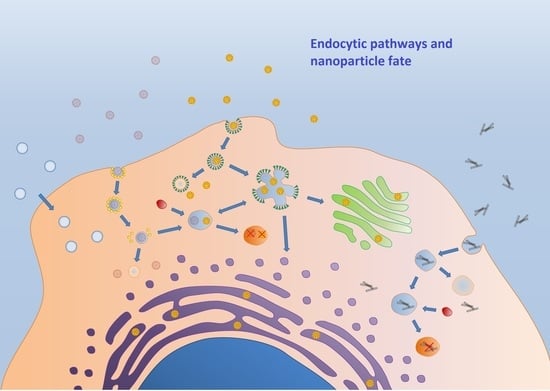
Endocytosis: The Nanoparticle and Submicron Nanocompounds Gateway into the Cell
Abstract
:1. Introduction
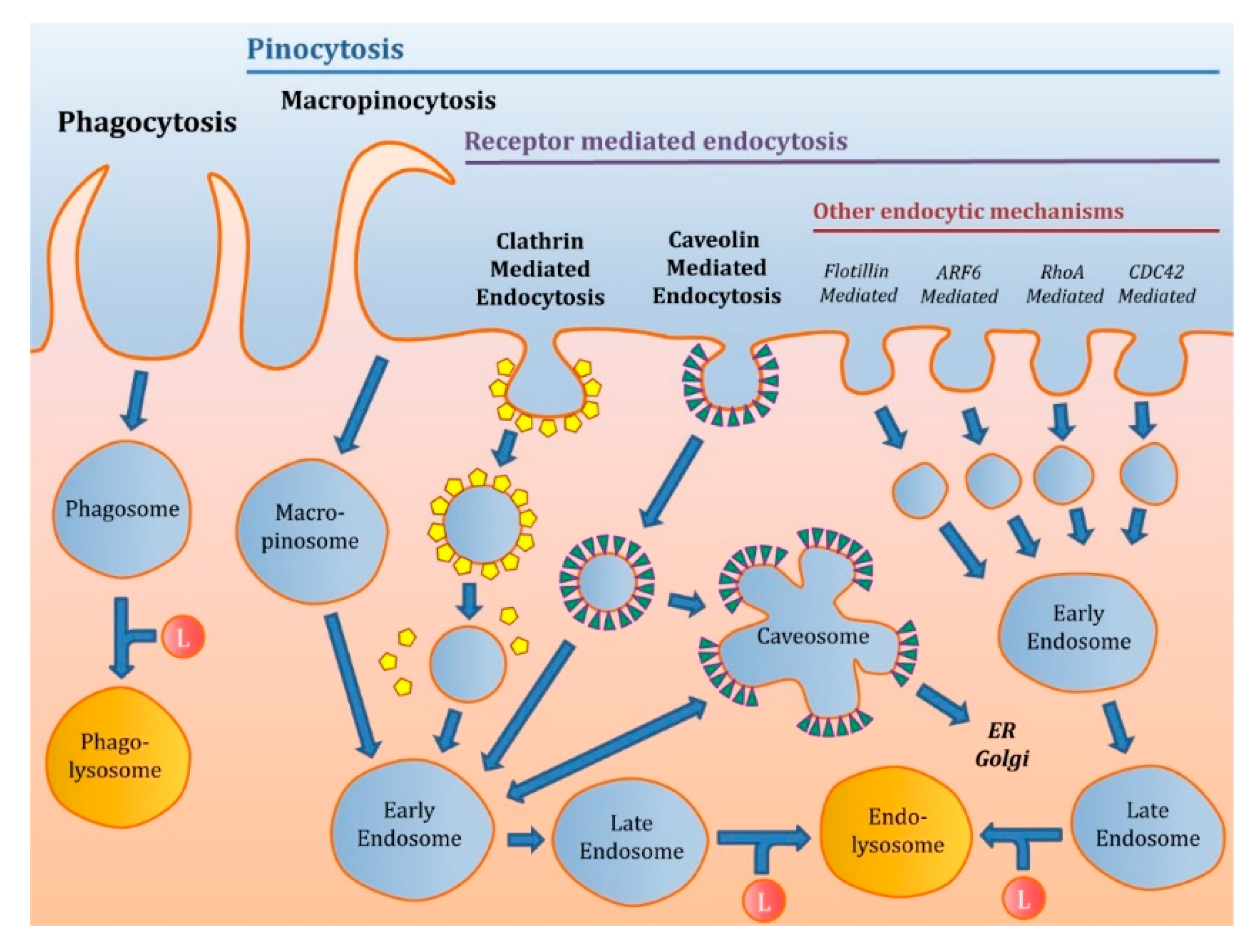
2. Classification of the Endocytic Pathways
2.1. Phagocytosis
2.2. Pinocytosis
2.2.1. Macropinocytosis
2.2.2. Receptor-Mediated Endocytosis (RME)
Clathrin-Mediated Endocytosis (CME)
Caveolin-Mediated Endocytosis (CVME)
Other Pathways
3. Methodology to Elucidate the Different Endocytosis Pathways of Nanoparticles (NPs) in Cells
4. Influence of NP Physical Properties on the Cellular Uptake
4.1. Size
4.2. Charge
4.3. Shape
4.4. Rigidity
4.5. Other Factors
5. Endocytosis Pathways for Nanoparticles
5.1. Polymeric NPs
5.1.1. Natural Polymers
Chitosan Submicron NPs (CSNPs)
Albumin-Based NPs
Alginate NPs
5.1.2. Synthetic Polymers
Polystyrene NPs
Poly(lactic-co-glycolic) (PLGA) NPs
Polyethylenimine (PEI) NPs
5.2. Dendrimers
5.3. Lipidic NPs
5.3.1. Liposomes
5.3.2. Solid Lipid NPs (SLNs)
5.4. Carbon Based Nanoparticles
5.4.1. Carbon Nanotubes
Single-Walled Carbon Nanotubes (SWCNTs)
Multi-Walled Carbon Nanotubes (MWCNTs)
5.4.2. Fullerenes
5.4.3. Carbon Oxide NPs
5.5. Quantum Dots (QDs)
5.6. Metallic NPs
5.6.1. Iron Oxide NPs (IONPs)
5.6.2. Gold NPs (AuNPs)
5.7. Mesoporous Silica NPs (MSNPs)
5.8. β-Cyclodextrin Based NPs (CDNPs)
5.9. Micelles
5.9.1. Gemini Surfactant Micelles
5.9.2. Polymeric Micelles
Pluronic
Polyethylene Glycol (PEG)
Hyaluronic Acid (HA)
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Borbas, E.; Sinko, B.; Tsinman, O.; Tsinman, K.; Kiserdei, E.; Demuth, B.; Balogh, A.; Bodak, B.; Domokos, A.; Dargo, G.; et al. Investigation and Mathematical Description of the Real Driving Force of Passive Transport of Drug Molecules from Supersaturated Solutions. Mol. Pharm. 2016, 13, 3816–3826. [Google Scholar] [CrossRef] [PubMed]
- Hunter, P. Next-generation nanotech meds: Diagnostic and therapeutic applications of non-organic nanoparticles are making their way into clinical use. EMBO Rep. 2017, 18, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Doherty, G.J.; McMahon, H.T. Mechanisms of endocytosis. Annu. Rev. Biochem. 2009, 78, 857–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kou, L.; Sun, J.; Zhai, Y.; He, Z. The endocytosis and intracellular fate of nanomedicines: Implication for rational design. Asian J. Pharm. Sci. 2013, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Varkouhi, A.K.; Scholte, M.; Storm, G.; Haisma, H.J. Endosomal escape pathways for delivery of biologicals. J. Control. Release 2011, 151, 220–228. [Google Scholar] [CrossRef]
- Mercer, J.; Helenius, A. Gulping rather than sipping: Macropinocytosis as a way of virus entry. Curr. Opin. Microbiol. 2012, 15, 490–499. [Google Scholar] [CrossRef]
- McMahon, H.T.; Boucrot, E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2011, 12, 517–533. [Google Scholar] [CrossRef]
- Popova, N.V.; Deyev, I.E.; Petrenko, A.G. Clathrin-mediated endocytosis and adaptor proteins. Acta Nat. 2013, 5, 62–73. [Google Scholar] [CrossRef]
- Pelkmans, L.; Burli, T.; Zerial, M.; Helenius, A. Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell 2004, 118, 767–780. [Google Scholar] [CrossRef] [Green Version]
- Vercauteren, D.; Vandenbroucke, R.E.; Jones, A.T.; Rejman, J.; Demeester, J.; De Smedt, S.C.; Sanders, N.N.; Braeckmans, K. The use of inhibitors to study endocytic pathways of gene carriers: Optimization and pitfalls. Mol. Ther. 2010, 18, 561–569. [Google Scholar] [CrossRef] [Green Version]
- Koivusalo, M.; Welch, C.; Hayashi, H.; Scott, C.C.; Kim, M.; Alexander, T.; Touret, N.; Hahn, K.M.; Grinstein, S. Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. J. Cell Biol. 2010, 188, 547–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillaireau, H.; Couvreur, P. Nanocarriers’ entry into the cell: Relevance to drug delivery. Cell. Mol. Life Sci. 2009, 66, 2873–2896. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef] [PubMed]
- Chithrani, D.B.; Dunne, M.; Stewart, J.; Allen, C.; Jaffray, D.A. Cellular uptake and transport of gold nanoparticles incorporated in a liposomal carrier. Nanomedicine 2010, 6, 161–169. [Google Scholar] [CrossRef]
- Foroozandeh, P.; Aziz, A.A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale. Res. Lett. 2018, 13, 339. [Google Scholar]
- Asati, A.; Santra, S.; Kaittanis, C.; Perez, J.M. Surface-charge-dependent cell localization and cytotoxicity of cerium oxide nanoparticles. ACS Nano 2010, 4, 5321–5331. [Google Scholar] [CrossRef] [Green Version]
- Billiet, L.; Gomez, J.P.; Berchel, M.; Jaffres, P.A.; Le, G.T.; Montier, T.; Bertrand, E.; Cheradame, H.; Guegan, P.; Mevel, M.; et al. Gene transfer by chemical vectors, and endocytosis routes of polyplexes, lipoplexes and lipopolyplexes in a myoblast cell line. Biomaterials 2012, 33, 2980–2990. [Google Scholar] [CrossRef]
- Li, Y.; Kroger, M.; Liu, W.K. Shape effect in cellular uptake of PEGylated nanoparticles: Comparison between sphere, rod, cube and disk. Nanoscale 2015, 7, 16631–16646. [Google Scholar] [CrossRef]
- Parakhonskiy, B.; Zyuzin, M.V.; Yashchenok, A.; Carregal-Romero, S.; Rejman, J.; Mohwald, H.; Parak, W.J.; Skirtach, A.G. The influence of the size and aspect ratio of anisotropic, porous CaCO3 particles on their uptake by cells. J. Nanobiotech. 2015, 13, 53. [Google Scholar] [CrossRef] [Green Version]
- Banquy, X.; Suarez, F.; Argaw, A.; Rabanel, J.; Grutter, P.; Bouchard, J.; Hildgen, P.; Giasson, S. Effect of mechanical properties of hydrogel nanoparticles on macrophage cell uptake. Soft Matter 2009, 5, 3984–3991. [Google Scholar] [CrossRef]
- Casals, E.; Pfaller, T.; Duschl, A.; Oostingh, G.J.; Puntes, V. Time evolution of the nanoparticle protein corona. ACS Nano 2010, 4, 3623–3632. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.J.; Hinner, M.J. Getting across the cell membrane: An overview for small molecules, peptides, and proteins. Methods Mol. Biol. 2015, 1266, 29–53. [Google Scholar] [PubMed] [Green Version]
- Kannan, R.M.; Nance, E.; Kannan, S.; Tomalia, D.A. Emerging concepts in dendrimer-based nanomedicine: From design principles to clinical applications. J. Intern. Med. 2014, 276, 579–617. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Miao, X.; Gao, Y.; Jia, H.; Liu, K.; Liu, Y. The protective effects of Trolox-loaded chitosan nanoparticles against hypoxia-mediated cell apoptosis. Nanomedicine 2014, 10, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.Q.; Wang, T.Y.; Webster, T.J.; Duan, H.J.; Qiu, J.Y.; Zhao, Z.M.; Yin, X.X.; Zheng, C.L. Intracellular disposition of chitosan nanoparticles in macrophages: Intracellular uptake, exocytosis, and intercellular transport. Int. J. Nanomed. 2017, 12, 6383–6398. [Google Scholar] [CrossRef] [Green Version]
- Lichtenberg, S.S.; Tsyusko, O.V.; Palli, S.R.; Unrine, J.M. Uptake and Bioactivity of Chitosan/Double-Stranded RNA Polyplex Nanoparticles in Caenorhabditis elegans. Environ. Sci. Technol. 2019, 53, 3832–3840. [Google Scholar] [CrossRef]
- Yang, C.; Gao, S.; Dagnaes-Hansen, F.; Jakobsen, M.; Kjems, J. Impact of PEG Chain Length on the Physical Properties and Bioactivity of PEGylated Chitosan/siRNA Nanoparticles in Vitro and in Vivo. ACS Appl. Mater. Interfaces 2017, 9, 12203–12216. [Google Scholar] [CrossRef]
- Muddineti, O.S.; Shah, A.; Rompicharla, S.V.K.; Ghosh, B.; Biswas, S. Cholesterol-grafted chitosan micelles as a nanocarrier system for drug-siRNA co-delivery to the lung cancer cells. Int. J. Biol. Macromol. 2018, 118, 857–863. [Google Scholar] [CrossRef]
- Huang, Y.; Deng, S.; Luo, X.; Liu, Y.; Xu, W.; Pan, J.; Wang, M.; Xia, Z. Evaluation of Intestinal Absorption Mechanism and Pharmacokinetics of Curcumin-Loaded Galactosylated Albumin Nanoparticles. Int. J. Nanomed. 2019, 14, 9721–9730. [Google Scholar] [CrossRef] [Green Version]
- Kushwah, V.; Agrawal, A.K.; Dora, C.P.; Mallinson, D.; Lamprou, D.A.; Gupta, R.C.; Jain, S. Novel Gemcitabine Conjugated Albumin Nanoparticles: A Potential Strategy to Enhance Drug Efficacy in Pancreatic Cancer Treatment. Pharm. Res. 2017, 34, 2295–2311. [Google Scholar] [CrossRef]
- Wan, X.; Zheng, X.; Pang, X.; Zhang, Z.; Zhang, Q. Incorporation of lapatinib into human serum albumin nanoparticles with enhanced anti-tumor effects in HER2-positive breast cancer. Colloids Surf. B Biointerfaces 2015, 136, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Barnett, M.E.; Takemoto, D.; Davidson, H.; Kompella, U.B. Human serum albumin nanoparticles for efficient delivery of Cu, Zn superoxide dismutase gene. Mol. Vis. 2007, 13, 746–757. [Google Scholar] [PubMed]
- Li, Q.; Liu, C.G.; Yu, Y. Separation of monodisperse alginate nanoparticles and effect of particle size on transport of vitamin E. Carbohydr. Polym. 2015, 124, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, L.; Li, X.; Hu, X.; Han, Y.; Luo, Y.; Wang, Z.; Li, Q.; Aldalbahi, A.; Wang, L.; et al. Size-Dependent Regulation of Intracellular Trafficking of Polystyrene Nanoparticle-Based Drug-Delivery Systems. ACS Appl. Mater. Interfaces 2017, 9, 18619–18625. [Google Scholar] [CrossRef]
- Forte, M.; Iachetta, G.; Tussellino, M.; Carotenuto, R.; Prisco, M.; De, F.M.; Laforgia, V.; Valiante, S. Polystyrene nanoparticles internalization in human gastric adenocarcinoma cells. Toxicol. In Vitro 2016, 31, 126–136. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, D.A.; Vanhecke, D.; Michen, B.; Blank, F.; Gehr, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Different endocytotic uptake mechanisms for nanoparticles in epithelial cells and macrophages. Beilstein J. Nanotechnol. 2014, 5, 1625–1636. [Google Scholar] [CrossRef] [Green Version]
- Fiorentino, I.; Gualtieri, R.; Barbato, V.; Mollo, V.; Braun, S.; Angrisani, A.; Turano, M.; Furia, M.; Netti, P.A.; Guarnieri, D.; et al. Energy independent uptake and release of polystyrene nanoparticles in primary mammalian cell cultures. Exp. Cell Res. 2015, 330, 240–247. [Google Scholar] [CrossRef] [Green Version]
- Platel, A.; Carpentier, R.; Becart, E.; Mordacq, G.; Betbeder, D.; Nesslany, F. Influence of the surface charge of PLGA nanoparticles on their in vitro genotoxicity, cytotoxicity, ROS production and endocytosis. J. Appl. Toxicol. 2016, 36, 434–444. [Google Scholar] [CrossRef]
- Dou, T.; Wang, J.; Han, C.; Shao, X.; Zhang, J.; Lu, W. Cellular uptake and transport characteristics of chitosan modified nanoparticles in Caco-2 cell monolayers. Int. J. Biol. Macromol. 2019, 138, 791–799. [Google Scholar] [CrossRef]
- Song, Y.; Shi, Y.; Zhang, L.; Hu, H.; Zhang, C.; Yin, M.; Chu, L.; Yan, X.; Zhao, M.; Zhang, X.; et al. Synthesis of CSK-DEX-PLGA Nanoparticles for the Oral Delivery of Exenatide to Improve Its Mucus Penetration and Intestinal Absorption. Mol. Pharm. 2019, 16, 518–532. [Google Scholar] [CrossRef]
- Li, S.; Zeng, Y.C.; Peng, K.; Liu, C.; Zhang, Z.R.; Zhang, L. Design and evaluation of glomerulus mesangium-targeted PEG-PLGA nanoparticles loaded with dexamethasone acetate. Acta Pharmacol. Sin. 2019, 40, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Thakur, N.S.; Patel, G.; Kushwah, V.; Jain, S.; Banerjee, U.C. Facile development of biodegradable polymer-based nanotheranostics: Hydrophobic photosensitizers delivery, fluorescence imaging and photodynamic therapy. J. Photochem. Photobiol. B 2019, 193, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Seib, F.P.; Jones, A.T.; Duncan, R. Comparison of the endocytic properties of linear and branched PEIs, and cationic PAMAM dendrimers in B16f10 melanoma cells. J. Control Release 2007, 117, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.W.; Fitzgerald, M.; Clemons, T.D.; House, M.J.; Padman, B.S.; Shaw, J.A.; Saunders, M.; Harvey, A.R.; Zdyrko, B.; Luzinov, I.; et al. Multimodal analysis of PEI-mediated endocytosis of nanoparticles in neural cells. ACS Nano. 2011, 5, 8640–8648. [Google Scholar] [CrossRef]
- Rejman, J.; Bragonzi, A.; Conese, M. Role of clathrin- and caveolae-mediated endocytosis in gene transfer mediated by lipo- and polyplexes. Mol. Ther. 2005, 12, 468–474. [Google Scholar] [CrossRef]
- Hwang, M.E.; Keswani, R.K.; Pack, D.W. Dependence of PEI and PAMAM Gene Delivery on Clathrin- and Caveolin-Dependent Trafficking Pathways. Pharm. Res 2015, 32, 2051–2059. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Liu, T.; Yu, X.; Gu, Y. A preliminary study on the interaction between Asn-Gly-Arg (NGR)-modified multifunctional nanoparticles and vascular epithelial cells. Acta Pharm. Sin. B 2017, 7, 361–372. [Google Scholar] [CrossRef]
- Lu, J.; Zhao, Y.; Zhou, X.; He, J.H.; Yang, Y.; Jiang, C.; Qi, Z.; Zhang, W.; Liu, J. Biofunctional Polymer-Lipid Hybrid High-Density Lipoprotein-Mimicking Nanoparticles Loading Anti-miR155 for Combined Antiatherogenic Effects on Macrophages. Biomacromolecules 2017, 18, 2286–2295. [Google Scholar] [CrossRef]
- Bengali, Z.; Rea, J.C.; Shea, L.D. Gene expression and internalization following vector adsorption to immobilized proteins: Dependence on protein identity and density. J. Gene Med. 2007, 9, 668–678. [Google Scholar] [CrossRef]
- Chae, S.Y.; Kim, H.J.; Lee, M.S.; Jang, Y.L.; Lee, Y.; Lee, S.H.; Lee, K.; Kim, S.H.; Kim, H.T.; Chi, S.C.; et al. Energy-independent intracellular gene delivery mediated by polymeric biomimetics of cell-penetrating peptides. Macromol. Biosci. 2011, 11, 1169–1174. [Google Scholar] [CrossRef]
- Vidal, F.; Vasquez, P.; Diaz, C.; Nova, D.; Alderete, J.; Guzman, L. Mechanism of PAMAM Dendrimers Internalization in Hippocampal Neurons. Mol. Pharm. 2016, 13, 3395–3403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, D.; Zhang, M.; Sun, Y.; Zhang, X.; Guan, G.; Zhao, X.; Qiao, M.; Chen, D.; Hu, H. The cellular uptake mechanism, intracellular transportation, and exocytosis of polyamidoamine dendrimers in multidrug-resistant breast cancer cells. Int. J. Nanomed. 2016, 11, 3677–3690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perumal, O.P.; Inapagolla, R.; Kannan, S.; Kannan, R.M. The effect of surface functionality on cellular trafficking of dendrimers. Biomaterials 2008, 29, 3469–3476. [Google Scholar] [CrossRef] [PubMed]
- Kitchens, K.M.; Foraker, A.B.; Kolhatkar, R.B.; Swaan, P.W.; Ghandehari, H. Endocytosis and interaction of poly (amidoamine) dendrimers with Caco-2 cells. Pharm. Res. 2007, 24, 2138–2145. [Google Scholar] [CrossRef]
- Gao, Y.G.; Lin, X.; Dang, K.; Jiang, S.F.; Tian, Y.; Liu, F.L.; Li, D.J.; Li, Y.; Miao, Z.P.; Qian, A.R. Structure-activity relationship of novel low-generation dendrimers for gene delivery. Org. Biomol. Chem. 2018, 16, 7833–7842. [Google Scholar] [CrossRef]
- Bastien, E.; Schneider, R.; Hackbarth, S.; Dumas, D.; Jasniewski, J.; Roder, B.; Bezdetnaya, L.; Lassalle, H.P. PAMAM G4.5-chlorin e6 dendrimeric nanoparticles for enhanced photodynamic effects. Photochem. Photobiol. Sci. 2015, 14, 2203–2212. [Google Scholar] [CrossRef]
- Hu, Q.; Chen, Q.; Yan, X.; Ding, B.; Chen, D.; Cheng, L. Chondrocyte affinity peptide modified PAMAM conjugate as a nanoplatform for targeting and retention in cartilage. Nanomedicine 2018, 13, 749–767. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [Green Version]
- Jose, A.; Labala, S.; Venuganti, V.V. Co-delivery of curcumin and STAT3 siRNA using deformable cationic liposomes to treat skin cancer. J. Drug Target. 2017, 25, 330–341. [Google Scholar] [CrossRef]
- Cui, S.; Wang, B.; Zhao, Y.; Chen, H.; Ding, H.; Zhi, D.; Zhang, S. Transmembrane routes of cationic liposome-mediated gene delivery using human throat epidermis cancer cells. Biotechnol. Lett. 2014, 36, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Alshehri, A.; Grabowska, A.; Stolnik, S. Pathways of cellular internalisation of liposomes delivered siRNA and effects on siRNA engagement with target mRNA and silencing in cancer cells. Sci. Rep. 2018, 8, 3748. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, L.; Tan, X.; Li, F.; Zhao, M.; Peng, S. Lipid rafts-mediated endocytosis and physiology-based cell membrane traffic models of doxorubicin liposomes. Biochim. Biophys. Acta 2016, 1858, 1801–1811. [Google Scholar] [CrossRef] [PubMed]
- Santiwarangkool, S.; Akita, H.; Khalil, I.A.; Abd Elwakil, M.M.; Sato, Y.; Kusumoto, K.; Harashima, H. A study of the endocytosis mechanism and transendothelial activity of lung-targeted GALA-modified liposomes. J. Control Release 2019, 307, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Watanabe, M.; Iwasaki, T.; Shudou, M.; Uda, R.M. Endosomal escape by photo-activated fusion of liposomes containing a malachite green derivative: A novel class of photoresponsive liposomes for drug delivery vehicles. Photochem. Photobiol. Sci. 2019, 18, 1471–1478. [Google Scholar] [CrossRef]
- Lu, M.; Zhao, X.; Xing, H.; Xun, Z.; Zhu, S.; Lang, L.; Yang, T.; Cai, C.; Wang, D.; Ding, P. Comparison of exosome-mimicking liposomes with conventional liposomes for intracellular delivery of siRNA. Int. J. Pharm. 2018, 550, 100–113. [Google Scholar] [CrossRef]
- Kang, J.H.; Jang, W.Y.; Ko, Y.T. The Effect of Surface Charges on the Cellular Uptake of Liposomes Investigated by Live Cell Imaging. Pharm. Res. 2017, 34, 704–717. [Google Scholar] [CrossRef]
- Radaic, A.; de Jesus, M.B. Solid lipid nanoparticles release DNA upon endosomal acidification in human embryonic kidney cells. Nanotechnology 2018, 29, 315102. [Google Scholar] [CrossRef]
- Chai, G.H.; Xu, Y.; Chen, S.Q.; Cheng, B.; Hu, F.Q.; You, J.; Du, Y.Z.; Yuan, H. Transport Mechanisms of Solid Lipid Nanoparticles across Caco-2 Cell Monolayers and their Related Cytotoxicology. ACS Appl. Mater. Interfaces 2016, 8, 5929–5940. [Google Scholar] [CrossRef]
- Martins, S.; Costa-Lima, S.; Carneiro, T.; Cordeiro-da-Silva, A.; Souto, E.B.; Ferreira, D.C. Solid lipid nanoparticles as intracellular drug transporters: An investigation of the uptake mechanism and pathway. Int. J. Pharm. 2012, 430, 216–227. [Google Scholar] [CrossRef]
- Neves, A.R.; Queiroz, J.F.; Lima, S.A.C.; Reis, S. Apo E-Functionalization of Solid Lipid Nanoparticles Enhances Brain Drug Delivery: Uptake Mechanism and Transport Pathways. Bioconj. Chem. 2017, 28, 995–1004. [Google Scholar] [CrossRef]
- Cha, C.; Shin, S.R.; Annabi, N.; Dokmeci, M.R.; Khademhosseini, A. Carbon-based nanomaterials: Multifunctional materials for biomedical engineering. ACS Nano 2013, 7, 2891–2897. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Wan, B.; Yang, Y.; Ren, X.; Guo, L.H. Length effects on the dynamic process of cellular uptake and exocytosis of single-walled carbon nanotubes in murine macrophage cells. Sci. Rep. 2017, 7, 1518. [Google Scholar] [CrossRef] [PubMed]
- Yaron, P.N.; Holt, B.D.; Short, P.A.; Losche, M.; Islam, M.F.; Dahl, K.N. Single wall carbon nanotubes enter cells by endocytosis and not membrane penetration. J. Nanobiotech. 2011, 9, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, B.; Chang, S.; Dai, Y.; Yu, D.; Chen, D. Cell response to carbon nanotubes: Size-dependent intracellular uptake mechanism and subcellular fate. Small 2010, 6, 2362–2366. [Google Scholar] [CrossRef] [PubMed]
- Haniu, H.; Saito, N.; Matsuda, Y.; Tsukahara, T.; Maruyama, K.; Usui, Y.; Aoki, K.; Takanashi, S.; Kobayashi, S.; Nomura, H.; et al. Culture medium type affects endocytosis of multi-walled carbon nanotubes in BEAS-2B cells and subsequent biological response. Toxicol. In Vitro 2013, 27, 1679–1685. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Yu, S.; Wang, C.; Kong, J. Tracking the endocytic pathway of recombinant protein toxin delivered by multiwalled carbon nanotubes. ACS Nano 2010, 4, 6483–6490. [Google Scholar] [CrossRef]
- Castro, E.; Hernandez, G.A.; Zavala, G.; Echegoyen, L. Fullerenes in Biology and Medicine. J. Mater. Chem. B 2017, 5, 6523–6535. [Google Scholar] [CrossRef]
- Russ, K.A.; Elvati, P.; Parsonage, T.L.; Dews, A.; Jarvis, J.A.; Ray, M.; Schneider, B.; Smith, P.J.; Williamson, P.T.; Violi, A.; et al. C60 fullerene localization and membrane interactions in RAW 264.7 immortalized mouse macrophages. Nanoscale 2016, 8, 4134–4144. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Chen, C.; Ye, C.; Wei, T.; Zhao, Y.; Lao, F.; Chen, Z.; Meng, H.; Gao, Y.; Yuan, H.; et al. The translocation of fullerenic nanoparticles into lysosome via the pathway of clathrin-mediated endocytosis. Nanotechnology 2008, 19, 145102. [Google Scholar] [CrossRef]
- Arayachukiat, S.; Seemork, J.; Pan-In, P.; Amornwachirabodee, K.; Sangphech, N.; Sansureerungsikul, T.; Sathornsantikun, K.; Vilaivan, C.; Shigyou, K.; Pienpinijtham, P.; et al. Bringing macromolecules into cells and evading endosomes by oxidized carbon nanoparticles. Nano Lett. 2015, 15, 3370–3376. [Google Scholar] [CrossRef]
- Saulite, L.; Dapkute, D.; Pleiko, K.; Popena, I.; Steponkiene, S.; Rotomskis, R.; Riekstina, U. Nano-engineered skin mesenchymal stem cells: Potential vehicles for tumour-targeted quantum-dot delivery. Beilstein J. Nanotechnol. 2017, 8, 1218–1230. [Google Scholar] [CrossRef] [Green Version]
- Karabanovas, V.; Zitkus, Z.; Kuciauskas, D.; Rotomskis, R.; Valius, M. Surface properties of quantum dots define their cellular endocytic routes, mitogenic stimulation and suppression of cell migration. J. Biomed. Nanotechnol. 2014, 10, 775–786. [Google Scholar] [CrossRef]
- Dalal, C.; Jana, N.R. Riboflavin-terminated, multivalent quantum dot as fluorescent cell imaging probe. Langmuir 2019, 35, 11380–11388. [Google Scholar] [CrossRef]
- Vrathasha, V.; Booksh, K.; Duncan, R.L.; Nohe, A. Mechanisms of Cellular Internalization of Quantum Dot(R) Conjugated Bone Formation Mimetic Peptide CK2.3. Nanomaterials 2018, 8, 513. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Huang, J.; Feng, Q.; Zhang, T.; Chen, X.; Li, X.; Liu, X.; Li, H.; Zhong, Z.; Xiao, K. Multi-Modal Visualization of Uptake and Distribution of Iron Oxide Nanoparticles in Macrophages, Cancer Cells, and Xenograft Models. J. Biomed. Nanotechnol. 2019, 15, 1801–1811. [Google Scholar] [CrossRef]
- Petters, C.; Dringen, R. Accumulation of iron oxide nanoparticles by cultured primary neurons. Neurochem. Int. 2015, 81, 1–9. [Google Scholar] [CrossRef]
- Calero, M.; Chiappi, M.; Lazaro-Carrillo, A.; Rodriguez, M.J.; Chichon, F.J.; Crosbie-Staunton, K.; Prina-Mello, A.; Volkov, Y.; Villanueva, A.; Carrascosa, J.L. Characterization of interaction of magnetic nanoparticles with breast cancer cells. J. Nanobiotechnol. 2015, 13, 16. [Google Scholar] [CrossRef] [Green Version]
- Chaves, N.L.; Estrela-Lopis, I.; Bottner, J.; Lopes, C.A.; Guido, B.C.; de Sousa, A.R.; Bao, S.N. Exploring cellular uptake of iron oxide nanoparticles associated with rhodium citrate in breast cancer cells. Int. J. Nanomed. 2017, 12, 5511–5523. [Google Scholar] [CrossRef] [Green Version]
- Bohmer, N.; Jordan, A. Caveolin-1 and CDC42 mediated endocytosis of silica-coated iron oxide nanoparticles in HeLa cells. Beilstein J. Nanotechnol. 2015, 6, 167–176. [Google Scholar] [CrossRef]
- Chithrani, B.D.; Chan, W.C. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 2007, 7, 1542–1550. [Google Scholar] [CrossRef]
- Ding, L.; Yao, C.; Yin, X.; Li, C.; Huang, Y.; Wu, M.; Wang, B.; Guo, X.; Wang, Y.; Wu, M. Size, Shape, and Protein Corona Determine Cellular Uptake and Removal Mechanisms of Gold Nanoparticles. Small 2018, 14, e1801451. [Google Scholar] [CrossRef]
- Wang, H.; Chen, B.; He, M.; Li, X.; Chen, P.; Hu, B. Study on uptake of gold nanoparticles by single cells using droplet microfluidic chip-inductively coupled plasma mass spectrometry. Talanta 2019, 200, 398–407. [Google Scholar] [CrossRef]
- Zhu, J.; Liao, L.; Zhu, L.; Zhang, P.; Guo, K.; Kong, J.; Ji, C.; Liu, B. Size-dependent cellular uptake efficiency, mechanism, and cytotoxicity of silica nanoparticles toward HeLa cells. Talanta 2013, 107, 408–415. [Google Scholar] [CrossRef]
- Li, L.; Xi, W.S.; Su, Q.; Li, Y.; Yan, G.H.; Liu, Y.; Wang, H.; Cao, A. Unexpected Size Effect: The Interplay between Different-Sized Nanoparticles in Their Cellular Uptake. Small 2019, 15, e1901687. [Google Scholar] [CrossRef]
- Zheng, N.; Li, J.; Xu, C.; Xu, L.; Li, S.; Xu, L. Mesoporous silica nanorods for improved oral drug absorption. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1132–1140. [Google Scholar] [CrossRef] [Green Version]
- Kasper, J.; Hermanns, M.I.; Bantz, C.; Koshkina, O.; Lang, T.; Maskos, M.; Pohl, C.; Unger, R.E.; Kirkpatrick, C.J. Interactions of silica nanoparticles with lung epithelial cells and the association to flotillins. Arch. Toxicol. 2013, 87, 1053–1065. [Google Scholar] [CrossRef] [Green Version]
- Poussard, S.; Decossas, M.; Le, B.O.; Mornet, S.; Naudin, G.; Lambert, O. Internalization and fate of silica nanoparticles in C2C12 skeletal muscle cells: Evidence of a beneficial effect on myoblast fusion. Int. J. Nanomed. 2015, 10, 1479–1492. [Google Scholar]
- Manzanares, D.; Araya-Duran, I.; Gallego-Yerga, L.; Jativa, P.; Marquez-Miranda, V.; Canan, J.; Jimenez Blanco, J.L.; Mellet, C.O.; Gonzalez-Nilo, F.D.; Garcia Fernandez, J.M.; et al. Molecular determinants for cyclo-oligosaccharide-based nanoparticle-mediated effective siRNA transfection. Nanomedicine 2017, 12, 1607–1621. [Google Scholar] [CrossRef]
- Mourtzis, N.; Paravatou, M.; Mavridis, I.M.; Roberts, M.L.; Yannakopoulou, K. Synthesis, characterization, and remarkable biological properties of cyclodextrins bearing guanidinoalkylamino and aminoalkylamino groups on their primary side. Chemistry 2008, 14, 4188–4200. [Google Scholar] [CrossRef]
- Zhang, Q.; Cai, Y.; Wang, X.J.; Xu, J.L.; Ye, Z.; Wang, S.; Seeberger, P.H.; Yin, J. Targeted Photodynamic Killing of Breast Cancer Cells Employing Heptamannosylated beta-Cyclodextrin-Mediated Nanoparticle Formation of an Adamantane-Functionalized BODIPY Photosensitizer. ACS Appl. Mater Interfaces 2016, 8, 33405–33411. [Google Scholar] [CrossRef]
- Xiong, Q.; Cui, M.; Bai, Y.; Liu, Y.; Liu, D.; Song, T. A supramolecular nanoparticle system based on beta-cyclodextrin-conjugated poly-l-lysine and hyaluronic acid for co-delivery of gene and chemotherapy agent targeting hepatocellular carcinoma. Colloids Surf. B Biointerfaces 2017, 155, 93–103. [Google Scholar] [CrossRef]
- Shi, Y.; Su, C.; Cui, W.; Li, H.; Liu, L.; Feng, B.; Liu, M.; Su, R.; Zhao, L. Gefitinib loaded folate decorated bovine serum albumin conjugated carboxymethyl-beta-cyclodextrin nanoparticles enhance drug delivery and attenuate autophagy in folate receptor-positive cancer cells. J. Nanobiotechnol. 2014, 12, 43. [Google Scholar] [CrossRef] [Green Version]
- Vert, M.; Yoshiharu, D.; Hellwich, K.-H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schue, F. Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Sci. J. IUPAC 2012, 84, 377–410. [Google Scholar] [CrossRef]
- Cardoso, A.M.; Morais, C.M.; Silva, S.G.; Marques, E.F.; de Lima, M.C.; Jurado, M.A. Bis-quaternary gemini surfactants as components of nonviral gene delivery systems: A comprehensive study from physicochemical properties to membrane interactions. Int. J. Pharm. 2014, 474, 57–69. [Google Scholar] [CrossRef]
- Cardoso, A.M.; Morais, C.M.; Cruz, A.R.; Cardoso, A.L.; Silva, S.G.; do Vale, M.L.; Marques, E.F.; Pedroso de Lima, M.C.; Jurado, A.S. Gemini surfactants mediate efficient mitochondrial gene delivery and expression. Mol. Pharm. 2015, 12, 716–730. [Google Scholar] [CrossRef]
- Arranja, A.; Denkova, A.G.; Morawska, K.; Waton, G.; van, V.S.; Dubruel, P.; Schosseler, F.; Mendes, E. Interactions of Pluronic nanocarriers with 2D and 3D cell cultures: Effects of PEO block length and aggregation state. J. Control Release 2016, 224, 126–135. [Google Scholar] [CrossRef]
- Yu, C.; He, B.; Xiong, M.H.; Zhang, H.; Yuan, L.; Ma, L.; Dai, W.B.; Wang, J.; Wang, X.L.; Wang, X.Q.; et al. The effect of hydrophilic and hydrophobic structure of amphiphilic polymeric micelles on their transport in epithelial MDCK cells. Biomaterials 2013, 34, 6284–6298. [Google Scholar] [CrossRef]
- Hu, X.; Yang, F.F.; Liu, C.Y.; Ehrhardt, C.; Liao, Y.H. In vitro uptake and transport studies of PEG-PLGA polymeric micelles in respiratory epithelial cells. Eur. J. Pharm. Biopharm. 2017, 114, 29–37. [Google Scholar] [CrossRef]
- Zhao, S.; Dai, W.; He, B.; Wang, J.; He, Z.; Zhang, X.; Zhang, Q. Monitoring the transport of polymeric micelles across MDCK cell monolayer and exploring related mechanisms. J. Control Release 2012, 158, 413–423. [Google Scholar] [CrossRef]
- Gu, Y.; Li, J.; Li, Y.; Song, L.; Li, D.; Peng, L.; Wan, Y.; Hua, S. Nanomicelles loaded with doxorubicin and curcumin for alleviating multidrug resistance in lung cancer. Int. J. Nanomed. 2016, 11, 5757–5770. [Google Scholar] [CrossRef] [Green Version]
- Starigazdova, J.; Nesporova, K.; Cepa, M.; Sinova, R.; Smejkalova, D.; Huerta-Angeles, G.; Velebny, V. In vitro investigation of hyaluronan-based polymeric micelles for drug delivery into the skin: The internalization pathway. Eur. J. Pharm. Sci. 2020, 143, 105168. [Google Scholar] [CrossRef]
- Zhang, M.; Asghar, S.; Jin, X.; Hu, Z.; Ping, Q.; Chen, Z.; Shao, F.; Xiao, Y. The enhancing effect of N-acetylcysteine modified hyaluronic acid-octadecylamine micelles on the oral absorption of paclitaxel. Int. J. Biol. Macromol. 2019, 138, 636–647. [Google Scholar] [CrossRef]


| NP Type | Main Endocytic Pathway | Size/Length | Charge | Shape | Reference(s) | |
|---|---|---|---|---|---|---|
| Natural polymers | CS | CME | 15–250 nm | Positive | Ellipsoidal and spherical | [25,26] |
| Albumin | CME | 140 nm | Positive | Spherical | [31] | |
| CME | 150 nm | Negative | Spherical | [30] | ||
| CVME | 120 nm | Negative | Spherical | [32] | ||
| Alginate | CME | 50–120 nm | Negative | Spherical | [33] | |
| CVME | 420 nm | Negative | Spherical | [33] | ||
| Macropinocytosis | 730 nm | Negative | Spherical | [33] | ||
| Synthetic polymers | Polystyrene | CME and passive diffusion | 40–150 nm | Negative | Not specified | [34,35,36,37] |
| PLGA | CME | 80 nm | Positive | Not specified | [38] | |
| Weak entrance CME and CVME independent | 80 nm | Negative | Not specified | [38] | ||
| PEI | CME and CVME | 100–130 nm (25 kDa) | Positive | Branched | [43,44,45,46] | |
| CME | 25 kDa | Positive | Linear | [43] | ||
| Dendrimers | PAMAM -NH2 | CME | G4 (5–150 nm) | Positive | Branched | [35,40,41,42] |
| CME and CVME | G2 | Positive | Branched | [43,44] | ||
| PAMAM -OH | CVME | G4 | Negative | Branched | [53] | |
| PAMAM -COOH | CVME | G3.5 | Negative | Branched | [53] | |
| CME | G1.5 | Negative | Branched | [54] | ||
| PAMAM | CME and CVME independent | G4 | Neutral | Branched | [53] | |
| Lipids | CME and macropinocytosis | 100–150 nm | Positive | Spherical | [59,60,66] | |
| Liposomes | CME and macropinocytosis | 100 nm | Negative | Spherical | [66] | |
| CME and CVME | 100 nm | Neutral | Spherical | [66] | ||
| SLNs | CME | 110–160 nm | Positive | Not specified | [67] | |
| CME, CVME and macropinocytosis | 85–90 nm | Negative | Not specified | [68] | ||
| Carbon based | SWCNTs | Macropinocytosis and non-specific interactions | 195–630 nm | Negative | Cylindrical | [72,73] |
| Passive diffusion | 50 nm | Negative | Cylindrical | [74] | ||
| MWCNTs | CME and CVME | 10 µm | Negative | Cylindrical | [75] | |
| Fullerenes | Passive diffusion | 1 nm (55 nm aggregates) | Negative | Icosaedral | [78] | |
| Carbon oxide NPs | Unspecific interactions | 38 nm (225 nm aggregates) | Negative | Irregular | [80] | |
| QDs | CVME and CME | 10–50 nm | Negative | Ellipsoidal | [81,82] | |
| Metallic | IONPS | CVME | 15–50 nm | Negative | Not specified | [85] |
| CME (Macropinocytosis in absence of FBS) | 15–45 nm | Negative | Spherical | [91,92] | ||
| AuNPs | Macropinocytosis | 80 nm | Negative | Spherical | [91] | |
| CME and CVME (Macropinocytosis in absence of FBS) | 15 nm | Negative | Star | [91] | ||
| CME (CME and CVME independent way in absence of FBS) | 33 × 10 nm | Negative | Rod | [91] | ||
| MSNPs | CME and CVME independent | 300 nm | Negative | Not specified | [93] | |
| RME, macropinocytosis and simple diffusion | 50–300 nm | Negative | Not specified | [93,94,97] | ||
| CVME | 200 nm | Negative | Rod | [95] | ||
| CME | 90–190 nm | Negative | Spherical | [95] | ||
| CDNPs | CME | 40–140 nm | Positive | Not specified | [99] | |
| Micelles | Gemini surfactants (14-2-14, 16-2-16, 12-2-12, 12-5-12, 12-10-12) | Direct translocation | 3 µm (1–6 µm) | Positive | Spherical | [104] |
| Gemini surfactants with HL (14-2-14, 16-2-16, 12-2-12, 12-5-12, 12-10-12) | Macropinocytosis | 3 µm (1–6 µm) | Negative | Spherical | [104] | |
| Gemini surfactant (14Ser)2N5/ DNA/HL | Energy independent processes | 200 nm | Negative | Spherical | [105] | |
| Gemini surfactant (16Ser)2N5/DNA | CME | 550 nm | Positive | Spherical | [105] | |
| Gemini surfactant 14-2-14/DNA (with or without HL) | Macropinocytosis and CVME | 555–800 nm | Positive | Spherical | [105] | |
| Pluronic | CVME | 2–5 nm | Neutral | Unimers | [106] | |
| CME | 15-50 nm | Neutral | Cross-linked micelles (spherical) | [106] | ||
| PEG-PCL PEG-DSPE | CME and CVME | 20–30 nm | Positive | Spherical | [107] | |
| CME and CVME | 20–30 nm | Positive | Spherical | [107] | ||
| mPEG-PLGA | CME and CVME | 30 nm | Not specified | Spherical | [108] | |
| PEG-PLA | CVME | 45 nm | Negative | Spherical | [109] | |
| PEG-D-tocopheryl succinate | CVME | 15 nm | Neutral | Spherical | [110] | |
| HA | CME and macropinocytosis | 130 nm | Neutral | Spherical | [111] | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzanares, D.; Ceña, V. Endocytosis: The Nanoparticle and Submicron Nanocompounds Gateway into the Cell. Pharmaceutics 2020, 12, 371. https://doi.org/10.3390/pharmaceutics12040371
Manzanares D, Ceña V. Endocytosis: The Nanoparticle and Submicron Nanocompounds Gateway into the Cell. Pharmaceutics. 2020; 12(4):371. https://doi.org/10.3390/pharmaceutics12040371
Chicago/Turabian StyleManzanares, Darío, and Valentín Ceña. 2020. "Endocytosis: The Nanoparticle and Submicron Nanocompounds Gateway into the Cell" Pharmaceutics 12, no. 4: 371. https://doi.org/10.3390/pharmaceutics12040371
APA StyleManzanares, D., & Ceña, V. (2020). Endocytosis: The Nanoparticle and Submicron Nanocompounds Gateway into the Cell. Pharmaceutics, 12(4), 371. https://doi.org/10.3390/pharmaceutics12040371