.P.; Annala, A.P.; Ylitalo, A.; Vahlberg, T.; Airaksinen, K.E.J. Long-term clinical outcome with titanium-nitride-oxide-coated stents and paclitaxel-eluting stents for coronary revascularization in an unselected population. Int. J. Cardiol. 2010, 144, 42–46. [Google Scholar] [CrossRef]
- Karjalainen, P.; Ylitalo, A.; Niemelä, M.; Kervinen, K.; Mäkikallio, T.; Pietili, M.; Sia, J.; Tuomainen, P.; Nyman, K.; Airaksinen, K. Titanium-nitride-oxide coated stents versus paclitaxel-eluting stents in acute myocardial infarction: A 12-months follow-up report from the TITAX AMI trial. Eurointervention 2008, 4, 234–241. [Google Scholar] [CrossRef]
- Karjalainen, P.P.; Annala, A.P.; Ylitalo, A.; Airaksinen, K.E.J. Long-term clinical outcome with titanium-nitride-oxide-coated stents, paclitaxel eluting stents and bare-metal stents for coronary revascularization in an unselected population. Eur. Heart J. 2008, 29, 456. [Google Scholar]
- Huang, N.; Yang, P.; Leng, Y.X.; Chen, J.Y.; Sun, H.; Wang, J.; Wang, G.J.; Ding, P.D.; Xi, T.F.; Leng, Y. Hemocompatibility of titanium oxide films. Biomaterials 2003, 24, 2177–2187. [Google Scholar] [CrossRef]
- Zhai, Z.; Zou, K.; Feng, W.; Wang, Q. Experimental Study on Nitrogen-Doped Nano-Scale TiO2 Prepared by Microwave-Assisted Process at Low Temperature. Mod. Appl. Sci. 2010, 4, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Pichugin, V.F.; Pustovalova, A.A.; Konishchev, M.E.; Khlusov, I.A.; Ivanova, N.M.; Zhilei, S.; Gutor, S.S. In-vitro Dissolution and Structural and Electrokinetic Characteristics of Titanium-Oxynitride Coatings Formed via Reactive Magnetron Sputtering. J. Surf. Invest. X-RaySynchroton Neutron Tech. 2016, 10, 282–291. [Google Scholar] [CrossRef]
- Pavanelli, W.R.; Silva, J.J.N. The Role of Nitric Oxide in Immune Response Against Trypanosoma Cruzi Infection. J. Nitric. Oxide 2010, 2, 1–10. [Google Scholar] [CrossRef]
- Tousoulis, D.; Kampoli, A.M.; Papageorgiou, C.T.N.; Stefanadis, C. The Role of Nitric Oxide on Endothelial Function. Curr. Vasc. Pharm. 2012, 10, 4–18. [Google Scholar]
- Sobrevia, L.; Ooi, L.; Ryan, S.; Steinert, J.R. Nitric Oxide: A Regulator of Cellular Function in Health and Disease. Oxid Med. Cell Longev. 2016, 2016, 9782346. [Google Scholar] [CrossRef] [Green Version]
- Pustovalova, A.A.; Pichugin, V.F.; Ivanova, N.M.; Bruns, M. Structural features of N-containing titanium dioxide thin films deposited by magnetron sputtering. Thin. Solid. Film. 2017, 627, 9–16. [Google Scholar] [CrossRef]
- Sarra-Bournet, C.; Haberl, B.; Charles, C.; Boswell, R. Characterization of nanocrystalline nitrogen-containing titanium oxide obtained by N2/O2/Ar low-field helicon plasma sputtering. J. Phys. D. Appl. Phys. 2011, 44. [Google Scholar] [CrossRef]
- Hauert, R.; Thorwarth, K.; Thorwarth, G. An overview on diamond-like carbon coatings in medical applications. Surf. Coat. Tech. 2013, 233, 119–130. [Google Scholar] [CrossRef]
- McLaughlin, J.A.; Maguire, P.D. Advances on the use of carbon based materials at the biological and surface interface for applications in medical implants. Diam. Relat. Mater. 2008, 17, 873–877. [Google Scholar] [CrossRef]
- Dearnley, P.A. A brief review of test methodologies for surface-engineered biomedical implant alloys. Surf. Coat. Tech. 2005, 198, 483–490. [Google Scholar] [CrossRef]
- Bociaga, D.; Komorowski, P.; Batory, D.; Szymanski, W.; Olejnik, A.; Jastrzebski, K.; Jakubowski, W. Silver-doped nanocomposite carbon coatings (Ag-DLC) for biomedical applications-Physiochemical and biological evaluation. Appl Surf. Sci. 2015, 355, 388–397. [Google Scholar] [CrossRef]
- Kwok, S.C.H.; Ha, P.C.T.; McKenzie, D.R.; Bilek, M.M.M.; Chu, P.K. Biocompatibility of calcium and phosphorus doped diamond-like carbon thin films synthesized by plasma immersion ion implantation and deposition. Diam. Relat. Mater. 2006, 15, 893–897. [Google Scholar] [CrossRef]
- Maguire, P.D.; McLaughlin, J.A.; Okpalugo, T.I.T.; Lemoine, P.; Papakonstantinou, P.; McAdams, E.T.; Needham, M.; Ogwu, A.A.; Ball, M.; Abbas, G.A. Mechanical stability, corrosion performance and bioresponse of amorphous diamond-like carbon for medical stents and guidewires. Diam. Relat. Mater. 2005, 14, 1277–1288. [Google Scholar] [CrossRef]
- Nagashima, S.; Hasebe, T.; Kamijo, A.; Yoshimoto, Y.; Hotta, A.; Morita, H.; Terada, H.; Tanaka, M.; Takahashi, K.; Suzuki, T. Effect of oxygen plasma treatment on non-thrombogenicity of diamond-like carbon films. Diam. Relat. Mater. 2010, 19, 861–865. [Google Scholar] [CrossRef]
- Hasebe, T.; Yohena, S.; Kamijo, A.; Okazaki, Y.; Hotta, A.; Takahashi, K.; Suzuki, T. Fluorine doping into diamond-like carbon coatings inhibits protein adsorption and platelet activation. J. Biomed. Mater. Res. A 2007, 83A, 1192–1199. [Google Scholar]
- Pandiyaraj, K.N.; Heeg, J.; Lampka, A.; Junge, F.; Barfels, T.; Wienecke, M.; Rhee, Y.H.; Kim, H.W. In vitro Cyto and Blood Compatibility of Titanium Containing Diamond-Like Carbon Prepared by Hybrid Sputtering Method. Plasma Sci. Technol 2012, 14, 829. [Google Scholar]
- Lackner, J.M.; Waldhauser, W.; Hartmann, P.; Bruckert, F.; Weidenhaupt, M.; Major, R.; Sanak, M.; Wiesinger, M.; Heim, D. Hemocompatibility of Inorganic Physical Vapor Deposition (PVD) Coatings on Thermoplastic Polyurethane Polymers. J. Funct Biomater. 2012, 3, 283–297. [Google Scholar]
- Salahas, A.; Vrahatis, A.; Karabinos, I.; Antonellis, I.; Ifantis, G.; Gavaliatsis, I.; Anthopoulos, P.; Tavernarakis, A. Success, safety, and efficacy of implantation of diamond-like carbon-coated stents. Angiology 2007, 58, 203–210. [Google Scholar]
- O’Brien, B.; Carroll, W. The evolution of cardiovascular stent materials and surfaces in response to clinical drivers: A review. Acta Biomater 2009, 5, 945–958. [Google Scholar]
- Sydow-Plum, G.; Tabrizian, M. Review of stent coating strategies: Clinical insights. Mater. Sci. Tech.-Lond 2008, 24, 1127–1143. [Google Scholar]
- Hulander, M.; Hong, J.; Andersson, M.; Gerven, F.; Ohrlander, M.; Tengvall, P.; Elwing, H. Blood Interactions with Noble Metals: Coagulation and Immune Complement Activation. Acs Appl. Mater. Inter. 2009, 1, 1053–1062. [Google Scholar]
- Seliger, C.; Schwennicke, K.; Schaffar, C.; Wolf, W.P.; Alt, E. Influence of a rough, ceramic-like stent surface made of iridium oxide on neointimal structure and thickening. Eur. Heart J. 2000, 21, 286. [Google Scholar]
- Di Mario, C.; Grube, E.; Nisanci, Y.; Reifart, N.; Colombo, A.; Rodermann, J.; Muller, R.; Umman, S.; Liistro, F.; Montorfano, M.; et al. MOONLIGHT: A controlled registry of an iridium oxide-coated stent with angiographic follow-up. Int. J. Cardiol. 2004, 95, 329–331. [Google Scholar]
- Unverdorben, M.; Sippel, B.; Degenhardt, R.; Sattler, K.; Fries, R.; Aht, B.; Wagner, E.; Koehler, H.; Daemgen, G.; Scholz, M.; et al. Comparison of a silicon carbide-coated stent versus a noncoated stent in human beings: The Tenax versus Nir Stent Study’s longterm outcome. Am. Heart J. 2003, 145, E7. [Google Scholar] [CrossRef]
- Asano, T.; Suwannasom, P.; Katagiri, Y.; Miyazaki, Y.; Sotomi, Y.; Kraak, R.P.; Wykrzykowska, J.; Rensing, B.J.; Piek, J.J.; Gyongyosi, M.; et al. First-in-Man Trial of SiO2 Inert-Coated Bare Metal Stent System in Native Coronary Stenosis-The AXETIS FIM Trial. Circ. J. 2018, 82, 477. [Google Scholar] [CrossRef]
- Rajtar, A.; Kaluza, G.L.; Yang, Q.; Hakimi, D.; Liu, D. Hydroxyapatite-coated cardiovascular stents. Eurointervention 2006, 2, 113–115. [Google Scholar]
- Xiangdong, Z.; Xiao, Y.; Yong, Z.; Cong, X.; Kun, Z.; Zhongqi, T.; Xingdong, Z. Nano hydroxyapatite-coated porous stent and preparation method and application thereof. CN Patent 109432493, 3 August 2019. [Google Scholar]
- Erne, P.; Schier, M.; Resink, T.J. The road to bioabsorbable stents: Reaching clinical reality? Cardiovasc. Inter. Rad. 2006, 29, 11–16. [Google Scholar] [CrossRef]
- Boland, E.L.; Shine, R.; Kelly, N.; Sweeney, C.A.; McHugh, P.E. A Review of Material Degradation Modelling for the Analysis and Design of Bioabsorbable Stents. Ann. Biomed. Eng. 2016, 44, 341–356. [Google Scholar] [CrossRef]
- Ho, M.Y.; Chen, C.C.; Wang, C.Y.; Chang, S.H.; Hsieh, M.J.; Lee, C.H.; Wu, V.C.C.; Hsieh, I.C. The Development of Coronary Artery Stents: From Bare-Metal to Bio-Resorbable Types. Met.-Basel. 2016, 6, 168. [Google Scholar] [CrossRef] [Green Version]
- Hytonen, J.P.; Taavitsainen, J.; Tarvainen, S.; Yla-Herttuala, S. Biodegradable coronary scaffolds: Their future and clinical and technological challenges. Cardiovasc. Res. 2018, 114, 1063–1072. [Google Scholar] [CrossRef]
- Mishra, S. Structural and Design Evolution of Bio-resorbable Scaffolds: The Journey so Far. Curr. Pharm. Des. 2018, 24, 402–413. [Google Scholar] [CrossRef]
- Habib, Y.M.; Ibrahim, E.-S. Nitric oxide-eluting bioresorbable stents for percutaneous coronary interventions. U.S. Patent 9,878,073, 30 January 2018. [Google Scholar]
- Elnaggar, M.A.; Seo, S.H.; Gobaa, S.; Lim, K.S.; Bae, I.H.; Jeong, M.H.; Han, D.K.; Joung, Y.K. Nitric oxide releasing coronary stent: A new approach using layer-by-layer coating and liposomal encapsulation. Small 2016, 12, 6012–6023. [Google Scholar] [CrossRef]
- Yang, T.; Zelikin, A.N.; Chandrawati, R. Progress and Promise of Nitric Oxide-Releasing Platforms. Adv. Sci. 2018, 5, 1701043. [Google Scholar] [CrossRef] [Green Version]
- Wong, P.C.; Tsai, P.H.; TH, T.H.L.; Cheng, C.K.; Jang, J.S.C.; Huang, J.C. Degradation behavior and mechanical strength of Mg-Zn-Ca bulk metallic glass composites with Ti particles as biodegradable materials. J. Alloy. Compd. 2017, 699, 914–920. [Google Scholar] [CrossRef]
- Liu, D.; Zhou, T.; Liu, Z.; Guo, B. Effect of solid-solution and aging treatment on corrosion behavior of orthogonal designed and vacuum melted Mg-Zn-Ca-Mn alloys. J. Appl Biomater. Func. 2020, 18, 1–13. [Google Scholar] [CrossRef]
- Gerold, B. Implant made of biodegradable magnesium alloy. U.S. Patent 2017/0157300 A1, 8 June 2017. [Google Scholar]
- Gerold, B. Implant made of a biodegradable magnesium alloy. U.S. Patent 8915953, 23 December 2014. [Google Scholar]
- Stekker, M.; Hort, N.; Feyerabend, F.; Hoffmann, E.; Hoffmann, M.; Horres, R. Resorbable stents which contain a magnesium alloy. WO Patent 2013024125A1, 21 February 2013. [Google Scholar]
- Kleine, K.; Kramer-Brown, P. Degradable medical device. U.S. Patent 20060271168, 30 December 2006. [Google Scholar]
- Wilcox, J. Controlled Degradation of Magnesium Stents. U.S. Patent 20090240323, 24 September 2009. [Google Scholar]
- Hoffmann, E.; Hoffmann, M.; Horres, R. Biodegradable vascular support. U.S. Patent 2010-0076544 A1, 25 March 2010. [Google Scholar]
- Orlowski, M.; Ruben, A. Bioresorbable metal stent with controlled resorption. U.S. Patent 20110076319, 31 March 2011. [Google Scholar]
- Klocke, B.; Borck, A. Polylactide-coated implant composed of a biocorrodible magnesium alloy. U.S. Patent EP 2 415 489 B1, 8 February 2012. [Google Scholar]
- Ghali, E. Understanding, Performance, and Testing; Wiley: New Jersey, NJ, USA, 2010. [Google Scholar]
- Ma, J.; Zhao, N.; Zhu, D.H. Biphasic responses of human vascular smooth muscle cells to magnesium ion. J. Biomed. Mater. Res. A 2016, 104, 347–356. [Google Scholar] [CrossRef]
- Kirkland, N.T.; Lespagnol, J.; Birbilis, N.; Staiger, M.P. A survey of bio-corrosion rates of magnesium alloys. Corros. Sci. 2010, 52, 287–291. [Google Scholar] [CrossRef]
- Zhang, S.X.; Zhang, X.N.; Zhao, C.L.; Li, J.A.; Song, Y.; Xie, C.Y.; Tao, H.R.; Zhang, Y.; He, Y.H.; Jiang, Y.; et al. Research on an Mg-Zn alloy as a degradable biomaterial. Acta Biomater. 2010, 6, 626–640. [Google Scholar] [CrossRef]
- Hort, N.; Huang, Y.; Fechner, D.; Stormer, M.; Blawert, C.; Witte, F.; Vogt, C.; Drucker, H.; Willumeit, R.; Kainer, K.U.; et al. Magnesium alloys as implant materials-Principles of property design for Mg-RE alloys. Acta Biomater. 2010, 6, 1714–1725. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.; Wang, J.F.; Zhu, S.J.; Yang, X.F.; Jia, Y.; Sun, Q.; Guan, S.K. A DFT study of the adsorption of short peptides on Mg and Mg-based alloy surfaces. Phys. Chem. Chem. Phys. 2018, 20, 3602–3607. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Y.H.; Bian, D.; Gao, S.; Leeflang, S.; Guo, H.; Zheng, Y.F.; Zhou, J. Study on the Mg-Li-Zn ternary alloy system with improved mechanical properties, good degradation performance and different responses to cells. Acta Biomater. 2017, 62, 418–433. [Google Scholar] [CrossRef]
- Torne, K.; Ornberg, A.; Weissenrieder, J. Influence of strain on the corrosion of magnesium alloys and zinc in physiological environments. Acta Biomater. 2017, 48, 541–550. [Google Scholar] [CrossRef]
- Liu, J.; Wang, P.; Chu, C.C.; Xi, T.F. A novel biodegradable and biologically functional arginine-based poly(ester urea urethane) coating for Mg-Zn-Y-Nd alloy: Enhancement in corrosion resistance and biocompatibility. J. Mater. Chem. B 2017, 5, 1787–1802. [Google Scholar] [CrossRef]
- Campos, C.M.; Muramatsu, T.; Iqbal, J.; Zhang, Y.J.; Onuma, Y.; Garcia-Garcia, H.M.; Haude, M.; Lemos, P.A.; Warnack, B.; Serruys, P.W. Bioresorbable Drug-Eluting Magnesium-Alloy Scaffold for Treatment of Coronary Artery Disease. Int. J. Mol. Sci. 2013, 14, 24492–24500. [Google Scholar] [CrossRef] [Green Version]
- Cao, N.Q.; Pham, D.N.; Kai, N.; Dinh, H.V.; Hiromoto, S.; Kobayashi, E. In Vitro Corrosion Properties of Mg Matrix In Situ Composites Fabricated by Spark Plasma Sintering. Met. –Basel. 2017, 7, 358. [Google Scholar] [CrossRef] [Green Version]
- Lewis, G. Reduction in the Corrosion Rate of Magnesium and Magnesium Alloy Specimens and Implications for Plain Fully Bioresorbable Coronary Artery Stents: A Review. J. Eng. Technol. 2016, 4, 572–597. [Google Scholar] [CrossRef] [Green Version]
- Patil, A.J.; Jackson, O.; Fulton, L.B.; Hong, D.; Desai, P.A.; Kelleher, S.A.; Chou, D.T.; Tan, S.S.; Kumta, P.N.; Beniash, E. Anticorrosive Self-Assembled Hybrid Alkylsilane Coatings for Resorbable Magnesium Metal Devices. Acs Biomater Sci. Eng. 2017, 3, 518–529. [Google Scholar] [CrossRef]
- Bow, D.R.; Macleod, C.B.; Jump, J. Metal alloy and medical device containing same. WO Patent 201904, 7 March 2017. [Google Scholar]
- Bowen, P.K.; Shearier, E.R.; Zhao, S.; Guillory, R.J.; Zhao, F.; Goldman, J.; Drelich, J.W. Biodegradable Metals for Cardiovascular Stents: From Clinical Concerns to Recent Zn-Alloys. Adv. Healthc Mater. 2016, 5, 1121–1140. [Google Scholar] [CrossRef] [Green Version]
- Bowen, P.K.; Drelich, J.; Goldman, J. Zinc Exhibits Ideal Physiological Corrosion Behavior for Bioabsorbable Stents. Adv. Mater. 2013, 25, 2577–2582. [Google Scholar] [CrossRef]
- Bowen, P.K.; Drelich, A.; Drelich, J.; Goldman, J. Rates of in vivo (arterial) and in vitro biocorrosion for pure magnesium. J. Biomed. Mater. Res. A 2015, 103, 341–349. [Google Scholar] [CrossRef]
- Vallee, B.L.; Falchuk, K.H. The Biochemical Basis of Zinc Physiology. Physiol Rev. 1993, 73, 79–118. [Google Scholar] [CrossRef]
- Levy, G.K.; Goldman, J.; Aghion, E. The Prospects of Zinc as a Structural Material for Biodegradable Implants-A Review Paper. Met. -Basel 2017, 7, 402. [Google Scholar] [CrossRef] [Green Version]
- Cetin, G.; Catalgol, Z.; Aydogdu, M.O.; Altun, E.; Koc, F.; Lin, C.-C.; Sengil, A.Z.; Gunduz, O. A novel antibacterial nanofibers mat made of co-axial electrospun polycaprolactone/silver nitrate/ zinc oxide composites. Adv. Nano Bio MD. 2018, 2, 275–286. [Google Scholar]
- Naskar, A.; Khan, H.; Jana, S. Cobalt doped ZnO–graphene nanocomposite: Synthesis, characterization and antibacterial activity on water borne bacteria. Adv. Nano Bio MD. 2017, 1, 182–190. [Google Scholar]
- Radousky, H.; Qian, F.; An, Y.; Zeng, Z.; Wang, G.; Li, Y.; Qu, L.; Zemanyi, G.; Wang, Y.M. Harvesting Mechanical and Thermal Energy by Combining ZnO Nanowires and NiTi Shape Memory Alloy. Adv. Nano Energy. 2017, 1, 13–20. [Google Scholar]
- Oprea, O.; Andronescu, E.; Ficai, D.; Ficai, A.; Oktar, F.N.; Yetmez, M. ZnO Applications and Challenges. Curr. Org. Chem 2014, 18, 192–203. [Google Scholar] [CrossRef]
- Guillory, R.J.; Bowen, P.K.; Hopkins, S.P.; Shearier, E.R.; Earley, E.J.; Gillette, A.A.; Aghion, E.; Bocks, M.; Drelich, J.W.; Goldman, J. Corrosion Characteristics Dictate the Long-Term Inflammatory Profile of Degradable Zinc Arterial Implants. Acs Biomater. Sci. Eng. 2016, 2, 2355–2364. [Google Scholar] [CrossRef]
- Drelich, A.J.; Zhao, S.; Guillory, R.J.; Drelich, J.W.; Goldman, J. Long-term surveillance of zinc implant in murine artery: Surprisingly steady biocorrosion rate. Acta Biomater. 2017, 58, 539–549. [Google Scholar] [CrossRef]
- Drelich, A.J.; Bowen, P.K.; LaLonde, L.; Goldman, J.; Drelich, J.W. Importance of oxide film in endovascular biodegradable zinc stents. Surf. Innov. 2016, 4, 133–140. [Google Scholar] [CrossRef]
- Werkhoven, R.J.; Sillekens, W.H.; Lieshout, J.B.J.M. Processing Aspects of Magnesium Alloy Stent Tube. Magnes. Technol. 2011, 2011, 419–424. [Google Scholar]
- Jarzebska, A.; Bieda, M.; Kawalko, J.; Rogal, L.; Koprowski, P.; Sztwiertnia, K.; Pachla, W.; Kulczyk, M. A new approach to plastic deformation of biodegradable zinc alloy with magnesium and its effect on microstructure and mechanical properties. Mater. Lett. 2018, 211, 58–61. [Google Scholar] [CrossRef]
- Jin, H.L.; Zhao, S.; Guillory, R.; Bowen, P.K.; Yin, Z.Y.; Griebel, A.; Schaffer, J.; Earley, E.J.; Goldman, J.; Drelich, J.W. Novel high-strength, low-alloys Zn-Mg (< 0.1 wt% Mg) and their arterial biodegradation. Mater. Sci. Eng. C-Mater. Biol. Appl. 2018, 84, 67–79. [Google Scholar]
- Bowen, P.K.; Seitz, J.M.; Guillory, R.J.; Braykovich, J.P.; Zhao, S.; Goldman, J.; Drelich, J.W. Evaluation of wrought Zn-Al alloys (1, 3, and 5 wt % Al) through mechanical and in vivo testing for stent applications. J. Biomed. Mater. Res. Part. B-Appl. Biomater. 2018, 106, 245–258. [Google Scholar] [CrossRef]
- Asgari, M.; Hang, R.Q.; Wang, C.; Yu, Z.T.; Li, Z.Y.; Xiao, Y. Biodegradable Metallic Wires in Dental and Orthopedic Applications: A Review. Met. -Basel 2018, 8, 212. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; McNamara, C.T.; Bowen, P.K.; Verhun, N.; Braykovich, J.P.; Goldman, J.; Drelich, J.W. Structural Characteristics and In Vitro Biodegradation of a Novel Zn-Li Alloy Prepared by Induction Melting and Hot Rolling. Met. Mater. Trans. A 2017, 48A, 1204–1215. [Google Scholar] [CrossRef]
- Yang, H.T.; Wang, C.; Liu, C.Q.; Chen, H.W.; Wu, Y.F.; Han, J.T.; Jia, Z.C.; Lin, W.J.; Zhang, D.Y.; Li, W.T.; et al. Evolution of the degradation mechanism of pure zinc stent in the one-year study of rabbit abdominal aorta model. Biomaterials 2017, 145, 92–105. [Google Scholar] [CrossRef]
- Shomali, A.A.; Guillory, R.J.; Seguin, D.; Goldman, J.; Drelich, J.W. Effect of PLLA coating on corrosion and biocompatibility of zinc in vascular environment. Surf. Innov. 2017, 5, 211–220. [Google Scholar] [CrossRef]
- Levy, G.K.; Leon, A.; Kafri, A.; Ventura, Y.; Drelich, J.W.; Goldman, J.; Vago, R.; Aghion, E. Evaluation of biodegradable Zn-1% Mg and Zn-1% Mg-0.5% Ca alloys for biomedical applications. J. Mater. Sci. -Mater. Med. 2017, 28, 174. [Google Scholar] [CrossRef]
- Yue, R.; Huang, H.; Ke, G.Z.; Zhang, H.; Pei, J.; Xue, G.H.; Yuan, G.Y. Microstructure, mechanical properties and in vitro degradation behavior of novel Zn-Cu-Fe alloys. Mater. Charact. 2017, 134, 114–122. [Google Scholar] [CrossRef]
- Shukla, S.K.; Shukla, S.K.; Govender, P.P.; Giri, N.G. Biodegradable polymeric nanostructures in therapeutic applications: Opportunities and challenges. Rsc Adv. 2016, 6, 94325–94351. [Google Scholar] [CrossRef]
- Tamai, H.; Igaki, K.; Tsuji, T.; Kyo, E.; Kosuga, K.; Kawashima, A.; Matsui, S.; Komori, H.; Motohara, S.; Uehata, H.; et al. A biodegradable poly-l-lactic acid coronary stent in the porcine coronary artery. J. Interv Cardiol. 1999, 12, 443–449. [Google Scholar] [CrossRef]
- Williams, S.F.; Rizk, S.; Martin, D.P. Poly-4-hydroxybutyrate (P4HB): A new generation of resorbable medical devices for tissue repair and regeneration. Biomed. Eng.-Biomed. Te. 2013, 58, 439–452. [Google Scholar] [CrossRef]
- Ong, A.T.L.; Serruys, P.W. Technology insight: An overview of research in drug-eluting stents. Nat. Clin. Pr. Card. 2005, 2, 647–658. [Google Scholar] [CrossRef]
- Lam, M.K.; Sen, H.; Tandjung, K.; van Houwelingen, K.G.; de Vries, A.G.; Danse, P.W.; Schotborgh, C.E.; Scholte, M.; Lowik, M.M.; Linssen, G.C.M.; et al. Comparison of 3 biodegradable polymer and durable polymer-based drug-eluting stents in all-comers (BIO-RESORT): Rationale and study design of the randomized TWENTE III multicenter trial. Am. Heart J. 2014, 167, 445–451. [Google Scholar] [CrossRef]
- Roy, I.; Nigmatullin, R.; Basnett, P.; Lukasiewicz, B. STENTS. WO Patent WO/2019/043384, 7 March 2019. [Google Scholar]
- Commandeur, S.; Van Beusekom, H.M.; Van Der Giessen, W.J. Polymers, drug release, and drug-eluting stents. J. Interv. Cardiol. 2006, 19, 500–506. [Google Scholar] [CrossRef]
- Hermawan, H.; Dube, D.; Mantovani, D. Degradable metallic biomaterials: Design and development of Fe-Mn alloys for stents. J. Biomed. Mater. Res. A 2010, 93A, 1–11. [Google Scholar] [CrossRef]
- Waksman, R.; Pakala, R.; Baffour, R.; Seabron, R.; Hellinga, D.; Tio, F.O. Short-term effects of biocorrodible iron stents in porcine coronary arteries. J. Interv. Cardiol. 2008, 21, 15–20. [Google Scholar] [CrossRef]
- Puranik, A.S.; Dawson, E.R.; Peppas, N.A. Recent advances in drug eluting stents. Int. J. Pharm. 2013, 441, 665–679. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Mo, Z.C.; Guo, F.F.; Shi, D.L.; Han, Q.Q.; Liu, Q. Drug loaded nanoparticle coating on totally bioresorbable PLLA stents to prevent in-stent restenosis. J. Biomed. Mater. Res. B 2018, 106, 88–95. [Google Scholar] [CrossRef]
- Wykrzykowska, J.J.; Kraak, R.P.; Hofma, S.H.; van der Schaaf, R.J.; Arkenbout, E.K.; Ijsselmuiden, A.J.; Elias, J.; van Dongen, I.M.; Tijssen, R.Y.G.; Koch, K.T.; et al. Bioresorbable Scaffolds versus Metallic Stents in Routine PCI. New Engl. J. Med. 2017, 376, 2319–2328. [Google Scholar] [CrossRef]
- Sorrentino, S.; Giustino, G.; Mehran, R.; Kini, A.S.; Sharma, S.K.; Faggioni, M.; Farhan, S.; Vogel, B.; Indolfi, C.; Dangas, G.D. Everolimus-Eluting Bioresorbable Scaffolds Versus Everolimus-Eluting Metallic Stents. J. Am. Coll Cardiol. 2017, 69, 3055–3066. [Google Scholar] [CrossRef]
- Feng, G.K.; Xiao, J.M.; Qin, C.S.; Lu, Z.; Li, J.; Zheng, X.X.; Liu, S.Z.; Wu, T.; Jiang, X.J. Long Term Comparison between Novel Fully Bioresorbable Scaffolds and Drug-Eluting Stents: A Twenty-Four-Month Study in Porcine Coronary Arteries. J. Am. Coll Cardiol. 2016, 68, C57–C58. [Google Scholar] [CrossRef]
- Gastaldi, D.; Sassi, V.; Petrini, L.; Vedani, M.; Trasatti, S.; Migliavacca, F. Continuum damage model for bioresorbable magnesium alloy devices-Application to coronary stents. J. Mech Behav. Biomed. 2011, 4, 352–365. [Google Scholar] [CrossRef]
- Ernst, A.; Bulum, J. New generations of drug-eluting stents-A brief review. Emj Int. Cardiol. 2014, 1, 100–106. [Google Scholar]
- Ormiston, J.A.; Serruys, P.W. Bioabsorbable coronary stents. Circ. Cardiovasc. Interv. 2009, 2, 255–260. [Google Scholar] [CrossRef] [Green Version]
- Waksman, R. Biodegradable stents: They do their job and disappear. J. Invasive. Cardiol. 2006, 18, 70–74. [Google Scholar]
- Finn, A.V.; Joner, M.; Nakazawa, G.; Kolodgie, F.; Newell, J.; John, M.C.; Gold, H.K.; Virmani, R. Pathological correlates of late drug-eluting stent thrombosis-Strut coverage as a marker of endothelialization. Circulation 2007, 115, 2435–2441. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, R.; Mintz, G.S.; Dussaillant, G.R.; Popma, J.J.; Pichard, A.D.; Satler, L.F.; Kent, K.M.; Griffin, J.; Leon, M.B. Patterns and mechanisms of in-stent restenosis-A serial intravascular ultrasound study. Circulation 1996, 94, 1247–1254. [Google Scholar] [CrossRef]
- Togni, M.; Windecker, S.; Cocchia, R.; Wenaweser, P.; Cook, S.; Billinger, M.; Meier, B.; Hess, O.M. Sirolimus-eluting stents associated with paradoxic coronary vasoconstriction. J. Am. Coll. Cardiol. 2005, 46, 231–236. [Google Scholar] [CrossRef] [Green Version]
- Barlis, P.; Tanigawa, J.; Di Mario, C. Coronary bioabsorbable magnesium stent: 15-month intravascular ultrasound and optical coherence tomography findings. Eur. Heart J. 2007, 28, 2319. [Google Scholar] [CrossRef]
- Garg, S.; Serruys, P. Biodegradable stents and non-biodegradable stents. Minerva. Cardioangiol. 2009, 57, 537–565. [Google Scholar]
- Serruys, P.W.; Garcia-Garcia, H.M.; Onuma, Y. From metallic cages to transient bioresorbable scaffolds: Change in paradigm of coronary revascularization in the upcoming decade? Eur. Heart J. 2012, 33, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Waksman, R.; Pakala, R.; Kuchulakanti, P.K.; Baffour, R.; Hellinga, D.; Seabron, R.; Tio, F.O.; Wittchow, E.; Hartwig, S.; Harder, C.; et al. Safety and efficacy of bioabsorbable magnesium alloy stents in porcine coronary arteries. Catheter. Cardio. Inte. 2006, 68, 607–617. [Google Scholar] [CrossRef]
- Waksman, R. Current state of the absorbable metallic (magnesium) stent. EuroIntervention 2009, 5, F94–F97. [Google Scholar] [CrossRef]
- Waksman, R.; Erbel, R.; Di Mario, C.; Bartunek, J.; de Bruyne, B.; Eberli, F.R.; Erne, P.; Haude, M.; Horrigan, M.; Ilsley, C.; et al. Early- and Long-Term Intravascular Ultrasound and Angiographic Findings After Bioabsorbable Magnesium Stent Implantation in Human Coronary Arteries. Jacc-Cardiovasc Inte. 2009, 2, 312–320. [Google Scholar] [CrossRef] [Green Version]
- Lan, Z.Y.; Lyu, Y.N.; Xiao, J.M.; Zheng, X.X.; He, S.Y.; Feng, G.K.; Zhang, Y.P.; Wang, S.H.; Kislauskis, E.; Chen, J.H.; et al. Novel Biodegradable Drug-Eluting Stent Composed of Poly-L-Lactic Acid and Amorphous Calcium Phosphate Nanoparticles Demonstrates Improved Structural and Functional Performance for Coronary Artery Disease. J. Biomed. Nanotechnol. 2014, 10, 1194–1204. [Google Scholar] [CrossRef]
- Carlquist, J.F.; Knight, S.; Horne, B.D.; Huntinghouse, J.A.; Rollo, J.S.; Muhlestein, J.B.; May, H.; Anderson, J.L. Cardiovascular risk among patients on clopidogrel anti-platelet therapy after placement of drug-eluting stents is modified by genetic variants in both the CYP2C19 and ABCB1 genes. Thromb. Haemost. 2013, 109, 744–754. [Google Scholar]
- Egashira, K.; Nakano, K.; Ohtani, K.; Funakoshi, K.; Zhao, G.; Ihara, Y.; Koga, J.I.; Kimura, S.; Tominaga, R.; Sunagawa, K. Local delivery of anti-monocyte chemoattractant protein-1 by gene-eluting Stents attenuates in-stent stenosis in rabbits and monkeys. Arter. Throm. Vas. 2007, 27, 2563–2568. [Google Scholar] [CrossRef] [Green Version]
- Goh, D.; Tan, A.; Farhatnia, Y.; Rajadas, J.; Alavijeh, M.S.; Seifalian, A.M. Nanotechnology-Based Gene-Eluting Stents. Mol. Pharm. 2013, 10, 1279–1298. [Google Scholar] [CrossRef]
- Ohtani, K.; Egashira, K.; Nakano, K.; Zhao, G.; Iwata, E.; Yamamoto, M.; Sunagawa, K. Anti-monocyte chemoattractant protein-1 strategy via local gene transfer with gene-eluting stents inhibits in-stent restenosis in hypercholesterolemic rabbits and monkeys. Circulation 2005, 112, U715. [Google Scholar]
- Sharif, F.; Hynes, S.O.; Cooney, R.; Howard, L.; McMahon, J.; Daly, K.; Crowley, J.; Barry, F.; O’Brien, T. Gene-eluting stents: Adenovirus-mediated delivery of eNOS to the blood vessel wall accelerates re-endothelialization and inhibits restenosis. Mol. Ther. 2008, 16, 1674–1680. [Google Scholar] [CrossRef]
- Sharif, F.; Hynes, S.O.; McCullagh, K.J.A.; Ganley, S.; Greiser, U.; McHugh, P.; Crowley, J.; Barry, F.; O’Brien, T. Gene-eluting stents: Non-viral, liposome-based gene delivery of eNOS to the blood vessel wall in vivo results in enhanced endothelialization but does not reduce restenosis in a hypercholesterolemic model. Gene Ther. 2012, 19, 321–328. [Google Scholar] [CrossRef] [Green Version]
- Sharif, F.; Hynes, S.O.; McMahon, J.; Cooney, R.; Conroy, S.; Dockery, P.; Duffy, G.; Daly, K.; Crowley, J.; Bartlett, J.S.; et al. Gene-eluting stents: Comparison of adenoviral and adeno-associated viral gene delivery to the blood vessel wall in vivo. Hum. Gene Ther. 2006, 17, 741–750. [Google Scholar] [CrossRef]
- Walter, D.H.; Cejna, M.; Diaz-Sandoval, L.; Willis, S.; Kirkwood, L.; Stratford, P.W.; Tietz, A.B.; Kirchmair, R.; Silver, M.; Curry, C.; et al. Local gene transfer of phVEGF-2 plasmid by gene-eluting stents-An alternative strategy for inhibition of restenosis. Circulation 2004, 110, 36–45. [Google Scholar] [CrossRef] [Green Version]
- Yin, R.X.; Yang, D.Z.; Wu, J.Z. Nanoparticle Drug-and Gene-eluting Stents for the Prevention and Treatment of Coronary Restenosis. Theranostics 2014, 4, 175–200. [Google Scholar] [CrossRef] [Green Version]
- Lekshmi, K.M.; Che, H.-L.; Choan, C.-S.; Park, I.-K. Drug- and gene-eluting stents for preventing coronary restenosis. Chonnam. Med. J. 2017, 53, 14–27. [Google Scholar] [CrossRef] [Green Version]
- Luderer, F.; Lobler, M.; Rohm, H.W.; Gocke, C.; Kunna, K.; Kock, K.; Kroemer, H.K.; Weitschies, W.; Schmitz, K.P.; Sternberg, K. Biodegradable Sirolimus-loaded Poly(lactide) Nanoparticles as Drug Delivery System for the Prevention of In-Stent Restenosis in Coronary Stent Application. J. Biomater. Appl. 2011, 25, 851–875. [Google Scholar] [CrossRef]
- Stolnik, S.; Davies, M.C.; Illum, L.; Davis, S.S.; Boustta, M.; Vert, M. The Preparation of Sub-200nm Biodegradable Colloidal Particles from Poly(Beta-Malic Acid-Co-Benzyl Malate) Copolymers and Their Surface Modification with Poloxamer and Poloxamine Surfactants. J. Control. Release. 1994, 30, 57–67. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Staiger, M.P.; Dias, G.J.; Hadisi, Z.; Saheban, M.; Kashefian, M. Drug release, cytocompatibility, bioactivity, and antibacterial activity of doxycycline loaded Mg-Ca-TiO2 composite scaffold. Mater. Des. 2018, 139, 212–221. [Google Scholar] [CrossRef]
- Martin, D.M.; Boyle, F.J. Drug-eluting stents for coronary artery disease: A review. Med. Eng. Phys. 2011, 33, 148–163. [Google Scholar] [CrossRef] [Green Version]
- Nakazawa, G.; Finn, A.V.; Vorpahl, M.; Ladich, E.R.; Kolodgie, F.D.; Virmani, R. Coronary Responses and Differential Mechanisms of Late Stent Thrombosis Attributed to First-Generation Sirolimus- and Paclitaxel-Eluting Stents. J. Am. Coll Cardiol. 2011, 57, 390–398. [Google Scholar] [CrossRef] [Green Version]
- Stefanini, G.G.; Holmes, D.R. Drug-Eluting Coronary-Artery Stents. N. Engl J. Med. 2013, 368, 254–265. [Google Scholar] [CrossRef] [Green Version]
- Baquet, M.; Jochheim, D.; Mehilli, J. Polymer-free drug-eluting stents for coronary artery disease. J. Interv. Cardiol. 2018, 31, 330–337. [Google Scholar] [CrossRef]
- Chen, W.L.; Habraken, T.C.J.; Hennink, W.E.; Kok, R.J. Polymer-Free Drug-Eluting Stents: An Overview of Coating Strategies and Comparison with Polymer-Coated Drug-Eluting Stents. Bioconjugate Chem. 2015, 26, 1277–1288. [Google Scholar] [CrossRef]
- Hausleiter, J.; Kastrati, A.; Wessely, R.; Dibra, A.; Mehilli, J.; Schratzenstaller, T.; Graf, I.; Renke-Gluszko, M.; Behnisch, B.; Dirschinger, J.; et al. FASTTRACK-Prevention of restenosis by a novel drug-eluting stent system with a dose-adjustable, polymerfree, on-site stent coating. Eur. Heart J. 2005, 26, 1475–1481. [Google Scholar] [CrossRef]
- Gershlick, A.; De Scheerder, I.; Chevalier, B.; Stephens-Lloyd, A.; Camenzind, E.; Vrints, C.; Reifart, N.; Missault, L.; Goy, J.J.; Brinker, J.A.; et al. Inhibition of restenosis with a paclitaxel-eluting, polymer-free coronary stent-The European evaLUation of pacliTaxel Eluting Stent (ELUTES) trial. Circulation 2004, 109, 487–493. [Google Scholar] [CrossRef] [Green Version]
- Song, D.; Hsu, L.F.; Au, J.L.S. Binding of taxol to plastic and glass containers and protein under in vitro conditions. J. Pharm. Sci. 1996, 85, 29–31. [Google Scholar] [CrossRef]
- Farah, S.; Khan, W.; Domb, A.J. Crystalline coating of rapamycin onto a stent: Process development and characterization. Int. J. Pharm. 2013, 445, 20–28. [Google Scholar] [CrossRef]
- Wu, S.; Harish, S.; Sanders-Millare, D.; Guruwaiya, J.; Mirzaee, D.; Hossainy, S.; Chen, C. Surface features of an implantable medical device. U.S. Patent 10/911,968, 10 February 2005. [Google Scholar]
- Mikhalovska, L.; Chorna, N.; Lazarenko, O.; Haworth, P.; Sudre, A.; Mikhalovsky, S. Inorganic coatings for cardiovascular stents: In vitro and in vivo studies. J. Biomed. Mater. Res. B 2011, 96B, 333–341. [Google Scholar] [CrossRef]
- Stevenson, C.L.; Santini, J.T.; Langer, R. Reservoir-based drug delivery systems utilizing microtechnology. Adv. Drug Deliv. Rev. 2012, 64, 1590–1602. [Google Scholar] [CrossRef] [Green Version]
- Bartorelli, A.L.; Trabattoni, D.; Fabbiocchi, F.; Montorsi, P.; Martini, S.D.; Calligaris, G.; Ravagnani, P. Synergy of passive coating and targeted drug delivery. J. Interv. Cardiol. 2003, 16, 499–505. [Google Scholar] [CrossRef]
- Kollum, M.; Farb, A.; Schreiber, R.; Terfera, K.; Arab, A.; Geist, A.; Haberstroh, J.; Wnendt, S.; Virmani, R.; Hehrlein, C. Particle debris from a nanoporous stent coating obscures potential antiproliferative effects of tacrolimus-eluting stents in a porcine model of restenosis. Catheter. Cardio. Inte. 2005, 64, 85–90. [Google Scholar] [CrossRef]
- Martin, F.; Walczak, R.; Boiarski, A.; Cohen, M.; West, T.; Cosentino, C.; Shapiro, J.; Ferrari, M. Tailoring width of microfabricated nanochannels to solute size can be used to control diffusion kinetics (vol 102, pg 123, 2005). J. Control. Release. 2005, 107, 183. [Google Scholar] [CrossRef]
- Ruan, C.M.; Bayer, T.; Meth, S.; Sukenik, C.N. Creation and characterization of n-alkylthiol and n-alkylamine self-assembled monolayers on 316L stainless steel. Thin Solid Film. 2002, 419, 95–104. [Google Scholar] [CrossRef]
- Arney, S.; Kroupenkine, T.N.; Weiss, D. Drug delivery stent. U.S. Patent 8915957B2, 15 September 2005. [Google Scholar]
- Byrne, R.A.; Kastrati, A.; Kufner, S.; Massberg, S.; Birkmeier, K.A.; Laugwitz, K.L.; Schulz, S.; Pache, J.; Fusaro, M.; Seyfarth, M.; et al. Randomized, non-inferiority trial of three limus agent-eluting stents with different polymer coatings: The Intracoronary Stenting and Angiographic Results: Test Efficacy of 3 Limus-Eluting Stents (ISAR-TEST-4) Trial. Eur. Heart J. 2009, 30, 2441–2449. [Google Scholar] [CrossRef]
- Krucoff, M.W.; Wijns, W.; Mehran, R.; Parhizgar, A.; Verheye, S.; Dubois, C.L.; Batchelor, W.B.; O’shaughnessy, C.D.; Petersen, J.L.; Kereiakes, D.J. Multicenter randomized evaluation of a novel paclitaxel eluting stent with bioresorbable polymer for the treatment of single and multivessel coronary disease: Primary results of the COSTAR II study. Eur. Heart J. 2007, 28, 574–575. [Google Scholar]
- Windecker, S.; Serruys, P.W.; Wandel, S.; Buszman, P.; Trznadel, S.; Linke, A.; Lenk, K.; Ischinger, T.; Klauss, V.; Eberli, F.; et al. Biolimus-eluting stent with biodegradable polymer versus sirolimus-eluting stent with durable polymer for coronary revascularisation (LEADERS): A randomised non-inferiority trial. Lancet 2008, 372, 1163–1173. [Google Scholar] [CrossRef]
- Heublein, B.; Evagorou, E.G.; Rohde, R.; Ohse, S.; Meliss, R.R.; Barlach, S.; Haverich, A. Polymerized degradable hyaluronan-a platform for stent coating with inherent inhibitory effects on neointimal formation in a porcine coronary model. Int. J. Artif. Organs. 2002, 25, 1166–1173. [Google Scholar] [CrossRef]
- Remuzzi, A.; Mantero, S.; Colombo, M.; Morigi, M.; Binda, E.; Camozzi, D.; Imberti, B. Vascular smooth muscle cells on hyaluronic acid: Culture and mechanical characterization of an engineered vascular construct. Tissue Eng. 2004, 10, 699–710. [Google Scholar] [CrossRef]
- Travis, J.A.; Hughes, M.G.; Wong, J.M.; Wagner, W.D.; Geary, R.L. Hyaluronan enhances contraction of collagen by smooth muscle cells and adventitial fibroblasts-Role of CD44 and implications for constrictive remodeling. Circ. Res. 2001, 88, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Hara, H.; Nakamura, M.; Palmaz, J.C.; Schwartz, R.S. Role of stent design and coatings on restenosis and thrombosis. Adv. Drug Deliv. Rev. 2006, 58, 377–386. [Google Scholar] [CrossRef]
- Acharya, G.; Park, K. Mechanisms of controlled drug release from drug-eluting stents. Adv. Drug Deliv. Rev. 2006, 58, 387–401. [Google Scholar] [CrossRef]
- Perrin, J.H. Sustained and controlled release drug delivery systems. J. Pharm. Sci. 1980, 69, 485. [Google Scholar] [CrossRef]
- Allen, L.; Ansel, H.C. Ansel’s Pharmaceutical Dosage forms and Drug Delivery Systems; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2013. [Google Scholar]
- Chien, Y. Novel Drug Delivery Systems, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Park, K. Controlled Drug Delivery: Challenges and Strategies; Amer Chemical Society: Washington, DC, USA, 1997. [Google Scholar]
- Saltzman, W.M. Drug Delivery: Engineering Principles for Drug Therapy; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Rathbone, M.J.; Hadgraft, J.; Roberts, M.S. Modified-release Drug Delivery Technology; Marcel Dekker: New York, NY, USA, 2003. [Google Scholar]
- Leon, M.B.; Abizaid, A.; Moses, J.W. The CYPHER™ stent: A New Gold Standard in the Treatment of Coronary Artery Disease; The Cardiovascular Research Foundation: New York, NY, USA, 2003. [Google Scholar]
- Russell, M.E. Comprehensive Review of the Polymer-Based Taxol Release Kinetics and Animal Data: INSIGHTS into Efficacy and Toxicity; TranscatheterCardiovascularTherapeutics (TCT): Miami, FL, USA, 2002. [Google Scholar]
- Heldman, A.W.; Cheng, L.; Jenkins, G.M.; Heller, P.F.; Kim, D.W.; Ware, M.; Nater, C.; Hruban, R.H.; Rezai, B.; Abella, B.S.; et al. Paclitaxel stent coating inhibits neointimal hyperplasia at 4 weeks in a porcine model of coronary restenosis. Circulation 2001, 103, 2289–2295. [Google Scholar] [CrossRef] [Green Version]
- Lansky, A.J.; Costa, R.A.; Mintz, G.S.; Tsuchiya, Y.; Midei, M.; Cox, D.A.; O’Shaughnessy, C.; Applegate, R.A.; Cannon, L.A.; Mooney, M.; et al. Non-polymer-based paclitaxel-coated coronary stents for the treatment of patients with de novo coronary lesions-Angiographic follow-up of the DELIVER clinical trial. Circulation 2004, 109, 1948–1954. [Google Scholar] [CrossRef] [Green Version]
- Farb, A.; Heller, P.F.; Shroff, S.; Cheng, L.; Kolodgie, F.D.; Carter, A.J.; Scott, D.S.; Froehlich, J.; Virmani, R. Pathological analysis of local delivery of paclitaxel via a polymer-coated stent. Circulation 2001, 104, 473–479. [Google Scholar] [CrossRef] [Green Version]
- Finkelstein, A.; McClean, D.; Kar, S.; Takizawa, K.; Varghese, K.; Baek, N.; Park, K.; Fishbein, M.C.; Makkar, R.; Litvack, F.; et al. Local drug delivery via a coronary stent with programmable release pharmacokinetics. Circulation 2003, 107, 777–784. [Google Scholar] [CrossRef] [Green Version]
- Whelan, D.M.; van der Giessen, W.J.; Krabbendam, S.C.; van Vliet, E.A.; Verdouw, P.D.; Serruys, P.W.; van Beusekom, H.M.M. Biocompatibility of phosphorylcholine coated stents in normal porcine coronary arteries. Heart 2000, 83, 338–345. [Google Scholar] [CrossRef] [Green Version]
- SCHIAVONE, A.; QIU, T.; ZHAO, L. Crimping and deployment of metallic and polymeric stents-finite element modelling. Vessel Plus 2017, 1, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Yang, J.; Huang, N.; Uhl, C.; Zhou, Y.H.; Liu, Y.L. Mechanical response of cardiovascular stents under vascular dynamic bending. Biomed. Eng. Online 2016, 15, 21. [Google Scholar] [CrossRef] [Green Version]
- Jayaraman, S. Structurally variable stents. U.S. Patent 6908480B2, 6 March 2003. [Google Scholar]
- Granada, J.F.; Huibregtse, B.A.; Dawkins, K.D. New stent design for use in small coronary arteries during percutaneous coronary intervention. Med. Devices: Evid. Res. 2010, 3, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Lacatus, R.; Papuc, I.; Purdoiu, R.C.; Pavaloiu, A.; Gal, A.; Antal, I.Z. Radiological diagnosis in experimental rabbit endocarditis. Sci. Work. Univ. Agron. Sci. Vet. Med. Buchar. C Vet. Med. 2010, 56, 186–195. [Google Scholar]
- Purdoiu, R.C.; Papuc, I.; Lacatuşu, R.; Pavaloiu, A.N. Radiological Diagnosis in Heart Conditions in Dogs. Cluj Vet. J. 2011, 1, 19. [Google Scholar]
- Hsiao, H.-M.; Chien, A.; Huang, B.-H.; Li, D.-R.; Chen, H.; Ko, C.-Y. Device Integrity of Drug-eluting Depot Stent for Smart Drug Delivery. In Smart Drug Delivery System; Sezer, A.D., Ed.; IntechOpen: London, UK, 2016. [Google Scholar]
- Zhao, S.J.; Gu, L.X.; Froemming, S.R. On the Importance of Modeling Stent Procedure for Predicting Arterial Mechanics. J. Biomech Eng.-T Asme 2012, 134, 121005 (6pg). [Google Scholar] [CrossRef]
- Mehta, A.; Gong, X.Y.; Imbeni, V.; Pelton, A.R.; Ritchie, R.O. Understanding the deformation and fracture of nitinol endovascular stents using in situ synchrotron X-ray microdiffraction. Adv. Mater. 2007, 19, 1183. [Google Scholar] [CrossRef]
- Xu, J.; Yang, J.; Sohrabi, S.; Zhou, Y.H.; Liu, Y.L. Finite Element Analysis of the Implantation Process of Overlapping Stents. J. Med. Devices 2017, 11, 021010. [Google Scholar] [CrossRef] [Green Version]
- Mehdizadeh, A.; Ali, M.S.M.; Takahata, K.; Al-Sarawi, S.; Abbott, D. A recoil resilient lumen support, design, fabrication and mechanical evaluation. J. Micromech. Microeng. 2013, 23, 065001. [Google Scholar] [CrossRef]
- Shi, Y.J.; Zhang, L.; Chen, J.H.; Zhang, J.; Yuan, F.; Shen, L.; Chen, C.X.; Pei, J.; Li, Z.H.; Tan, J.Y.; et al. In vitro and in vivo degradation of rapamycin-eluting Mg-Nd-Zn-Zr alloy stents in porcine coronary arteries. Mat. Sci. Eng. C-Mater 2017, 80, 1–6. [Google Scholar] [CrossRef]




| Mg–Alloy | Key Features | Ref. |
|---|---|---|
| Mg–Zn (up to 3% Zn) | Higher affinity of adsorption to the surface of Mg–Zn alloy with the increase of Zn concentration (up to 3%). | [123] |
| Mg–Y (1% Y) | Adsorption of peptides is slightly weakened compared to that on the clean Mg (0001) surfaces. | |
| Mg–Nd (1% Nd) | ||
| Mg (3.5 or 6.5%)-Li (0.5, 2 or 4%)-Zn | Good mechanical properties, degradation behavior, cytocompatibility, and hemocompatibility. Enhanced mechanical properties—yield strength, ultimate strength and elongation (twice as compared to pure Zn) and corrosion resistance without losing the viability of the Human Umbilical Vein Endothelial Cells (HUVECS) and Human Aorta Vascular Smooth Muscle Cells (VSMCS). | [124] |
| Mg–Al alloy AZ61 | Highly susceptible to stress corrosion cracking (SCC) as compared to Zn, which is highly ductile with limited susceptibility to SCC. | [125] |
| MgZnYNd (coated with arginine (Arg)-based poly (ester urea urethane) (Arg–PEUU)) | Super corrosion retardation, high hemocompatibility, high cytocompatibility. | [126] |
| Mg stent (coated with phytic acid (PA)); heparin loaded PA and bivalirudin loaded PA | Effective control on corrosion rate, biofunctional effect, good hemocompatibility, inhibits platelets adhesion, promotes endothelial cells growth superior stents compared with the bare Mg stents, super-hydrophilic surface (the contact angle being very close to zero). Hydrogen evolution vs. immersion time exhibit a slightly linear release between 5 and 10 days as compared to uncoated samples where an exponential hydrogen release was noticed within this interval. | [124] |
| Zn-Alloy | Key Features | Ref. |
|---|---|---|
| Pure Zn | Stents maintained mechanical integrity while no severe inflammation, platelet aggregation, thrombosis formation, or intimal hyperplasia were observed in abdominal aorta of rabbits. Good mechanical integrity for 6 months. After 12 months of implantation, the degraded volume of the stents was 41.75 ± 29.72%. | [150] |
| Zinc wires coated with PLLA/MPS | Corrodes at half the rate of uncoated Zn. Reduction of the biocompatibility and increasing cell toxicity and neointimal hyperplasia takes place. | [151] |
| Zn-1% Mg and Zn-1% Mg-0.5% Ca | These zinc alloys can be considered as good candidates for biodegradable implants. | [152] |
| Zn-Li alloy | Increase of ultimate tensile strength from <120 MPa (pure Zn) to >560 MPa. In vitro corrosion was evaluated by immersion tests in simulated body fluid and reveal higher resistance to corrosion compared to pure Zn. Samples containing 4% Li have shown the best results. | [149] |
| Zn-3Cu-xFe (x = 0, 0.5 and 1 wt %) alloys | The mechanical characteristics and in vitro behavior of Zn-3Cu-xFe alloys are more suitable than that of Zn-3Cu alloys as candidates for biodegradable materials. | [153] |
| Zn–Al alloys (containing up to 5.5 wt% Al) | Important mechanical characteristics: Yield strength 190–240 MPa; ultimate tensile strength 220–300 MPa, elongation 15–30%, elastic ranges 0.19–0.27%. Intergranular corrosion of Zn–Al alloys and cracking related with corrosion are observed. Absences of necrosis traces, though chronic and acute inflammatory indications were present. | [147] |
| Stent (Manufacturer) | Type/Generation | Drug | Material (Bulk/Polymer) | FDA | Trials | Drug-Eluting Time | Ref. |
|---|---|---|---|---|---|---|---|
| CYPHERTM (Cordis) | SES/First | Sirolimus | Stainless steel/Parylene C | 2003 | FIM (First-In Man), RAVEL, SIRIUS, E-SIRIUS, C-SIRIUS | 80% of sirolimus elutes over ~30 days; remainder released by end of 90 days | [163,169,195] |
| Taxus® (Boston Scientific) | PES/First | Paclitaxel | Stainless steel or platinum-chromium/TransluteTM polymer | 2004 | TAXUS I-VI, TAXUS ATLAS, PERSEUS | elutes over ~90 days | [163,169,195,196] |
| Endeavor® (Medtronic Inc., Minneapolis, MN) | ZES/Second | Zotarolimus | cobalt-chromium/phosphorylcholine | 2008 | ENDEAVOR I–IV | 80% during first 10 days | [195,197] |
| XienceTM V (Abbott Laboratories) | EES/Second | Everolimus | L-605 Co-Cr/Poly (vinylidenefluoride-co-hexafluoropropylene) (PVDF-HFP) | 2008 | SPIRIT I-IV (Standard Protocol Items: Recommendations for Interventional Trials I-IV) | 80% during first 30 days | [195,197] |
| Axxion (Biosensors International) | PF-DES | Paclitaxel | 316L SS | - | - | 40–50% in the first week 100% after 4 weeks | [198] |
| Achieve (Cook Inc.) | PF-DES | Paclitaxel | 316L SS | - | 8 months DELIVER (DELiverability of the Resolute Integrity Stent in All-Comer Vessels and Cross-OvER Stenting) Clinical Trial | 28% within 4 days 69% within 2 weeks | [198,199] |
| Amazonia PAX (MINVASYS) | PF-DES | Paclitaxel | L605 Co-Cr | - | Pax A and Pax B Clinical Study Design | 60% within 48 h, 100% within 7 weeks | [198,199] |
| Biofreedom (Biosensors International) | PF-DES | Biolimus A9 | 316L SS | - | BioFreedom FIM | 98% of drug within 4 weeks | [195,198] |
| Polymer-free DFS (Medtronic) | PF-DES | Sirolimus | Co-Cr, Tantalum | - | Medtronic RevElution Trial | N/A | [8,198] |
| Cre8 (Alvimedica) | PF-DES | Amphilimus | L605 Co-Cr | - | Clinical performance of CRE8 drug-eluting stent in all comer population (PARTICIPATE) (phase 4) | 50% of drug on 1st day 100% within 3 weeks | [8,198] |
| JANUS (Sorin Biomedica) | PF-DES | Tacrolimus | 316L SS | - | JUPITER I, JUPITER II | 50% over 4 weeks | [195,198] |
| NANO + (LEPU Medical) | PF-DES | Sirolimus | 316L SS | - | Clinical performance of nano plus sirolimus-eluting stents in patients with coronary artery disease | 85% in 4 weeks | [8,198,199] |
| Supra-G (Cook Inc.) | PF-DES | Paclitaxel | 316L SS | 6 months ASPECT (Asian Paclitaxel-Eluting Stent Clinical Trial) | N/A | [198,199] | |
| VEST Async (MIV Therapeutics) | PF-DES | Sirolimus | 316L SS | - | 9 months Vest Saync II Clinical Trial | 100% in 3–4 weeks | [198,199] |
| V-Flex Plus (Cook Inc) | PF-DES | Paclitaxel | 316L SS | - | 6 months Clinical Trial | 28% within 4 days 69% within 2 weeks | [198,199] |
| YUKON (Translumina GmbH) | PF-DES | Sirolimus, Probucol | 316L SS | ISAR-TEST, ISAR-TEST 3, ISAR-TEST 4, ISAR-PEACE (Posthumous Evaluation of Advanced Cancer Environment | 66% in 2 weeks 100% over 3 weeks | [195,198] | |
| YINYI (Liaoning Biomedical Materials) | PF-DES | Paclitaxel | 316L SS | Safety and Efficacy Registry of Yinyi Stent (SERY-II) (SERY-II) | 42% in 24 h 100% in 9 weeks | [8,198] |
| Coating | Technology | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| Direct coating | Stent dipping into the drug solution followed by solvent evaporation | Ideal for drugs with a very low aqueous solubility | Limited loaded drug amount; burst drug release kinetics | [8,198,199] |
| Crystallization | Direct temperature-dependent or micro drop spray drug crystallization on the stent surface | Slower release than amorphous drug layers due to lower dissolution rate | Limited loaded drug amount; burst drug release kinetics | [8,198,199] |
| Nano-/micro-porous coating | Micro/nanopores on the stent surface produced by sandblasting or mechanical modification | Higher amount of drug loading; sustained drug release due to a longer diffusion time; rough surface induces early endothelialization | Possible release of aluminum oxide particles | [8,198,199] |
| Inorganic porous coating | Pores are localized in an inorganic coating on the metal stent surface | Reduction of platelet activation due to a decreased release of metal ions | Release of inorganic particles after implantation pose a major challenge | [8,198,199] |
| Macroporous drug reservoir | Drug reservoir in form of abluminal stent grooves, holes or channels | Single and multidrug loading; slower drug elution by barriers like nanopores | Release of ions may cause local irritation | [8,198,199] |
| Nano-particle coating | Surface coating with a porous composite matrix based magnetic silicon and carbon nanoparticles or self-assembled monolayers | High drug adsorption and flexibility of the nanoparticle coating; rapid endothelialization | Nanocarrier properties are critical since the polymer may trigger mild immune response | [8,198,199] |
| Drug filling/ internal coating | A drug coats an internal lumen of the stent, diffusing through abluminal microholes directly into the vessel wall | Slower drug elution by barriers like microholes | N/A | [8,198,199] |
| Self-assembled monolayers | Deposition of self-assembled hydrocarbon chains on a stent surface | Controlled release and rapid endothelialization | Low drug loading | [8] |
| Polymer DES Coating | Features | Ref. |
|---|---|---|
| Polylactic acid | Effective in short and mid-term follow-ups | [190,212,213,214] |
| Poly-l-lactic acid (PLLA) | Feasible, safe, and effective implantation | [190] |
| Poly (lactic-co-glycolic acid) | Slow releasing capability for hydrophobic drugs | [190] |
| Hyaluronic acid | Good degradation efficiency, enhances the proliferation and migration of endothelial cells | [190,215,216,217] |
| Polyzene-F | Highly biocompatible, anti-inflammatory, bacteria-resistant and pro-healing | [190] |
| Drug | Binding Target | Structural Formula | Mode of Action |
|---|---|---|---|
| Sirolimus | FK-506 Binding Protein 12 |  | Anti-proliferative, immunosuppressive |
| Umirolimus/ Biolimus A9/ Biolimus/BA9 | FK-506 Binding Protein 12 |  | Immunosuppressive |
| Zotarolimus | FK-506 Binding Protein 12 |  | Anti-proliferative, immunosuppressive |
| Everolimus | FK-506 Binding Protein 12 |  | Immunosuppressive |
| Novolimus | FK-506 Binding Protein 12 |  | Anti-proliferative, anti-inflammatory |
| Tacrolimus | FK-506 Binding Protein 12 |  | Anti-proliferative, immunosuppressive |
| Pimecrolimus | Macrophilin-12 |  | Immuno-modulating agent of the calcineurin inhibitor |
| Paclitaxel | Microtubules |  | Anti-proliferative agent |
| Dexamethasone | Specific steroid-binding protein receptor |  | Anti-inflammatory |
| Curcumin | Microtubules |  | N/A |
| Terumo statin | -- | - | Anti-proliferative |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beshchasna, N.; Saqib, M.; Kraskiewicz, H.; Wasyluk, Ł.; Kuzmin, O.; Duta, O.C.; Ficai, D.; Ghizdavet, Z.; Marin, A.; Ficai, A.; et al. Recent Advances in Manufacturing Innovative Stents. Pharmaceutics 2020, 12, 349. https://doi.org/10.3390/pharmaceutics12040349
Beshchasna N, Saqib M, Kraskiewicz H, Wasyluk Ł, Kuzmin O, Duta OC, Ficai D, Ghizdavet Z, Marin A, Ficai A, et al. Recent Advances in Manufacturing Innovative Stents. Pharmaceutics. 2020; 12(4):349. https://doi.org/10.3390/pharmaceutics12040349
Chicago/Turabian StyleBeshchasna, Natalia, Muhammad Saqib, Honorata Kraskiewicz, Łukasz Wasyluk, Oleg Kuzmin, Oana Cristina Duta, Denisa Ficai, Zeno Ghizdavet, Alexandru Marin, Anton Ficai, and et al. 2020. "Recent Advances in Manufacturing Innovative Stents" Pharmaceutics 12, no. 4: 349. https://doi.org/10.3390/pharmaceutics12040349
APA StyleBeshchasna, N., Saqib, M., Kraskiewicz, H., Wasyluk, Ł., Kuzmin, O., Duta, O. C., Ficai, D., Ghizdavet, Z., Marin, A., Ficai, A., Sun, Z., Pichugin, V. F., Opitz, J., & Andronescu, E. (2020). Recent Advances in Manufacturing Innovative Stents. Pharmaceutics, 12(4), 349. https://doi.org/10.3390/pharmaceutics12040349










