Topical Application of Hyaluronic Acid-RGD Peptide-Coated Gelatin/Epigallocatechin-3 Gallate (EGCG) Nanoparticles Inhibits Corneal Neovascularization via Inhibition of VEGF Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Preparation of Gelatin/EGCG NPs with Surface HA-RGD-Conjugation (GEH-RGD)
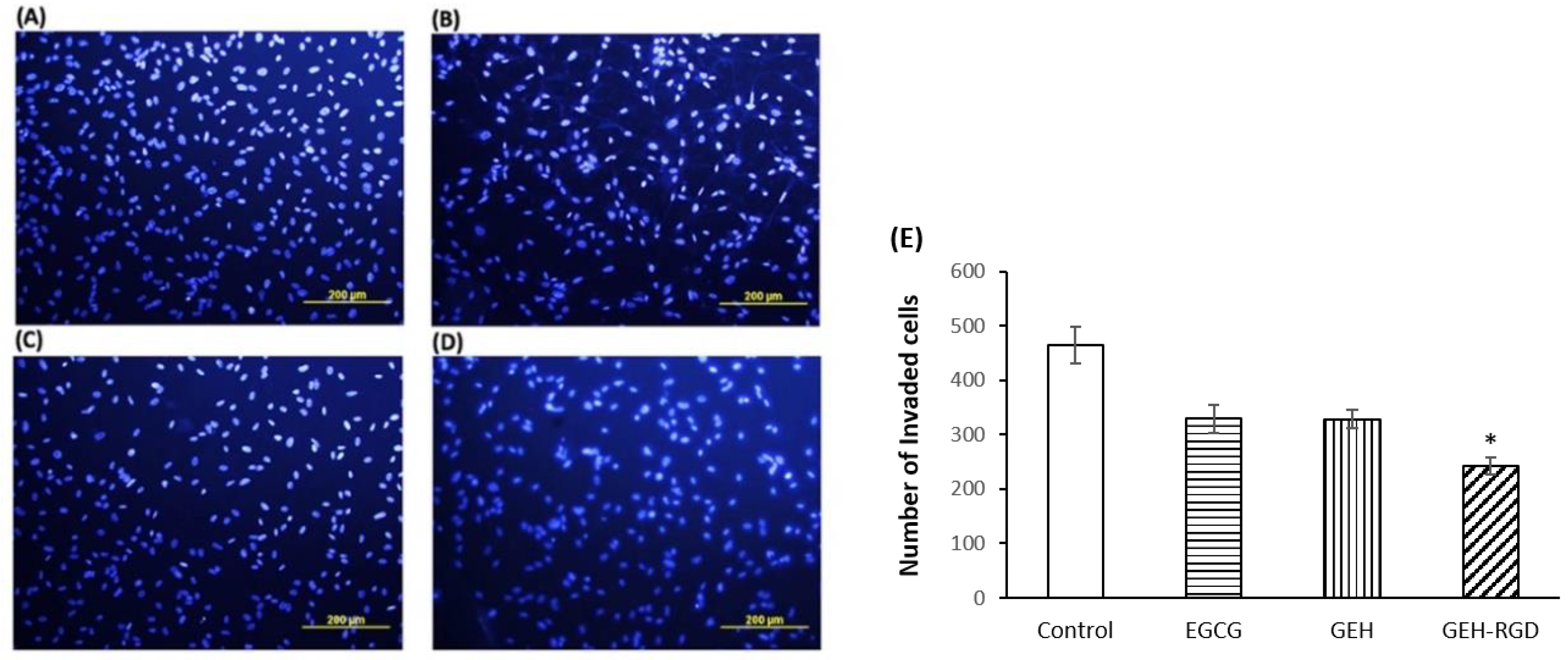
2.3. Functional Evaluation of GEH-RGD NPs on HUVECs
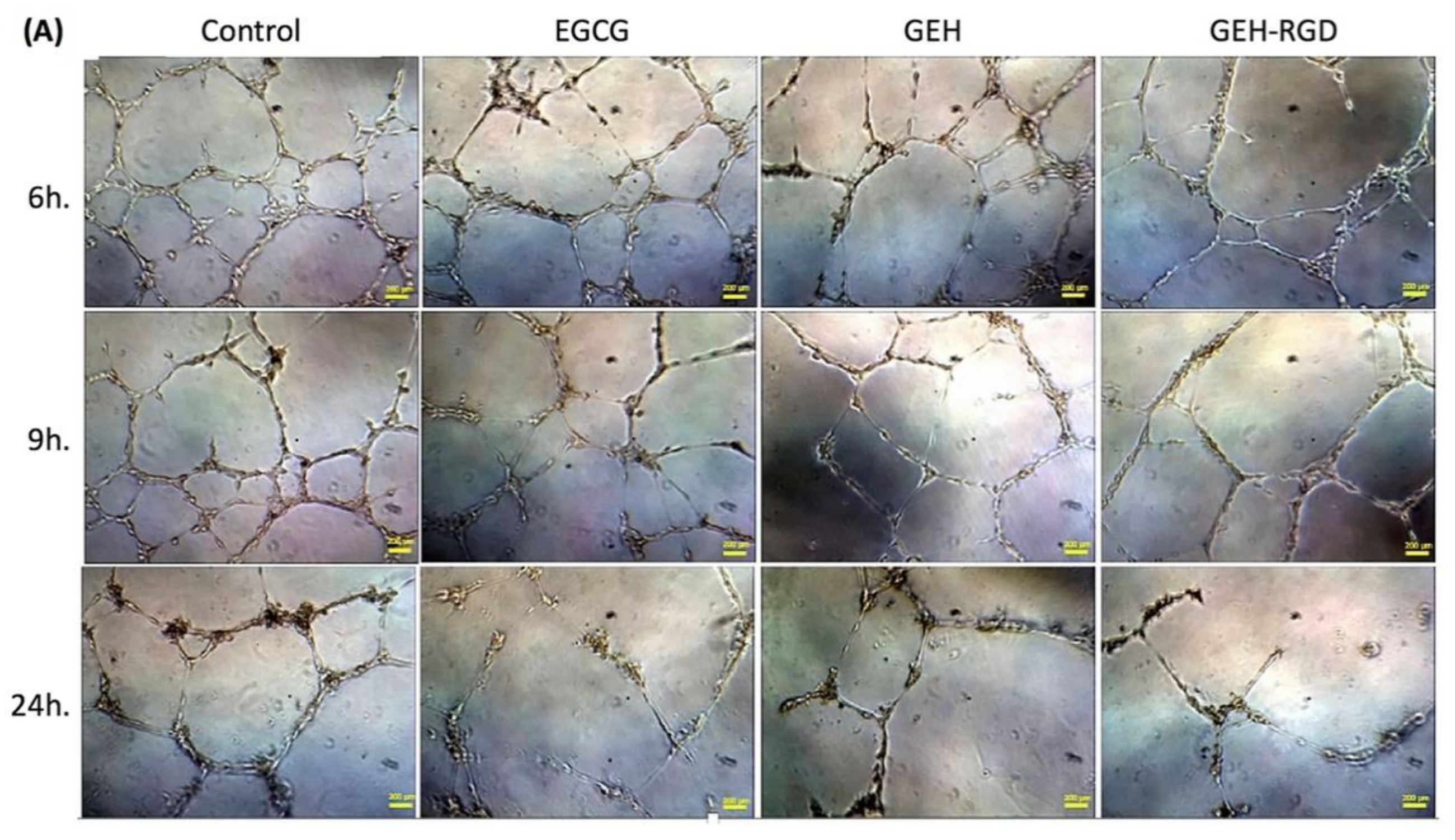
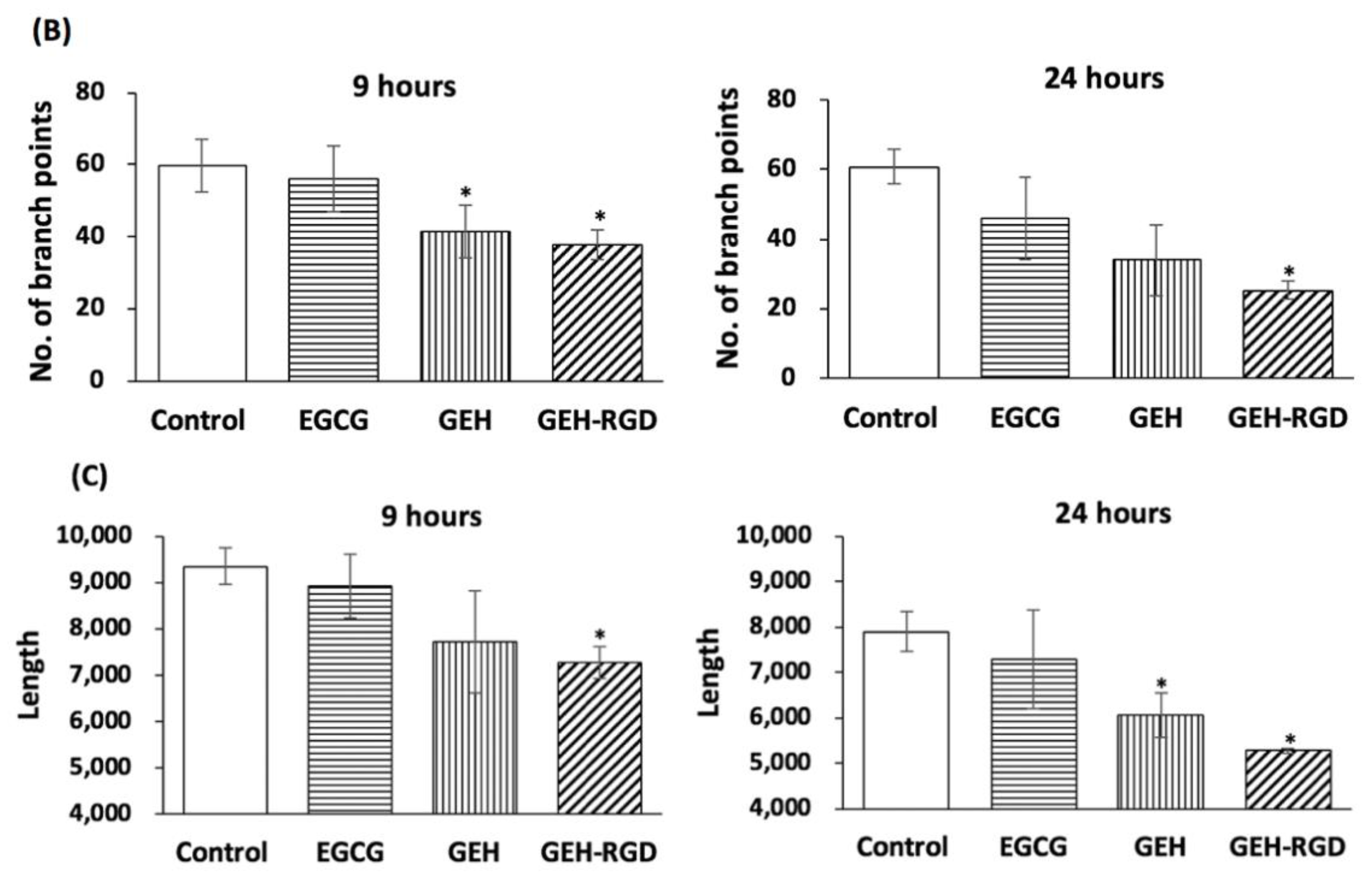
2.3.1. Tube Formation Assay
2.3.2. Gelatin Zymography
2.4. Topical Delivery of NPs in a Mouse Model of Corneal NV
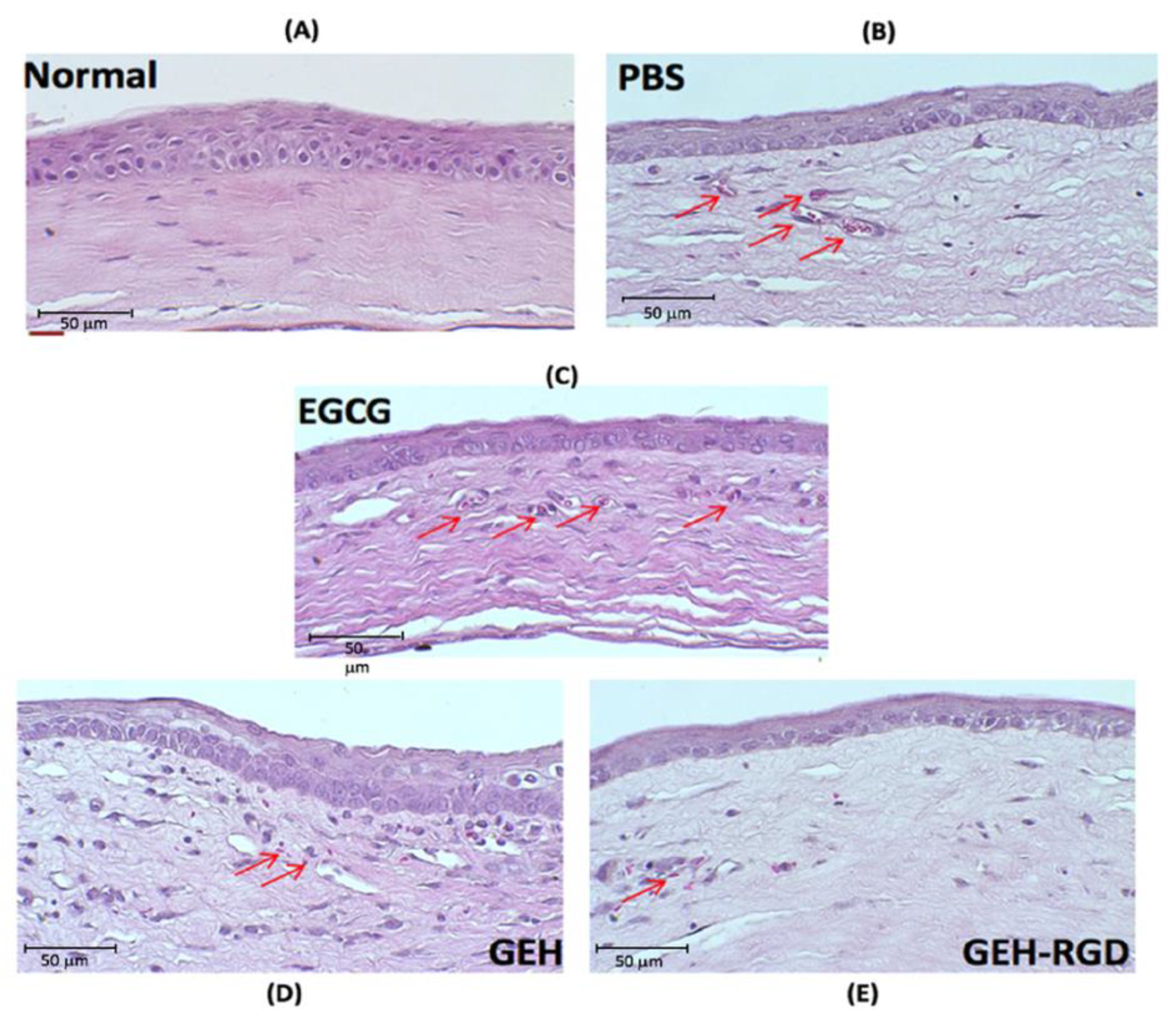
2.4.1. Histopathology Examination of Corneal Sections
2.4.2. Quantification of VEGF and MMP-9 in Cornea Extraction
2.5. Statistical Analysis
3. Results
3.1. Characterization of EGCG-Loaded GEH-RGD NPs
3.2. GEH-RGD NPs Inhibit In Vitro Angiogenetic Activity
3.3. GEH-RGD NPs Inhibit MMP-2 and MMP-9 Activities
3.4. Topical Application of GEH-RGD NPs Suppresses the Corneal NV in a Mouse Model of Chemical Cauterization
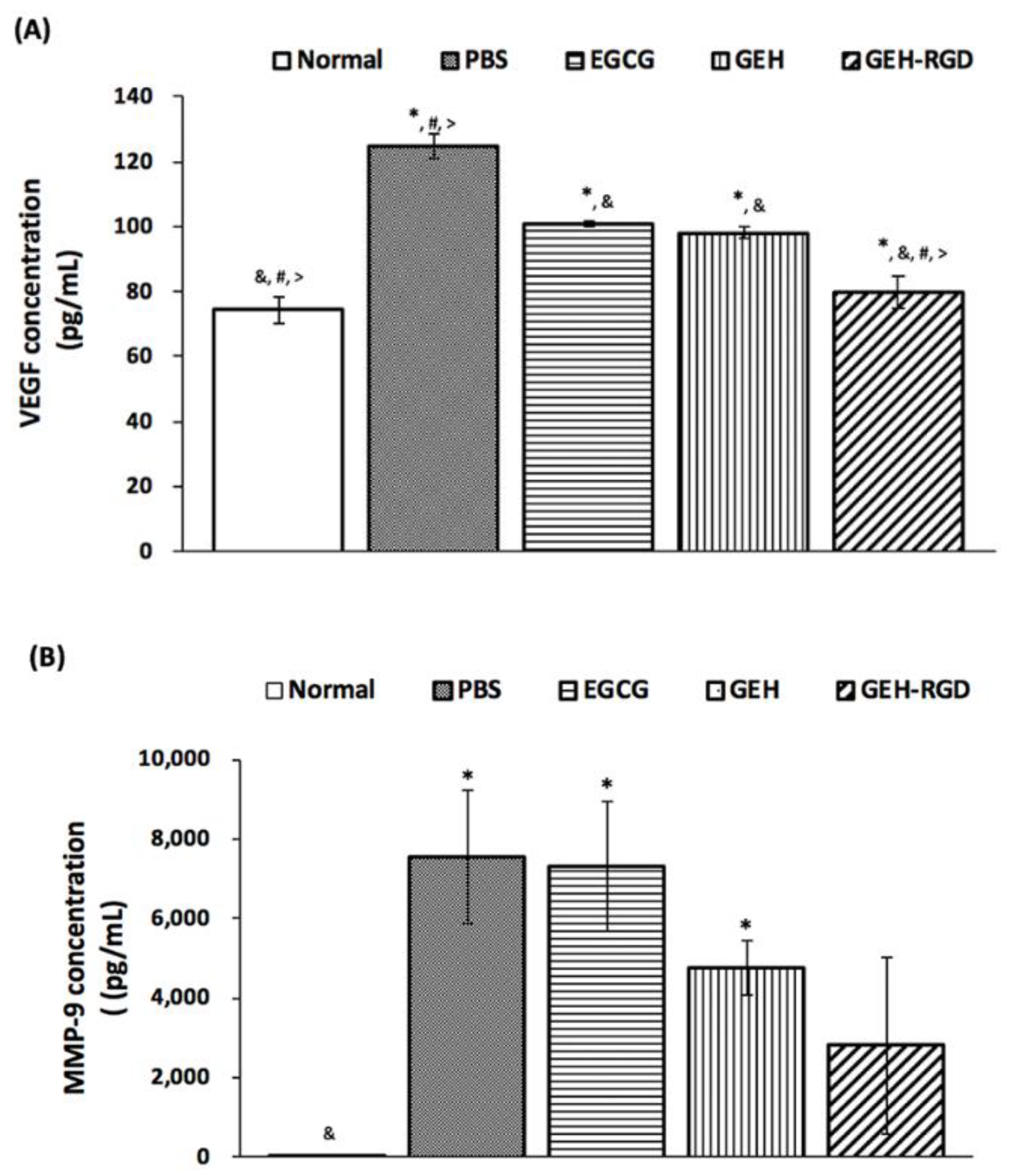
3.5. Topical Application of GEH-RGD NPs Attenuates the Expression of VEGF and MMP-9 in the Chemical Cauterized Corneas
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Supplement 1—The Protocol for EGCG Quantification by ABST+ Method
Appendix A.2. Supplement 2—Transwell Migration Assay
References
- Menzel-Severing, J. Emerging techniques to treat corneal neovascularisation. Eye 2012, 26, 2–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevenson, W.; Cheng, S.F.; Dastjerdi, M.H.; Ferrari, G.; Dana, R.; Cheng, S.F.; Dastjerdi, M.H.; Ferrari, G.; Dana, R. Corneal neovascularization and the utility of topical VEGF inhibition: Ranibizumab (Lucentis) vs. bevacizumab (Avastin). Ocul. Surf. 2012, 10, 67–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, L.; Loza, R.J.; Han, K.Y.; Sunoqrot, S.; Cunningham, C.; Purta, P.; Drake, J.; Jain, S.; Hong, S.; Chang, J.-H. Nanotechnology in Corneal Neovascularization Therapy—A Review. J. Ocular Pharmacol. Ther. 2013, 29, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.H.; Garg, N.K.; Lunde, E.; Han, K.Y.; Jain, S.; Azar, D.T. Corneal neovascularization: An anti-VEGF therapy review. Surv. Ophthalmol. 2012, 57, 415–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shakiba, Y.; Mansouri, K.; Arshadi, D.; Rezaei, N. Corneal neovascularization: Molecular events and therapeutic options. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Whitcher, J.P.; Srinivasan, M.; Upadhyay, M.P. Corneal blindness: A global perspective. Bull. World Health Organ. 2001, 79, 214–221. [Google Scholar] [PubMed]
- Bachmann, B.O.; Bock, F.; Wiegand, S.J.; Maruyama, K.; Dana, M.R.; Kruse, F.E.; Luetjen-Drecoll, E.; Cursiefen, C. Promotion of graft survival by vascular endothelial growth factor a neutralization after high-risk corneal transplantation. Arch. Ophthalmol. 2008, 126, 71–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feizi, S.; Azari, A.A.; Safapour, S. Therapeutic approaches for corneal neovascularization. Eye Vis. (Lond.) 2017, 4, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Abdelfattah, N.S.; Amgad, M.; Zayed, A.A.; Salem, H.; Elkhanany, A.E.; Hussein, H.; El-Baky, N.A. Clinical correlates of common corneal neovascular diseases: A literature review. Int. J. Ophthalmol. 2015, 8, 182–193. [Google Scholar]
- Cope, J.R.; Collier, S.A.; Rao, M.M.; Chalmers, R.; Mitchell, L.; Richdale, K.; Wagner, H.; Kinoshita, B.T.; Lam, D.Y.; Sorbara, L.; et al. Contact lens wearer demographics and risk behaviors for contact lens-related eye infections—United States, 2014. MMWR Morb. Mortal Wkly Rep. 2015, 64, 865–870. [Google Scholar] [CrossRef] [Green Version]
- Ang, J.H.; Efron, N. Corneal hypoxia and hypercapnia during contact lens wear. Optom. Vis. Sci. 1990, 67, 512–521. [Google Scholar] [PubMed]
- Singh, N.; Amin, S.; Richter, E.; Rashid, S.; Scoglietti, V.; Jani, P.D.; Wang, J.; Kaur, R.; Ambati, J.; Dong, Z.; et al. Flt-1 intraceptors inhibit hypoxia-induced VEGF expression in vitro and corneal neovascularization in vivo. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1647–1652. [Google Scholar] [CrossRef] [PubMed]
- Moreddu, R.; Vigolo, D.; Yetisen, A.K. Contact Lens Technology: From fundamentals to Applications. Adv. Healthc. Mater. 2019, 8, 1900368. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.K.; Bergmanson, J.P.; Miller, W.L.; Marsack, J.D.; Johnson, L.A. Complications and fitting challenges associated with scleral contact lenses: A review. Cont. Lens. Anterior Eye 2016, 39, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Alipour, F.; Khaheshi, S.; Soleimanzadeh, M.; Heidarzadeh, S.; Heydarzadeh, S. Contact lens-related complications: A review. J. Ophthalmic. Vis. Res. 2017, 12, 193–204. [Google Scholar] [PubMed]
- Lee, P.; Wang, C.C.; Adamis, A.P. Ocular neovascularization: An epidemiologic review. Surv. Ophthalmol. 1998, 43, 245–269. [Google Scholar] [CrossRef]
- Lau, C.M.L.; Yu, Y.; Jahanmir, G.; Chau, Y. Controlled release technology for anti-angiogenesis treatment of posterior eye diseases: Current status and challenges. Adv. Drug Deliv. Rev. 2018, 126, 145–161. [Google Scholar] [CrossRef]
- Ferrari, G.; Dastjerdi, M.H.; Okanobo, A.; Cheng, S.-F.; Amparo, F.; Nallasamy, N.; Dana, R. Topical 4anibizumab as a treatment of corneal neovascularization. Cornea 2013, 32, 992–997. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Huerta, V.; Gutiérrez-Sánchez, L.; Flores-Estrada, J. (-)-Epigallocatechin 3-gallate (EGCG) at the ocular surface inhibits corneal neovascularization. Med. Hypotheses 2011, 76, 311–313. [Google Scholar] [CrossRef]
- Chang, C.Y.; Wang, M.C.; Miyagawa, T.; Chen, Z.Y.; Lin, F.H.; Chen, K.H.; Liu, G.S.; Tseng, C.L. Preparation of RGD modified biopolymeric nanoparticles contained epigalloccatechin-3-gallate for targeting vascular endothelium cells applied in corneal angiogenesis inhibition. Int. J. Nanomed. 2017, 12, 279–294. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.C.; Yu, S.H.; Tsai, G.J.; Tang, D.W.; Mi, F.L.; Peng, Y.P. Novel technology for the preparation of self-assembled catechin/gelatin nanoparticles and their characterization. J. Agric. Food Chem. 2010, 58, 6728–6734. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, S.K.; Guo, W.; Liu, L.; Band, M.A.; Paulson, E.K.; Meydani, M. Green tea catechin, epigallocatechin-3-gallate, inhibits vascular endothelial growth factor angiogenic signaling by disrupting the formation of a receptor complex. Int. J. Cancer 2006, 118, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Jun, J.-H.; Jung, E.-H.; Koo, B.A.; Kim, Y.S. Epigalloccatechin-3-gallate inhibits ocular neovascularization and vascular permeability in human retinal pigment epithelial and human retinal microvascular endothelial cells via suppression of MMP-9 and VEGF activation. Molecules 2014, 19, 12150–12172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, R.T.; Kambampati, R.; Murphy, C.J.; Bentley, E. Expression of matrix metalloproteinase 2 and 9 in experimentally wounded canine corneas and spontaneous chronic corneal epithelial defects. Cornea 2007, 26, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Cavet, M.E.; Harrington, K.L.; Vollmer, T.R.; Ward, K.W.; Zhang, J.Z. Anti-inflammatory and anti-oxidative effects of the green tea polyphenol epigallocatechin gallate in human corneal epithelial cells. Mol. Vis. 2011, 17, 533–542. [Google Scholar] [PubMed]
- Friedlander, M.; Theesfeld, C.L.; Sugita, M.; Fruttiger, M.; Thomas, M.A. Involvement of integrins alpha v beta 3 and alpha v beta 5 in ocular neovascular diseases. Proc. Natl. Acad. Sci. USA 1996, 93, 9764–9769. [Google Scholar] [CrossRef] [Green Version]
- Stepp, M.A. Corneal integrins and their functions. Exp. Eye Res. 2006, 83, 3–15. [Google Scholar] [CrossRef]
- Fan, T.P.; Yeh, J.C.; Leung, K.W.; Yue, P.Y.; Wong, R.N. Angiogenesis: From plants to blood vessels. Trends Pharmacol. Sci. 2006, 27, 297–309. [Google Scholar] [CrossRef]
- Danhier, F.; Breton, A.L.; Préat, V. RGD-based strategies to target alpha(v) beta(3) integrin in cancer therapy and diagnosis. Mol. Pharm. 2012, 9, 2961–2973. [Google Scholar] [CrossRef]
- Guo, Z.; He, B.; Jin, H.; Zhang, H.; Dai, W.; Zhang, L.; Zhang, H.; Wang, X.; Wang, J.; Zhang, X.; et al. Targeting efficiency of RGD-modified nanocarriers with different ligand intervals in response to integrin αvβ3 clustering. Biomaterials 2014, 35, 6106–6117. [Google Scholar] [CrossRef]
- Tucker, G.C. Alpha v integrin inhibitors and cancer therapy. Curr. Opin. Investig. Drugs 2003, 4, 722–731. [Google Scholar] [PubMed]
- Bellis, S.L. Advantages of RGD peptides for directing cell association with biomaterials. Biomaterials 2011, 32, 4205–4210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, N.M. Biopharmaceutical considerations in topical ocular drug delivery. Clin. Exp. Pharmacol. Physiol. 2000, 27, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.; Gilger, B.; Robinson, M. Novel approaches to ocular drug delivery. Curr. Opin. Mol. Ther. 2004, 6, 195–205. [Google Scholar]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular drug delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef]
- Tsai, C.H.; Wang, P.Y.; Lin, I.C.; Huang, H.; Liu, G.S.; Tseng, C.L. Ocular Drug Delivery: Role of Degradable Polymeric Nanocarriers for Ophthalmic Application. Int. J. Mol. Sci. 2018, 19, 2830. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.Y.; Wang, M.C.; Chen, Z.Y.; Chiu, W.Y.; Chen, K.H.; Lin, I.C.; Yang, W.C.V.; Wu, C.C.; Tseng, C.L. Gelatin–epigallocatechin gallate nanoparticles with hyaluronic acid decoration as eye drops can treat rabbit dry-eye syndrome effectively via inflammatory relief. Int. J. Nanomed. 2018, 13, 7251–7273. [Google Scholar] [CrossRef] [Green Version]
- Shutava, T.G.; Balkundi, S.S.; Lvov, Y.M. (-)-Epigallocatechin gallate/gelatin layer-by-layer assembled films and microcapsules. J. Colloid Interface Sci. 2009, 330, 276–283. [Google Scholar] [CrossRef]
- Yang, C.S.; Lambert, J.D.; Ju, J.; Lu, G.; Sang, S. Tea and cancer prevention: Molecular mechanisms and human relevance. Toxicol. Appl. Pharmacol. 2007, 224, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Gu, L. Fabrication of self-assembled (-)-epigallocatechin gallate (EGCG) ovalbumin-dextran conjugate nanoparticles and their transport across monolayers of human intestinal epithelial Caco-2 cells. J. Agric. Food Chem. 2014, 62, 1301–1309. [Google Scholar] [CrossRef]
- Sanna, V.P.G.; Roggio, A.M.; Punzoni, S.; Posadino, A.M.; Arca, A.M.S.; Bandiera, P.; Uzzau, S.; Sechi, M. Targeted biocompatible nanoparticles for the delivery of (−)-epigallocatechin 3-gallate to prostate cancer cells. J. Med. Chem. 2011, 54, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Kojima-Yuasa, A.; Hua, J.J.; Kennedy, D.O.; Matsui-Yuas, I. Green tea extract inhibits angiogenesis of human umbilical vein endothelial cells through reduction of expression of VEGF receptors. Life Sci. 2003, 73, 1299–1313. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.-P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Salam, A.; Mathew, R.; Sivaprasad, S. Treatment of proliferative diabetic retinopathy with anti-VEGF agents. Acta Ophthalmol. 2011, 89, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Kwak, N.; Okamoto, N.; Wood, J.M.; Campochiaro, P.A. VEGF is major stimulator in model of choroidal neovascularization. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3158–3164. [Google Scholar]
- Spragg, D.D.; Alford, D.R.; Greferath, R.; Larsen, C.E.; Lee, K.-D.; Gurtner, G.C.; Cybulsky, M.I.; Tosi, P.F.; Nicolau, C.; Michael, A.; et al. Immunotargeting of liposomes to activated vascular endothelial cells: A strategy for site-selective delivery in the cardiovascular system. Proc. Natl. Acad. Sci. USA 1997, 94, 8795–8800. [Google Scholar] [CrossRef] [Green Version]
- Kelly, K.A.; Allport, J.R.; Tsourkas, A.; Shinde-Patil, V.R.; Josephson, L.; Weissleder, R. Detection of vascular adhesion molecule-1 expression using a novel multimodal nanoparticle. Circ. Res. 2005, 96, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Tsourkas, A.; Shinde-Patil, V.R.; Kelly, K.A.; Patel, P.; Wolley, A.; Allport, J.R.; Weissleder, R. In vivo imaging of activated endothelium using an anti-VCAM-1 magnetooptical probe. Bioconjug. Chem. 2005, 16, 576–581. [Google Scholar] [CrossRef]
- Runnels, J.M.; Zamiri, P.; Spencer, J.A.; Veilleux, I.; Wei, X.; Bogdanov, A.; Lin, C.P. Imaging molecular expression on vascular endothelial cells by in vivo immunofluorescence microscopy. Mol. Imaging 2006, 5, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Eslani, M.; Baradaran-Rafii, A.; Movahedan, A.; Djalilian, A.R. The ocular surface chemical burns. J Ophthalmol. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Tseng, C.L.; Chen, K.H.; Su, W.Y.; Lee, Y.H.; ChangWu, C.; Lin, F.H. Cationic gelatin nanoparticles for drug delivery to the ocular surface: In vitro and In vivo evaluation. J. Nanomater. 2013, 2013, 1–11. [Google Scholar]
- Neuhaus, T.; Pabst, S.; Stier, S.; Weber, A.-A.; Schrör, K.; Sachinidis, A.; Vetter, H.D.; Ko, Y. Inhibition of the vascular-endothelial growth factor-induced itracellualr signaling and mitogenesis of human endothelial cells by epigallocatechin-3 gallate. Eur. J. Pharmacol. 2004, 483, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Koh, C.H.; Lee, H.S.; Chung, S.K. Effect of topical epigallocatechin gallate on corneal neovascularization in rabbits. Cornea 2014, 33, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, N.; Guha, R.; Chowdhury, S.; Nandi, S.; Konar, A.; Hazra, S. Curcumin nanoparticles inhibit corneal neovascularization. J. Mol. Med. 2015, 93, 1095–1106. [Google Scholar] [CrossRef]




| NP’s Group | Particle Size (nm) | Zeta Potential (mV) | PDI |
|---|---|---|---|
| GE | 91.90 ± 44.53 | 18.4 ± 4.4 | 0.30 ± 0.20 |
| GEH | 277.40 ± 73.00 | −13.2 ± 4.1 | 0.38 ± 0.18 |
| GEH-RGD | 158.10 ± 11.06 | 12.9 ± 4.1 | ± 0.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyagawa, T.; Chen, Z.-Y.; Chang, C.-Y.; Chen, K.-H.; Wang, Y.-K.; Liu, G.-S.; Tseng, C.-L. Topical Application of Hyaluronic Acid-RGD Peptide-Coated Gelatin/Epigallocatechin-3 Gallate (EGCG) Nanoparticles Inhibits Corneal Neovascularization via Inhibition of VEGF Production. Pharmaceutics 2020, 12, 404. https://doi.org/10.3390/pharmaceutics12050404
Miyagawa T, Chen Z-Y, Chang C-Y, Chen K-H, Wang Y-K, Liu G-S, Tseng C-L. Topical Application of Hyaluronic Acid-RGD Peptide-Coated Gelatin/Epigallocatechin-3 Gallate (EGCG) Nanoparticles Inhibits Corneal Neovascularization via Inhibition of VEGF Production. Pharmaceutics. 2020; 12(5):404. https://doi.org/10.3390/pharmaceutics12050404
Chicago/Turabian StyleMiyagawa, Takuya, Zhi-Yu Chen, Che-Yi Chang, Ko-Hua Chen, Yang-Kao Wang, Guei-Sheung Liu, and Ching-Li Tseng. 2020. "Topical Application of Hyaluronic Acid-RGD Peptide-Coated Gelatin/Epigallocatechin-3 Gallate (EGCG) Nanoparticles Inhibits Corneal Neovascularization via Inhibition of VEGF Production" Pharmaceutics 12, no. 5: 404. https://doi.org/10.3390/pharmaceutics12050404
APA StyleMiyagawa, T., Chen, Z.-Y., Chang, C.-Y., Chen, K.-H., Wang, Y.-K., Liu, G.-S., & Tseng, C.-L. (2020). Topical Application of Hyaluronic Acid-RGD Peptide-Coated Gelatin/Epigallocatechin-3 Gallate (EGCG) Nanoparticles Inhibits Corneal Neovascularization via Inhibition of VEGF Production. Pharmaceutics, 12(5), 404. https://doi.org/10.3390/pharmaceutics12050404





