Influence of Carbamazepine Dihydrate on the Preparation of Amorphous Solid Dispersions by Hot Melt Extrusion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Carbamazepine (CBZ) Dihydrate
2.3. Karl Fischer Titration (KFT)
2.4. Thermal Analysis
2.4.1. Differential Scanning Calorimetry (DSC)
2.4.2. Thermal Gravimetric Analysis (TGA)
2.5. X-ray Powder Diffraction (XRPD)
2.6. Polarized Light Microscopy (PLM) with Hot Stage
2.7. Processing by Hot Melt Extrusion (HME)
2.8. High-Performance Liquid Chromatography (HPLC)
2.9. Intrinsic Dissolution Rates (IDR) Measurement
2.10. Rheology Studies
2.11. Wide Angle X-ray Scattering (WAXS) with Temperature Control
2.12. Study Design and Excipients Selection
3. Results
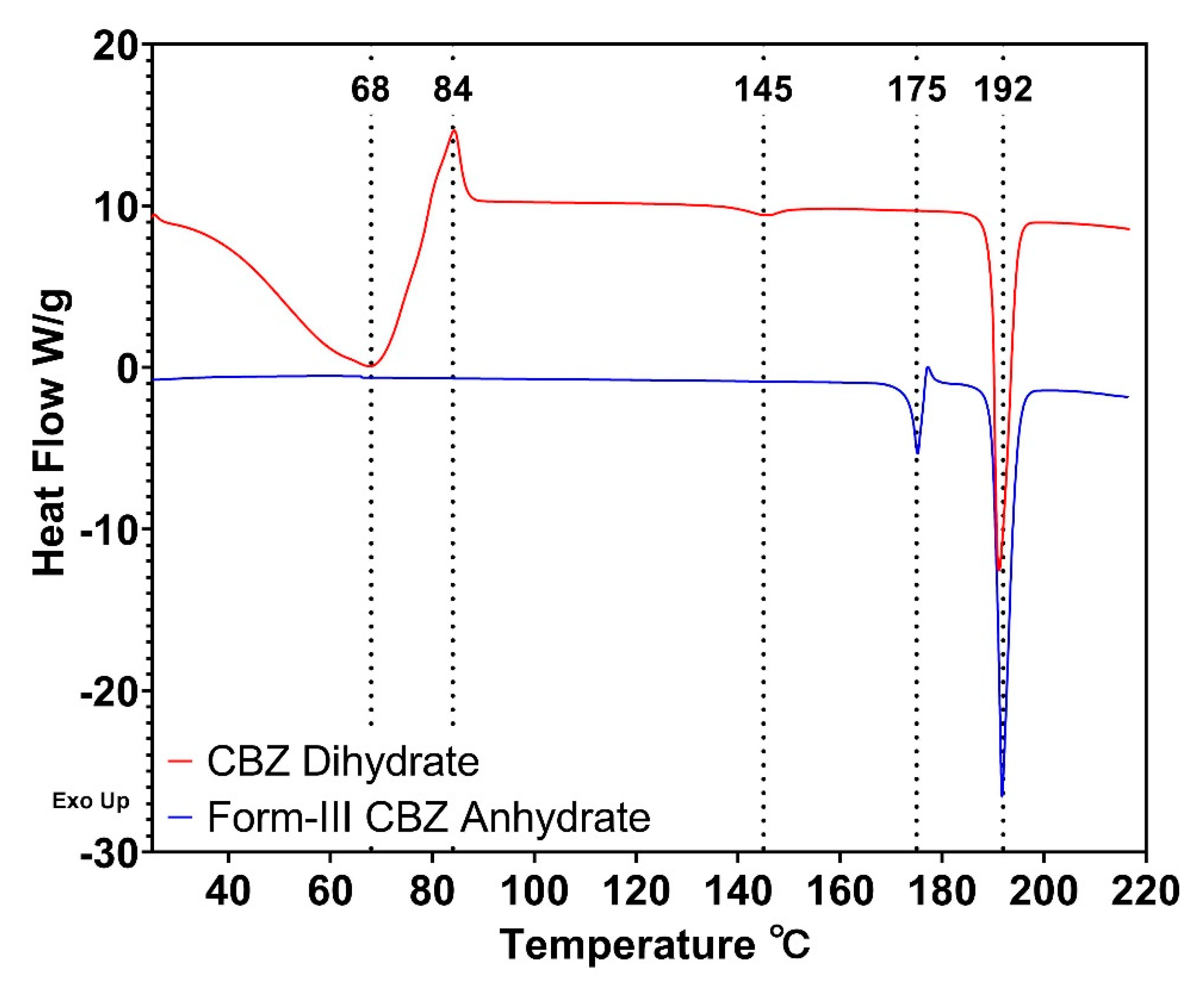
3.1. Formation of CBZ Dihydrate
3.2. Dehydration Kinetics of CBZ Dihydrate
3.3. CBZ ASDs Prepared by HME using CBZ Dihydrate
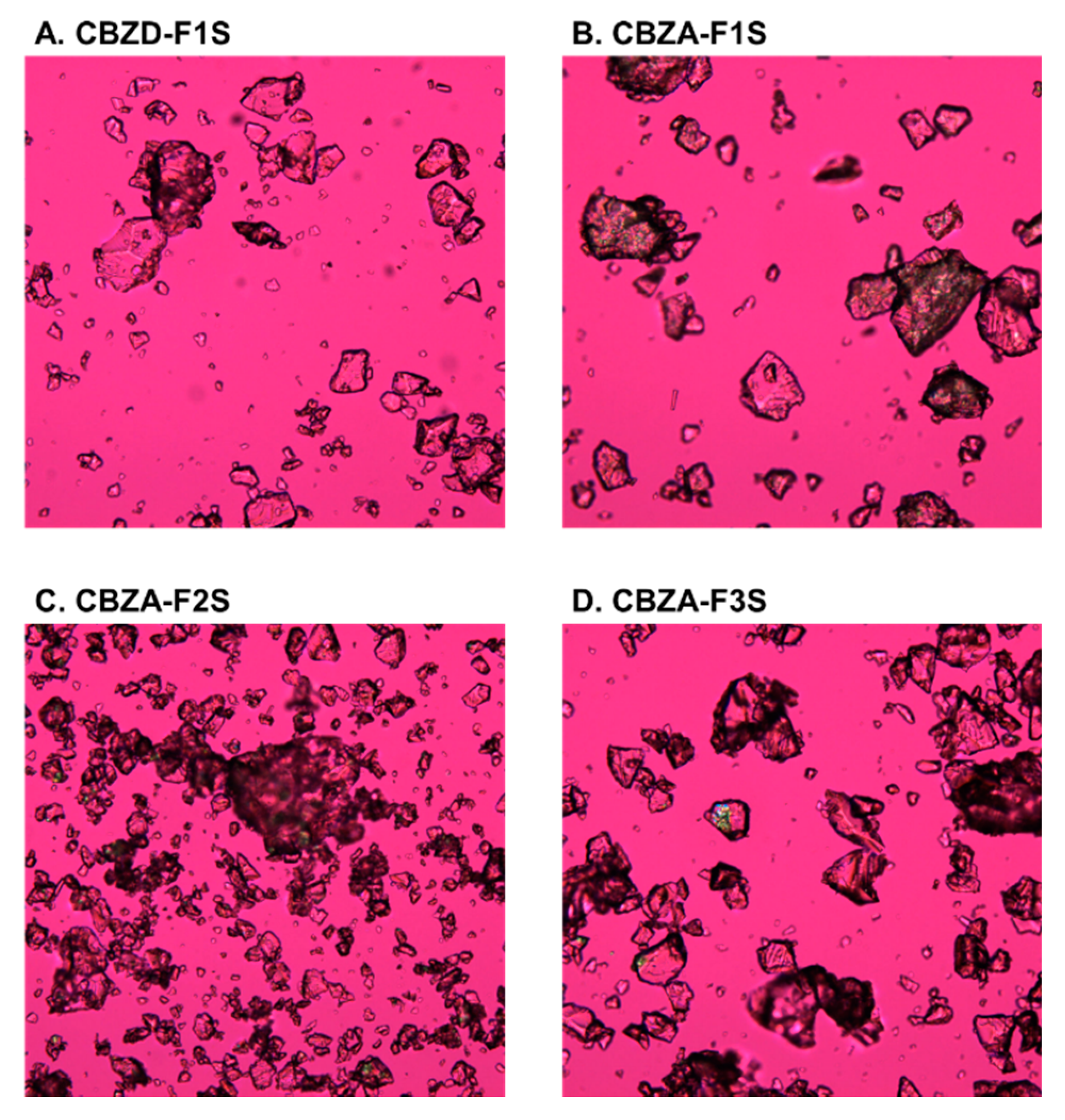
3.3.1. Summary of Extrusion Processes
3.3.2. CBZ ASDs at Different Drug Loading
3.3.3. CBZ ASDs Prepared using Different Screw Designs
3.4. In-situ Dehydration Studies of CBZ Dihydrate-polymer System
4. Discussion
4.1. Extrusion Design
4.2. In situ Dehydration of CBZ Dihydrate in HME
4.2.1. Form-I CBZ anhydrate
4.2.2. Miscibility of the CBZ Dihydrate-polymer System
4.2.3. Disordered State upon the Dehydration of CBZ Dihydrate in Polymer Matrices
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Keserü, G.M.; Makara, G.M. The influence of lead discovery strategies on the properties of drug candidates. Nat. Rev. Drug Discov. 2009, 8, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins: Basic science and product development. J. Pharm. Pharmacol. 2010, 62, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, Y.; Wada, K.; Nakatani, M.; Yamada, S.; Onoue, S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: Basic approaches and practical applications. Int. J. Pharm. 2011, 420, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Boyd, B.J.; Bergström, C.A.; Vinarov, Z.; Kuentz, M.; Brouwers, J.; Augustijns, P.; Brandl, M.; Bernkop-Schnürch, A.; Shrestha, N.; Préat, V. Successful oral delivery of poorly water-soluble drugs both depends on the intraluminal behavior of drugs and of appropriate advanced drug delivery systems. Eur. J. Pharm. Sci. 2019, 137, 104967. [Google Scholar] [CrossRef] [PubMed]
- Jermain, S.V.; Brough, C.; Williams, R.O., III. Amorphous solid dispersions and nanocrystal technologies for poorly water-soluble drug delivery–an update. Int. J. Pharm. 2018, 535, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Ponnammal, P.; Kanaujia, P.; Yani, Y.; Ng, W.K.; Tan, R.B. Orally disintegrating tablets containing melt extruded amorphous solid dispersion of tacrolimus for dissolution enhancement. Pharmaceutics 2018, 10, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dedroog, S.; Huygens, C.; Van den Mooter, G. Chemically identical but physically different: A comparison of spray drying, hot melt extrusion and cryo-milling for the formulation of high drug loaded amorphous solid dispersions of naproxen. Eur. J. Pharm. Biopharm. 2019, 135, 1–12. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, L.; Zhang, F. Reactive melt extrusion to improve the dissolution performance and physical stability of naproxen amorphous solid dispersions. Mol. Pharm. 2017, 14, 658–673. [Google Scholar] [CrossRef]
- DiNunzio, J.C.; Hughey, J.R.; Brough, C.; Miller, D.A.; Williams, R.O., III; McGinity, J.W. Production of advanced solid dispersions for enhanced bioavailability of itraconazole using KinetiSol® Dispersing. Drug Dev. Ind. Pharm. 2010, 36, 1064–1078. [Google Scholar] [CrossRef]
- Zhang, M.; Li, H.; Lang, B.; O’Donnell, K.; Zhang, H.; Wang, Z.; Dong, Y.; Wu, C.; Williams, R.O., III. Formulation and delivery of improved amorphous fenofibrate solid dispersions prepared by thin film freezing. Eur. J. Pharm. Biopharm. 2012, 82, 534–544. [Google Scholar] [CrossRef]
- Hanada, M.; Jermain, S.V.; Lu, X.; Su, Y.; Williams, R.O., III. Predicting physical stability of ternary amorphous solid dispersions using specific mechanical energy in a hot melt extrusion process. Int. J. Pharm. 2018, 548, 571–585. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Su, Y.; Zhang, J.; DiNunzio, J.; Leone, A.; Huang, C.; Brown, C.D. Rheology guided rational selection of processing temperature to prepare copovidone–nifedipine amorphous solid dispersions via hot melt extrusion (HME). Mol. Pharm. 2016, 13, 3494–3505. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Huang, S.; Lowinger, M.B.; Liu, X.; Lu, X.; Su, Y.; Williams, R.O., III. Influence of mechanical and thermal energy on nifedipine amorphous solid dispersions prepared by hot melt extrusion: Preparation and physical stability. Int. J. Pharm. 2019, 561, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Crowley, M.M.; Zhang, F.; Repka, M.A.; Thumma, S.; Upadhye, S.B.; Kumar Battu, S.; McGinity, J.W.; Martin, C. Pharmaceutical applications of hot-melt extrusion: Part I. Drug Dev. Ind. Pharm. 2007, 33, 909–926. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; O’Donnell, K.P.; de Vaux, S.M.D.; O’Brien, J.; Stutzman, J.; Williams, R.O., III. Processing thermally labile drugs by hot-melt extrusion: The lesson with gliclazide. Eur. J. Pharm. Biopharm. 2017, 119, 56–67. [Google Scholar] [CrossRef]
- Haser, A.; Huang, S.; Listro, T.; White, D.; Zhang, F. An approach for chemical stability during melt extrusion of a drug substance with a high melting point. Int. J. Pharm. 2017, 524, 55–64. [Google Scholar] [CrossRef]
- Stahly, G.P. Diversity in single-and multiple-component crystals. The search for and prevalence of polymorphs and cocrystals. Cryst. Growth Des. 2007, 7, 1007–1026. [Google Scholar] [CrossRef] [Green Version]
- Pina, M.F.; Pinto, J.F.; Sousa, J.J.; Fábián, L.; Zhao, M.; Craig, D.Q. Identification and characterization of stoichiometric and nonstoichiometric hydrate forms of paroxetine HCl: Reversible changes in crystal dimensions as a function of water absorption. Mol. Pharm. 2012, 9, 3515–3525. [Google Scholar] [CrossRef]
- Fujii, K.; Uekusa, H.; Itoda, N.; Yonemochi, E.; Terada, K. Mechanism of dehydration–hydration processes of lisinopril dihydrate investigated by ab initio powder X-ray diffraction analysis. Cryst. Growth Des. 2012, 12, 6165–6172. [Google Scholar] [CrossRef]
- Taylor, L.S.; York, P. Characterization of the phase transitions of trehalose dihydrate on heating and subsequent dehydration. J. Pharm. Sci. 1998, 87, 347–355. [Google Scholar] [CrossRef]
- Raijada, D.; Arnfast, L.; Bond, A.D.; Aho, J.; Bøtker, J.; Sandler, N.; Rantanen, J. Dehydration of nitrofurantoin monohydrate during melt extrusion. Cryst. Growth Des. 2017, 17, 3707–3715. [Google Scholar] [CrossRef]
- Serajuddin, A.T. Comparative thermal properties of the monohydrates of sodium theophylline and theophylline. J. Pharm. Pharmacol. 1986, 38, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.; Suryanarayanan, R. Influence of processing-induced phase transformations on the dissolution of theophylline tablets. AAPS PharmSciTech 2004, 5, 39. [Google Scholar]
- Saleki-Gerhardt, A.; Stoweell, J.G.; Byrn, S.R.; Zografi, G. Hydration and dehydration of crystalline and amorphous forms of raffinose. J. Pharm. Sci. 1995, 84, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.-P.; Fan, J.; Green, J.; Lu, Q.; Sanchez, E.; Angell, C. Vitrification of trehalose by water loss from its crystalline dihydrate. J. Therm. Anal. Calorim. 1996, 47, 1391–1405. [Google Scholar] [CrossRef]
- Sussich, F.; Cesàro, A. Trehalose amorphization and recrystallization. Carbohydr. Res. 2008, 343, 2667–2674. [Google Scholar] [CrossRef]
- Garner, W. The kinetics of endothermic solid reactions. In Chemistry of the Solid State; Academic Press: New York, NY, USA, 1955; pp. 213–231. [Google Scholar]
- Li, Y.; Han, J.; Zhang, G.G.; Grant, D.J.; Suryanarayanan, R. In situ dehydration of carbamazepine dihydrate: A novel technique to prepare amorphous anhydrous carbamazepine. Pharm. Dev. Technol. 2000, 5, 257–266. [Google Scholar] [CrossRef]
- Einfalt, T.; Planinšek, O.; Hrovat, K. Methods of amorphization and investigation of the amorphous state. Acta Pharm. 2013, 63, 305–334. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Suryanarayanan, R. Influence of environmental conditions on the kinetics and mechanism of dehydration of carbamazepine dihydrate. Pharm. Dev. Technol. 1998, 3, 587–596. [Google Scholar] [CrossRef]
- Surana, R.; Pyne, A.; Suryanarayanan, R. Solid-vapor interactions: Influence of environmental conditions on the dehydration of carbamazepine dihydrate. AAPS PharmSciTech 2003, 4, 539–548. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; O’Donnell, K.P.; Keen, J.M.; Rickard, M.A.; McGinity, J.W.; Williams, R.O. A new extrudable form of hypromellose: AFFINISOL™ HPMC HME. AAPS PharmSciTech 2016, 17, 106–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kachrimanis, K.; Griesser, U. Dehydration kinetics and crystal water dynamics of carbamazepine dihydrate. Pharm. Res. 2012, 29, 1143–1157. [Google Scholar] [CrossRef] [PubMed]
- Khoo, J.Y.; Shah, U.V.; Schaepertoens, M.; Williams, D.R.; Heng, J.Y. Process-induced phase transformation of carbamazepine dihydrate to its polymorphic anhydrates. Powder Technol. 2013, 236, 114–121. [Google Scholar] [CrossRef]
- Horstman, E.; Fung, P.; Lapina, O.; Khuth, T.; Touba, S.; Morrison, H. Application of Twin Screw Extruders to Scale-Up Amorphous GS-Z. In Proceedings of the AAPS PharmSci360, San Antonio, TX, USA, 3–6 November 2019. [Google Scholar]
- Edwards, A.D.; Shekunov, B.Y.; Forbes, R.T.; Grossmann, J.G.; York, P. Time-resolved X-ray scattering using synchrotron radiation applied to the study of a polymorphic transition in carbamazepine. J. Pharm. Sci. 2001, 90, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Grzesiak, A.L.; Lang, M.; Kim, K.; Matzger, A.J. Comparison of the four anhydrous polymorphs of carbamazepine and the crystal structure of form I. J. Pharm. Sci. 2003, 92, 2260–2271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arlin, J.-B.; Price, L.S.; Price, S.L.; Florence, A.J. A strategy for producing predicted polymorphs: Catemeric carbamazepine form V. Chem. Commun. 2011, 47, 7074–7076. [Google Scholar] [CrossRef] [Green Version]
- Gelbrich, T.; Hursthouse, M.B. Systematic investigation of the relationships between 25 crystal structures containing the carbamazepine molecule or a close analogue: A case study of the XPac method. CrystEngComm 2006, 8, 448–460. [Google Scholar] [CrossRef]
- Tian, F.; Zeitler, J.; Strachan, C.; Saville, D.; Gordon, K.; Rades, T. Characterizing the conversion kinetics of carbamazepine polymorphs to the dihydrate in aqueous suspension using Raman spectroscopy. J. Pharm. Biomed. Anal. 2006, 40, 271–280. [Google Scholar] [CrossRef]
- McMahon, L.E.; Timmins, P.; Williams, A.C.; York, P. Characterization of dihydrates prepared from carbamazepine polymorphs. J. Pharm. Sci. 1996, 85, 1064–1069. [Google Scholar] [CrossRef]
- Khoo, J.Y.; Heng, J.Y.; Williams, D.R. Agglomeration effects on the drying and dehydration stability of pharmaceutical acicular hydrate: Carbamazepine dihydrate. Ind. Eng. Chem. Res. 2010, 49, 422–427. [Google Scholar] [CrossRef]
- Khoo, J.Y.; Williams, D.R.; Heng, J.Y. Dehydration kinetics of pharmaceutical hydrate: Effects of environmental conditions and crystal forms. Drying Technol. 2010, 28, 1164–1169. [Google Scholar] [CrossRef]
- Sahakijpijarn, S.; Moon, C.; Koleng, J.J.; Williams, R.O. Formulation Composition and Process Affect Counterion for CSP7 Peptide. Pharmaceutics 2019, 11, 498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, H.; Wang, C.; Sun, C.C. Fast Determination of Phase Stability of Hydrates Using Intrinsic Dissolution Rate Measurements. Cryst. Growth Des. 2019, 19, 5471–5476. [Google Scholar] [CrossRef]
- Bartolomei, M.; Bertocchi, P.; Antoniella, E.; Rodomonte, A. Physico-chemical characterisation and intrinsic dissolution studies of a new hydrate form of diclofenac sodium: Comparison with anhydrous form. J. Pharm. Biomed. Anal. 2006, 40, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Djuris, J.; Nikolakakis, I.; Ibric, S.; Djuric, Z.; Kachrimanis, K. Preparation of carbamazepine–Soluplus® solid dispersions by hot-melt extrusion, and prediction of drug–polymer miscibility by thermodynamic model fitting. Eur. J. Pharm. Biopharm. 2013, 84, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Comyn, J. Introduction to polymer permeability and the mathematics of diffusion. In Polymer Permeability; Springer: Berlin/Heidelberg, Germany, 1985; pp. 1–10. [Google Scholar]








| Lot # | Formulation | Total Feed Rate (g/min) | Barrel Temperature (°C) | Screw Design | Screw Speed (rpm) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Zone 1 | Zone 2 | Feeding (Drug) | Zone 3 | Endplate | Die | |||||
| CBZA-F1 | 10% w/w form-III CBZ anhydrate 90% w/w Soluplus®-Vitamin E succinate (7:3) | 6 | 140 | 60 | 1 | 100 | ||||
| CBZA-F2 | 120 | |||||||||
| CBZA-F3 | 140 | |||||||||
| CBZA-F4 | 25% w/w form-III CBZ anhydrate 75% w/w Soluplus®-Vitamin E succinate (7:3) | 60 | 100 | |||||||
| CBZA-F5 | 120 | |||||||||
| CBZA-F6 | 140 | |||||||||
| CBZD-F1 | 10% w/w CBZ dihydrate 90% w/w Soluplus®-Vitamin E succinate (7:3) | 60 | 100 | |||||||
| CBZD-F2 | 120 | |||||||||
| CBZD-F3 | 140 | |||||||||
| CBZD-F4 | 25% w/w CBZ dihydrate 75% w/w Soluplus®-Vitamin E succinate (7:3) | 60 | 100 | |||||||
| CBZD-F5 | 120 | |||||||||
| CBZD-F6 | 140 | |||||||||
| CBZA-F1S | 10% w/w form-III CBZ anhydrate 90% w/w Soluplus®-Vitamin E succinate (7:3) | 60 | 2 | 200 | ||||||
| CBZA-F2S | 100 | |||||||||
| CBZA-F3S | 140 | |||||||||
| CBZD-F1S | 10% w/w CBZ dihydrate 90% w/w Soluplus®-Vitamin E succinate (7:3) | 60 | 200 | |||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Müller, F.; Huang, S.; Lowinger, M.; Liu, X.; Schooler, R.; Williams, R.O., III. Influence of Carbamazepine Dihydrate on the Preparation of Amorphous Solid Dispersions by Hot Melt Extrusion. Pharmaceutics 2020, 12, 379. https://doi.org/10.3390/pharmaceutics12040379
Ma X, Müller F, Huang S, Lowinger M, Liu X, Schooler R, Williams RO III. Influence of Carbamazepine Dihydrate on the Preparation of Amorphous Solid Dispersions by Hot Melt Extrusion. Pharmaceutics. 2020; 12(4):379. https://doi.org/10.3390/pharmaceutics12040379
Chicago/Turabian StyleMa, Xiangyu, Felix Müller, Siyuan Huang, Michael Lowinger, Xu Liu, Rebecca Schooler, and Robert O. Williams, III. 2020. "Influence of Carbamazepine Dihydrate on the Preparation of Amorphous Solid Dispersions by Hot Melt Extrusion" Pharmaceutics 12, no. 4: 379. https://doi.org/10.3390/pharmaceutics12040379






