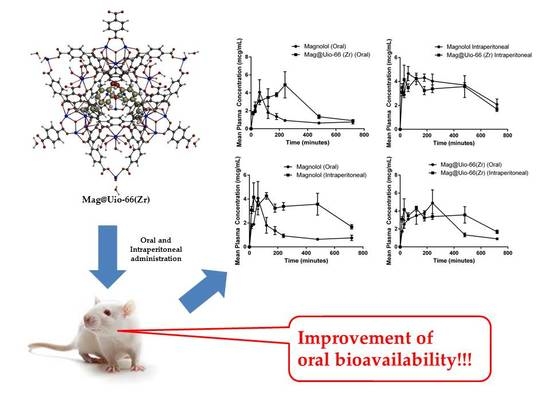
Enhanced Oral Bioavailability of the Pharmacologically Active Lignin Magnolol via Zr-Based Metal Organic Framework Impregnation
Abstract
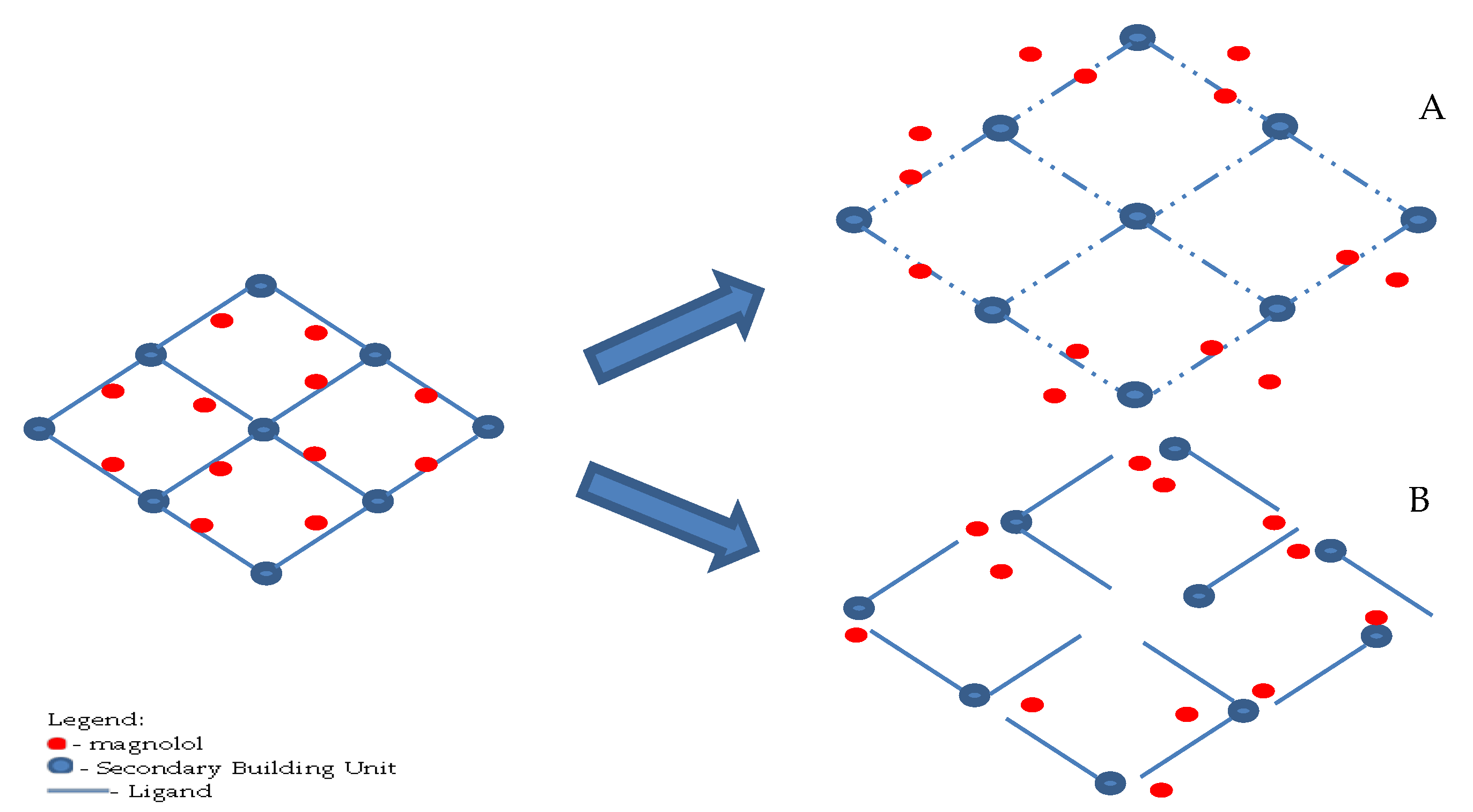
:1. Introduction
2. Materials and Methods
2.1. Material and Reagents
2.2. Animals
2.3. Methods
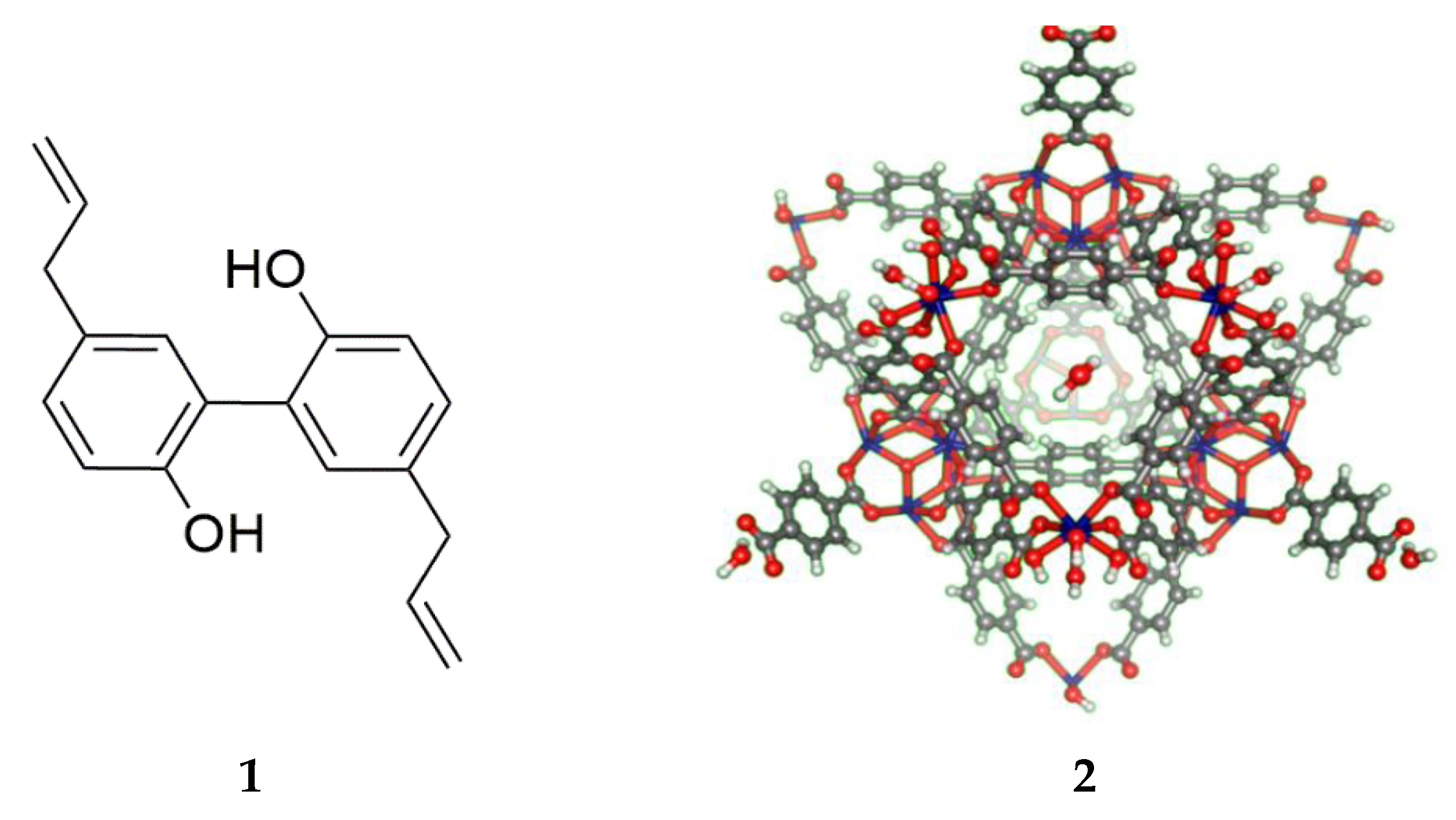
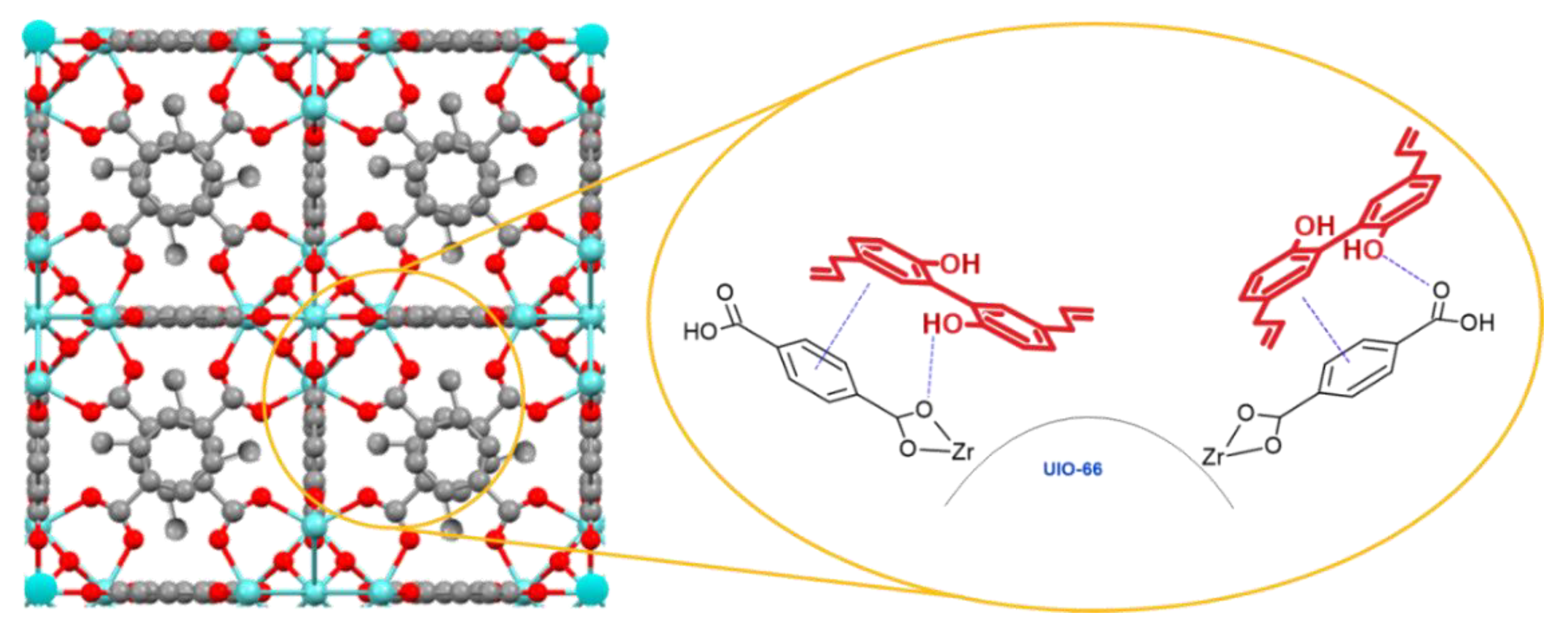
2.3.1. Synthesis of Uio-66(Zr)
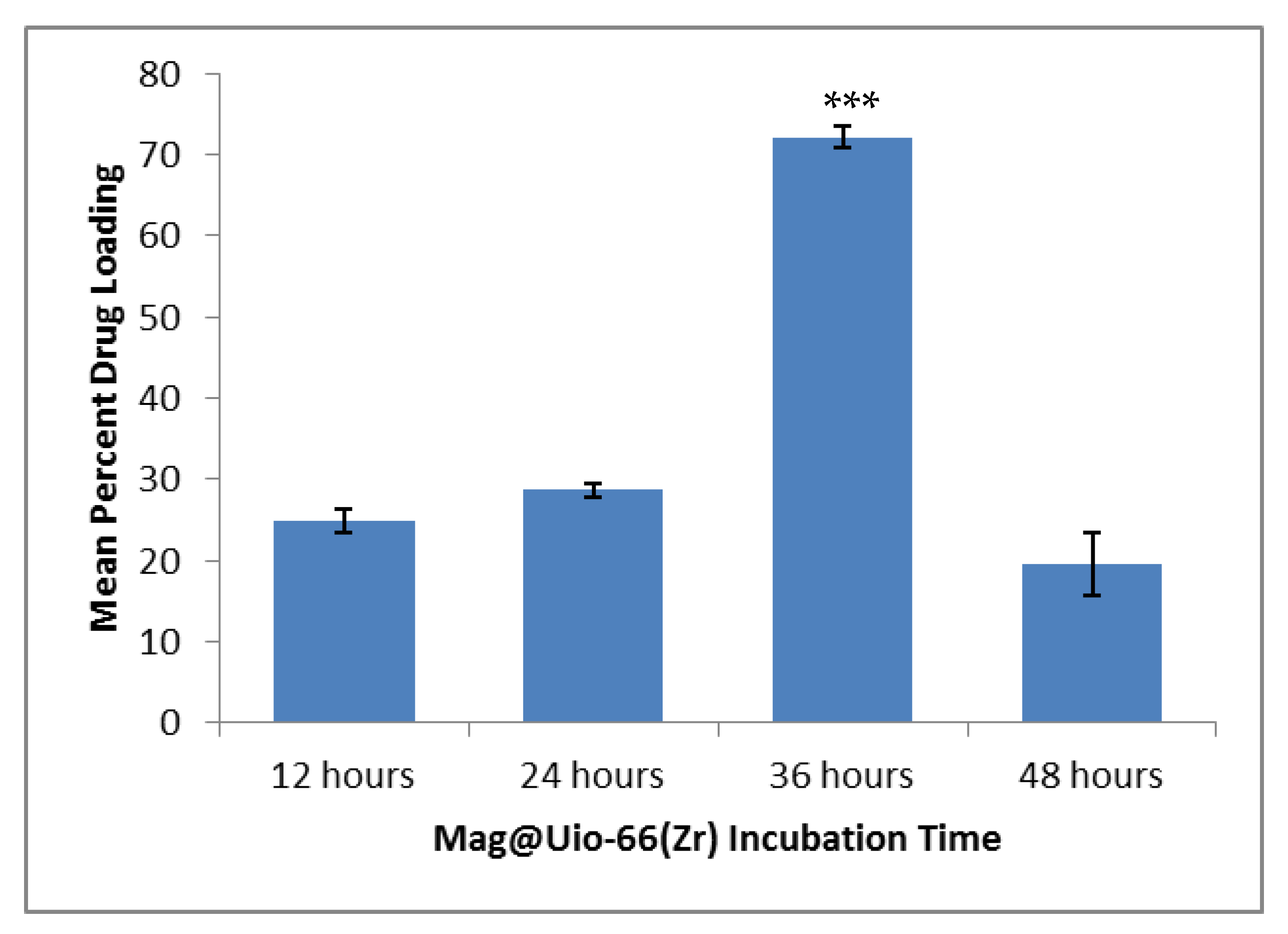
2.3.2. Magnolol Impregnation and Quantification
2.3.3. Characterization of Magnolol-Loaded MOF
Nitrogen Sorption Isotherms
Thermogravimetric Analysis
Powder X-ray Diffraction (PXRD)
Scanning Electron Microscopy (SEM)
Particle Size Determination
2.3.4. In Vitro Drug Release
2.3.5. Acute Oral Toxicity
2.3.6. Oral Bioavailability and Tissue Distribution
Collection of Serum Samples
Collection of Tissue Samples
Magnolol Quantification
2.3.7. Statistical Analysis
3. Results
3.1. Magnolol Impregnation and Quantification
3.2. Characterization of mag@Uio-66(Zr)
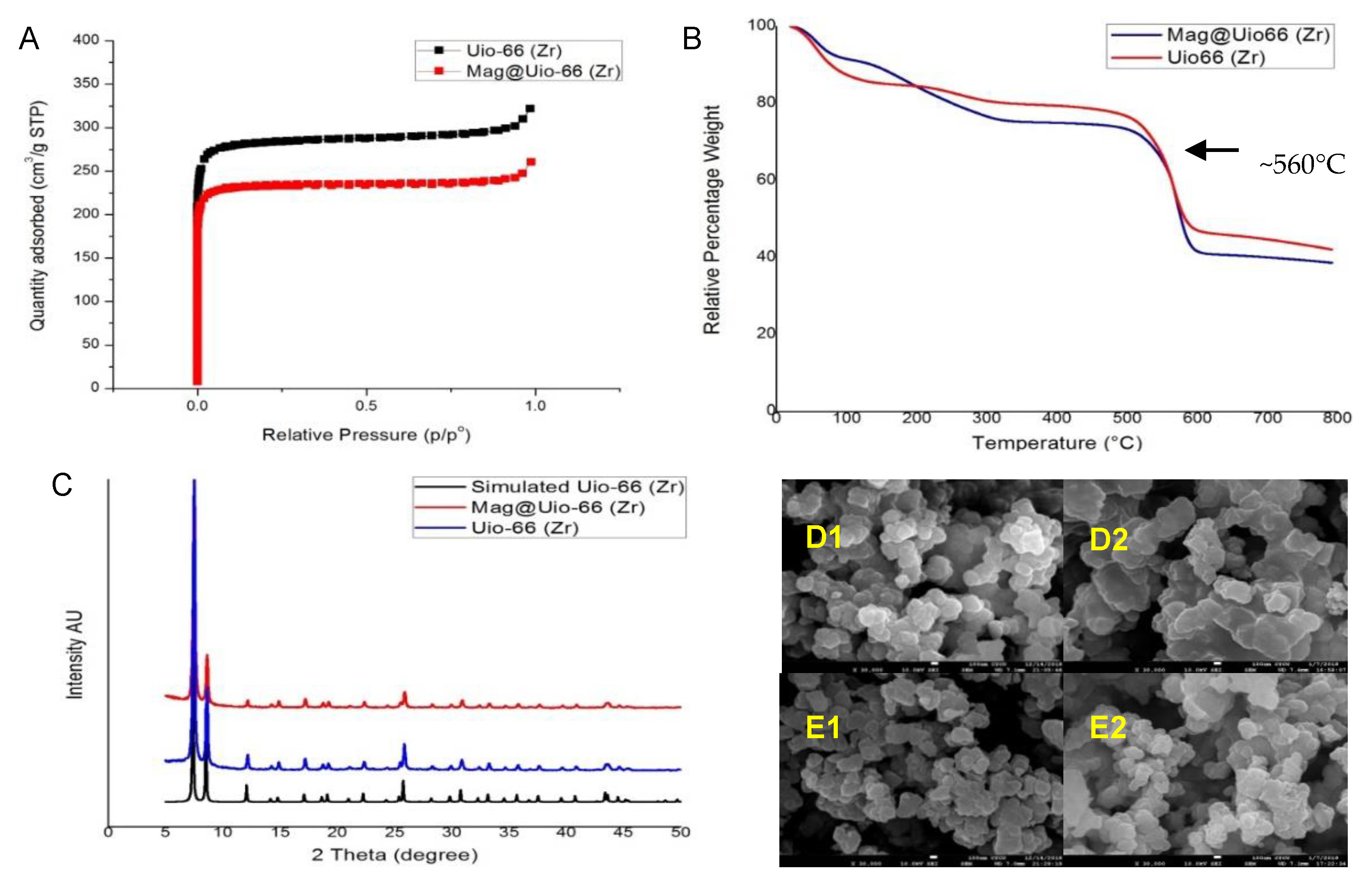
3.2.1. Nitrogen Sorption Isotherms
3.2.2. Thermogravimetric Analysis (TGA)
3.2.3. Powder X-ray Diffraction (PXRD)
3.2.4. Scanning Electron Microscopy (SEM)
3.2.5. Particle Size Determination
3.3. In Vitro Drug Release
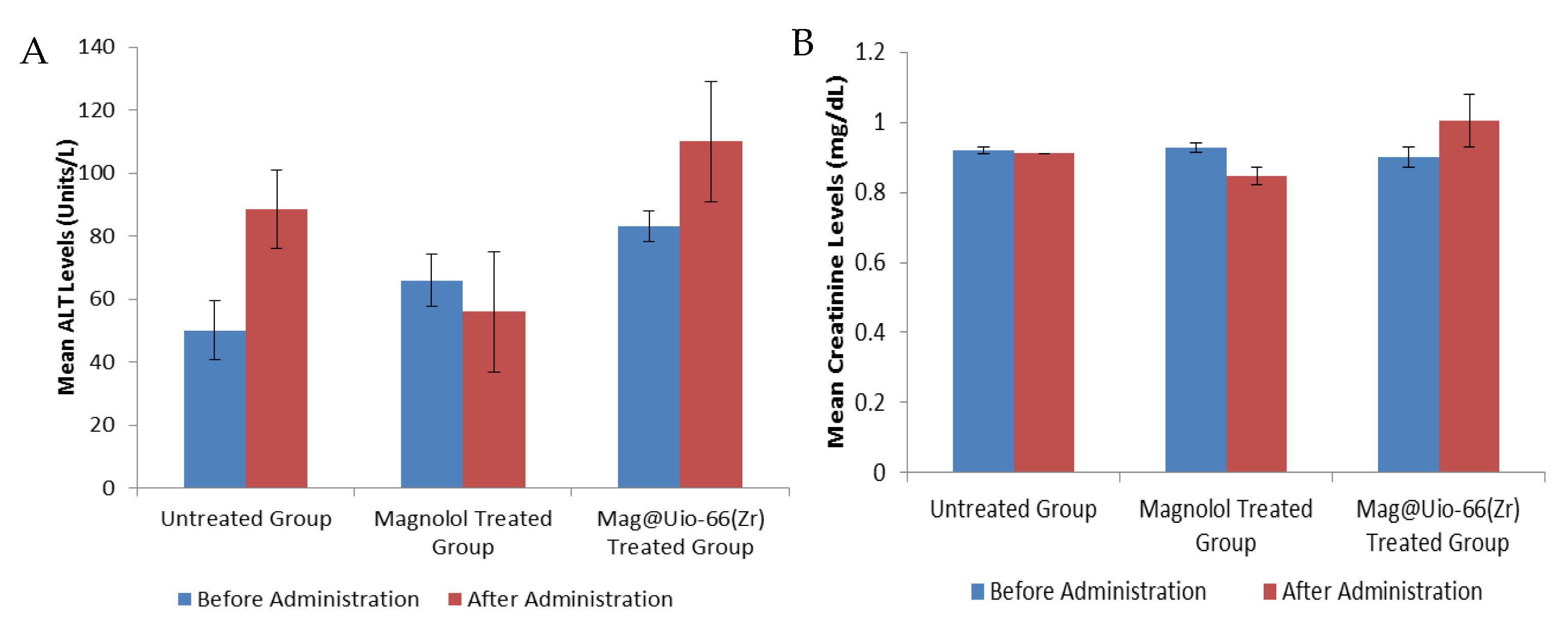
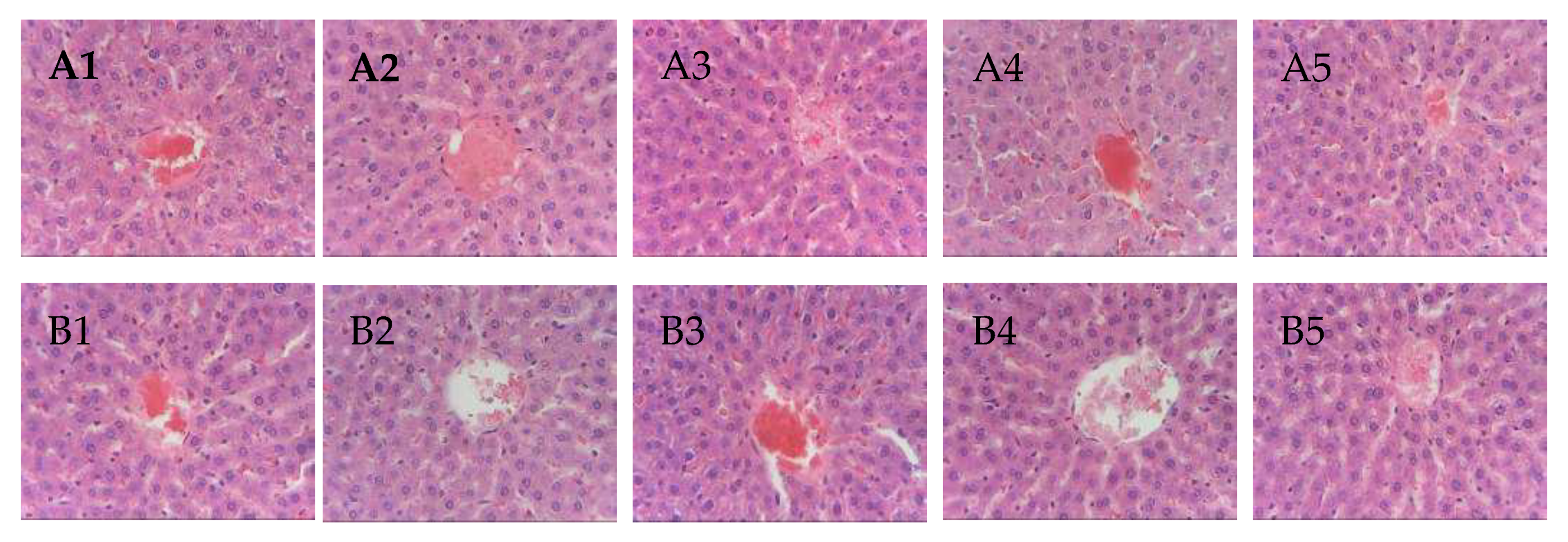
3.4. Acute Oral Toxicity
3.5. Oral Bioavailability and Tissue Distribution
3.5.1. Oral Bioavailability
3.5.2. Tissue Distribution
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gupta, S.; Kesarla, R.; Omri, A. Formulation strategies to improve the bioavailability of poorly absorbed drugs with special emphasis on self-emulsifying systems. ISRN Pharm. 2013, 2013, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Hu, M.; Li, X. Series in drug discovery and development. In Oral Bioavailability: Basic Principles, Advanced Concepts, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 978-0-470-26099-9. [Google Scholar]
- Gao, S.; Hu, M. Bioavailability challenges associated with development of anti-cancer phenolics. Mini Rev. Med. Chem. 2010, 10, 550–567. [Google Scholar] [CrossRef] [Green Version]
- Ko, C.H.; Chen, H.H.; Lin, Y.R.; Chan, M.H. Inhibition of smooth muscle contraction by magnolol and honokiol in porcine trachea. Planta Med. 2003, 69, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Syu, W.-J.; Shen, C.-C.; Lu, J.-J.; Lee, G.-H.; Sun, C.-M. Antimicrobial and cytotoxic activities of neolignans from Magnolia officinalis. Chem. Biodivers. 2004, 1, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Homma, M.; Minami, M.; Taniguchi, C.; Oka, K.; Morita, S.; Niitsuma, T.; Hayashi, T. Inhibitory effects of lignans and flavonoids in saiboku-to, a herbal medicine for bronchial asthma, on the release of leukotrienes from human polymorphonuclear leukocytes. Planta Med. 2000, 66, 88–91. [Google Scholar] [CrossRef]
- Haraguchi, H.; Ishikawa, H.; Shirataki, N.; Fukuda, A. Antiperoxidative activity of neolignans from Magnolia obovata. J. Pharm. Pharmacol. 1997, 49, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, K.; Ding, X.; Tang, H.; Xu, Z. Magnolol inhibits growth and induces apoptosis in esophagus cancer KYSE-150 cell lines via the MAP kinase pathway. J. Thorac. Dis. 2019, 11, 3030–3038. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jung, E.; Park, J.; Jung, K.; Lee, S.; Hong, S.; Park, J.; Park, E.; Kim, J.; Park, S.; et al. Anti-inflammatory effects of magnolol and honokiol are mediated through inhibition of the downstream pathway of MEKK-1 in NF-κB activation signaling. Planta Med. 2005, 71, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.H.; Chou, C.J.; Lee, T.F.; Wang, L.C.H.; Chen, C.F. Pharmacokinetic and pharmacodynamic studies of magnolol after oral administration in rats. Pharm. Sci. 1996, 2, 191–193. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Z.; Huang, X.; Shi, W.; Zhang, R.; Chen, M.; Huang, H.; Wu, L. Insights on the multifunctional activities of magnolol. BioMed Res. Int. 2019, 2019, 1847130. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.; Liu, S.; Ding, P.; Wang, L.; Ju, J.; Liang, G. Enhancement of oral bioavailability of magnolol by encapsulation in mixed micelles containing Pluronic F127 and L61. J. Pharm. Pharmacol. 2018, 70, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.; Shen, H.; Wang, J.; Ju, J. Improved oral bioavailability of magnolol by using a binary mixed micelle system. Artif. Cells Nanomed. Biotechnol. 2018, 46, 668–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefanache, A.; Ignat, M.; Peptu, C.; Diaconu, A.; Stoleriu, I.; Ochiuz, L. Development of a prolonged-release drug delivery system with magnolol loaded in amino-functionalized Mesoporous Silica. Appl. Sci. 2017, 7, 237. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.J.; Chien, Y.C.; Wu, C.H.; Liu, D.M. Magnolol-loaded core-shell hydrogel nanoparticles: Drug release, intracellular uptake, and controlled cytotoxicity for the inhibition of migration of vascular smooth muscle cells. Mol. Pharm. 2011, 8, 2339–2349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.-W.; Hu, S.C.-S.; Yen, F.-L.; Hsu, L.-F.; Lee, I.-T.; Lin, Z.-C.; Tsai, M.-H.; Huang, C.-L.; Liang, C.-J.; Chiang, Y.-C. Magnolol nanoparticles exhibit improved water solubility and suppress TNF-α-induced VCAM-1 expression in endothelial cells. J. Biomed. Nanotechnol. 2017, 13, 255–268. [Google Scholar] [CrossRef]
- Lin, S.P.; Hou, Y.C.; Liao, T.Y.; Tsai, S.Y. Enhancing the bioavailability of magnolol in rabbits using melting solid dispersion with polyvinylpyrrolidone. Drug Dev. Ind. Pharm. 2014, 40, 330–337. [Google Scholar] [CrossRef]
- Wang, W.; Cui, C.; Li, M.; Zhang, Z.; Lv, H. Study of a novel disintegrable oleanolic acid-polyvinylpolypyrrolidone solid dispersion. Drug Dev. Ind. Pharm. 2017, 43, 1178–1185. [Google Scholar] [CrossRef]
- Chen, C.Y.-C.; Wu, C.-H. Magnolol encapsulated by liposome in inhibiting smooth muscle cell proliferation. J. Chin. Chem. Soc. 2008, 55, 517–521. [Google Scholar] [CrossRef]
- Gangu, K.K.; Maddila, S.; Mukkamala, S.B.; Jonnalagadda, S.B. A review on contemporary metal-organic framework materials. Inorg. Chim. Acta 2016, 446, 61–74. [Google Scholar] [CrossRef]
- Janiak, C.; Vieth, J.K. MOFs, MILs and more: Concepts, properties and applications for porous coordination networks (PCNs). New J. Chem. 2010, 34, 2366. [Google Scholar] [CrossRef]
- Seth, S.; Matzger, A.J. Metal-organic frameworks: Examples, counterexamples, and an actionable definition. Cryst. Growth Des. 2017, 17, 4043–4048. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, M.; Xie, Z. Nanoscale metal-organic frameworks for drug delivery: A conventional platform with new promise. J. Mater. Chem. B 2018, 6, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Shearer, G.C.; Chavan, S.; Bordiga, S.; Svelle, S.; Olsbye, U.; Lillerud, K.P. Defect engineering: Tuning the porosity and composition of the metal–organic framework UiO-66 via modulated synthesis. Chem. Mater. 2016, 28, 3749–3761. [Google Scholar] [CrossRef]
- Cochran, K.W.; Doull, J.; Mazur, M.; Dubois, K.P. Acute toxicity of zirconium, columbium, strontium, lanthanum, cesium, tantalum and yttrium. Arch Ind. Hyg. Occup. Med. 1950, 1, 637–650. [Google Scholar] [PubMed]
- Ghosh, S.; Sharma, A.; Talukder, G. Zirconium: An abnormal trace element in biology. Biol. Trace Elem. Res. 1992, 35, 247–271. [Google Scholar] [CrossRef]
- Orellana-Tavra, C.; Mercado, S.A.; Fairen-Jimenez, D. Endocytosis mechanism of nano metal-organic frameworks for drug delivery. Adv. Healthc. Mater. 2016, 5, 2261–2270. [Google Scholar] [CrossRef] [Green Version]
- Abánades-Lázaro, I.; Wells, C.J.R.; Forgan, R.S. Multivariate modulation of the Zr MOF UiO-66 for defect-controlled combination anticancer drug delivery. Angew. Chem. Int. Ed. 2020. [Google Scholar] [CrossRef]
- Dong, H.; Yang, G.; Zhang, X.; Meng, X.; Sheng, J.; Sun, X.; Feng, Y.; Zhang, F. Folic acid functionalized zirconium-based metal-organic frameworks as drug carriers for active tumor-targeted drug delivery. Chem. Eur. J. 2018, 24, 17148–17154. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, S.; Wang, H.; Peng, Y.; Tan, Z.; Tang, B. Functional groups influence and mechanism research of UiO-66-type metal-organic frameworks for ketoprofen delivery. Colloids Surf. B Biointerfaces 2019, 178, 1–7. [Google Scholar] [CrossRef]
- Nasrabadi, M.; Ghasemzadeh, M.A.; Zand Monfared, M.R. The preparation and characterization of UiO-66 metal-organic frameworks for the delivery of the drug ciprofloxacin and an evaluation of their antibacterial activities. New J. Chem. 2019, 43, 16033–16040. [Google Scholar] [CrossRef]
- Ahmed, I.; Tong, M.; Jun, J.W.; Zhong, C.; Jhung, S.H. Adsorption of nitrogen-containing compounds from model fuel over sulfonated metal-organic framework: Contribution of hydrogen-bonding and acid-base interactions in adsorption. J. Phys. Chem. C 2016, 120, 407–415. [Google Scholar] [CrossRef]
- Bellido, E.; Hidalgo, T.; Lozano, M.V.; Guillevic, M.; Simón-Vázquez, R.; Santander-Ortega, M.J.; González-Fernández, Á.; Serre, C.; Alonso, M.J.; Horcajada, P. Heparin-engineered mesoporous iron metal-organic framework nanoparticles: Toward stealth drug nanocarriers. Adv. Healthc. Mater. 2015, 4, 1246–1257. [Google Scholar] [CrossRef] [PubMed]
- Singco, B.; Liu, L.-H.; Chen, Y.-T.; Shih, Y.-H.; Huang, H.-Y.; Lin, C.-H. Approaches to drug delivery: Confinement of aspirin in MIL-100 (Fe) and aspirin in the de novo synthesis of metal-organic frameworks. Microporous Mesoporous Mater. 2016, 223, 254–260. [Google Scholar] [CrossRef]
- Al Haydar, M.; Abid, H.; Sunderland, B.; Wang, S. Metal organic frameworks as a drug delivery system for flurbiprofen. Drug Des. Dev. Ther. 2017, 11, 2685–2695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, B.; Wang, X.; Zhang, Q.; Xi, X.; Cai, J.; Qi, H.; Shi, S.; Wang, J.; Yuan, D.; Fang, M. Synthesis and characterization of the interpenetrated MOF-5. J. Mater. Chem. 2010, 20, 3758. [Google Scholar] [CrossRef]
- Danhier, F.; Lecouturier, N.; Vroman, B.; Jérôme, C.; Marchand-Brynaert, J.; Feron, O.; Préat, V. Paclitaxel-loaded PEGylated PLGA-based nanoparticles: In vitro and in vivo evaluation. J. Control. Release 2009, 133, 11–17. [Google Scholar] [CrossRef]
- Baishya, H. Application of mathematical models in drug release kinetics of carbidopa and levodopa ER tablets. J. Dev. Drugs 2017, 6. [Google Scholar] [CrossRef]
- Organization for Economic Cooperation and Development. Test no. 425: Acute oral toxicity: Up-and-down procedure. In OECD Guidelines for the Testing of Chemicals, 4th ed.; OECD Publishing: Paris, France, 2008; ISBN 9789264071049. [Google Scholar]
- Higashi, Y. Simultaneous analysis of honokiol and magnolol in rat serum by HPLC with fluorescence detection after solid-phase extraction for pharmacokinetic studies. Austin J. Anal. Pharm. Chem. 2015, 2, 1–5. [Google Scholar]
- Kotani, A.; Kojima, S.; Hakamata, H.; Jin, D.; Kusu, F. Determination of honokiol and magnolol by micro HPLC with electrochemical detection and its application to the distribution analysis in branches and leaves of Magnolia obovata. Chem. Pharm. Bull. 2005, 53, 319–322. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.-P.; Tsai, S.-Y.; Chao, L.P.-D.; Chen, Y.-C.; Hou, Y.-C. Pharmacokinetics, bioavailability, and tissue distribution of magnolol following single and repeated dosing of magnolol to rats. Planta Med 2011, 77, 1800–1805. [Google Scholar] [CrossRef] [Green Version]
- Kurisingal, J.F.; Babu, R.; Kim, S.-H.; Li, Y.X.; Chang, J.-S.; Cho, S.J.; Park, D.-W. Microwave-induced synthesis of a bimetallic charge-transfer metal organic framework: A promising host for the chemical fixation of CO2. Catal. Sci. Technol. 2018, 8, 591–600. [Google Scholar] [CrossRef]
- Miri, B.; Motakef-Kazemi, N.; Shojaosadati, S.A.; Morsali, A. Application of a nanoporous metal organic framework based on iron carboxylate as drug delivery system. Iran J. Pharm. Res. 2018, 17, 1164–1171. [Google Scholar] [PubMed]
- Li, Z.; Peng, Y.; Xia, X.; Cao, Z.; Deng, Y.; Tang, B. Sr/PTA metal organic framework as a drug delivery system for osteoarthritis treatment. Sci. Rep. 2019, 9, 17570. [Google Scholar] [CrossRef] [PubMed]
- Rojas, S.; Colinet, I.; Cunha, D.; Hidalgo, T.; Salles, F.; Serre, C.; Guillou, N.; Horcajada, P. Toward understanding drug incorporation and delivery from biocompatible metal-organic frameworks in view of cutaneous administration. ACS Omega 2018, 3, 2994–3003. [Google Scholar] [CrossRef] [PubMed]
- Abrams, D.B.; Turner, J.R.; Baumann, L.C.; Karel, A.; Collins, S.E.; Witkiewitz, K.; Fulmer, T.; Tanenbaum, M.L.; Commissariat, P.; Kupperman, E.; et al. Area under the curve (AUC). In Encyclopedia of Behavioral Medicine; Gellman, M.D., Turner, J.R., Eds.; Springer: New York, NY, USA, 2013; pp. 125–126. ISBN 978-1-4419-1004-2. [Google Scholar]
- Isaeva, V.I.; Kustov, L.M. The application of metal-organic frameworks in catalysis (Review). Pet. Chem. 2010, 50, 167–180. [Google Scholar] [CrossRef]
- Giménez-Marqués, M.; Hidalgo, T.; Serre, C.; Horcajada, P. Nanostructured metal-organic frameworks and their bio-related applications. Coord. Chem. Rev. 2016, 307, 342–360. [Google Scholar] [CrossRef]
- Wittmann, T.; Tschense, C.B.L.; Zappe, L.; Koschnick, C.; Siegel, R.; Stäglich, R.; Lotsch, B.V.; Senker, J. Selective host-guest interactions in metal-organic frameworks via multiple hydrogen bond donor-acceptor recognition sites. J. Mater. Chem. A 2019, 7, 10379–10388. [Google Scholar] [CrossRef]
- Liu, J.; Canfield, N.; Liu, W. Preparation and characterization of a hydrophobic metal-organic framework membrane supported on a thin porous metal sheet. Ind. Eng. Chem. Res. 2016, 55, 3823–3832. [Google Scholar] [CrossRef]
- Sun, W.; Li, H.; Li, H.; Li, S.; Cao, X. Adsorption mechanisms of ibuprofen and naproxen to UiO-66 and UiO-66-NH2: Batch experiment and DFT calculation. Chem. Eng. J. 2019, 360, 645–653. [Google Scholar] [CrossRef]
- Cunha, D.; Ben Yahia, M.; Hall, S.; Miller, S.R.; Chevreau, H.; Elkaïm, E.; Maurin, G.; Horcajada, P.; Serre, C. Rationale of drug encapsulation and release from biocompatible porous metal-organic frameworks. Chem. Mater. 2013, 25, 2767–2776. [Google Scholar] [CrossRef]
- Chen, D.; Yang, D.; Dougherty, C.A.; Lu, W.; Wu, H.; He, X.; Cai, T.; van Dort, M.E.; Ross, B.D.; Hong, H. In vivo targeting and positron emission tomography imaging of tumor with intrinsically radioactive metal-organic frameworks nanomaterials. ACS Nano 2017, 11, 4315–4327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Gu, J.; Wang, Y.; Li, B.; Li, Y.; Zhao, W.; Shi, J. Inherent anchorages in UiO-66 nanoparticles for efficient capture of alendronate and its mediated release. Chem. Commun. 2014, 50, 8779–8782. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.; Kao, C.-Y.; Chou, C.-L.; Liu, L.-C.; Chou, T.-C. Protective effect of magnolol-loaded polyketal microparticles on lipopolysaccharide-induced acute lung injury in rats. J. Microencapsul. 2016, 33, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Song, H.; Cui, Y.; Deng, Y.; Chen, X. Magnolol entrapped ultra-fine fibrous mats electrospun from poly (ethylene glycol)-b-poly (L-lactide) and in vitro release. Chin. J. Polym. Sci. 2011, 29, 173–179. [Google Scholar] [CrossRef]
- De Coste, J.B.; Peterson, G.W.; Jasuja, H.; Glover, T.G.; Huang, Y.; Walton, K.S. Stability and degradation mechanisms of metal-organic frameworks containing the Zr6O4(OH)4 secondary building unit. J. Mater. Chem. A 2013, 1, 5642. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Qin, C.; Wang, X.-L.; Yang, G.-S.; Shao, K.-Z.; Lan, Y.-Q.; Su, Z.-M.; Huang, P.; Wang, C.-G.; Wang, E.-B. Zeolitic imidazolate framework-8 as efficient pH-sensitive drug delivery vehicle. Dalton Trans. 2012, 41, 6906. [Google Scholar] [CrossRef]
- Orellana-Tavra, C.; Marshall, R.J.; Baxter, E.F.; Lázaro, I.A.; Tao, A.; Cheetham, A.K.; Forgan, R.S.; Fairen-Jimenez, D. Drug delivery and controlled release from biocompatible metal-organic frameworks using mechanical amorphization. J. Mater. Chem. B 2016, 4, 7697–7707. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Thakur, K.; Kush, P.; Jain, U.K. Docetaxel loaded chitosan nanoparticles: Formulation, characterization and cytotoxicity studies. Int. J. Biol. Macromol. 2014, 69, 546–553. [Google Scholar] [CrossRef]
- Danyuo, Y.; Ani, C.J.; Salifu, A.A.; Obayemi, J.D.; Dozie-Nwachukwu, S.; Obanawu, V.O.; Akpan, U.M.; Odusanya, O.S.; Abade-Abugre, M.; McBagonluri, F.; et al. Anomalous release kinetics of prodigiosin from poly-N-isopropyl-acrylamid based hydrogels for the treatment of triple negative breast cancer. Sci. Rep. 2019, 9, 3862. [Google Scholar] [CrossRef] [Green Version]
- Peppas, N.A. Analysis of fickian and non-fickian drug release from polymers. Pharm. Acta Helv. 1985, 60, 110–111. [Google Scholar]
- Klech, C.M.; Simonelli, A.P. Examination of the moving boundaries associated with non-fickian water swelling of glassy gelatin beads: Effect of solution pH. J. Membr. Sci. 1989, 43, 87–101. [Google Scholar] [CrossRef]
- Low, J.J.; Benin, A.I.; Jakubczak, P.; Abrahamian, J.F.; Faheem, S.A.; Willis, R.R. Virtual high throughput screening confirmed experimentally: Porous coordination polymer hydration. J. Am. Chem. Soc. 2009, 131, 15834–15842. [Google Scholar] [CrossRef] [PubMed]
- Antwi-Baah, R.; Liu, H. Recent hydrophobic metal-organic frameworks and their applications. Materials 2018, 11, 2250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvey, J.A.; Pearce, C.J.; Hall, M.G.; Bruni, E.J.; De Coste, J.B.; Sava-Gallis, D.F. Insights into the solvent-assisted degradation of organophosphorus compounds by a Zr-based metal–organic framework. Dalton Trans. 2019, 48, 16153–16157. [Google Scholar] [CrossRef] [PubMed]
- Brotzel, F.; Mayr, H. Nucleophilicities of amino acids and peptides. Org. Biomol. Chem. 2007, 5, 3814. [Google Scholar] [CrossRef]
- Kalmutzki, M.J.; Hanikel, N.; Yaghi, O.M. Secondary building units as the turning point in the development of the reticular chemistry of MOFs. Sci. Adv. 2018, 4, 9180. [Google Scholar] [CrossRef] [Green Version]
- Schaate, A.; Roy, P.; Godt, A.; Lippke, J.; Waltz, F.; Wiebcke, M.; Behrens, P. Modulated synthesis of Zr-based metal-organic frameworks: From nano to single crystals. Chemistry 2011, 17, 6643–6651. [Google Scholar] [CrossRef]


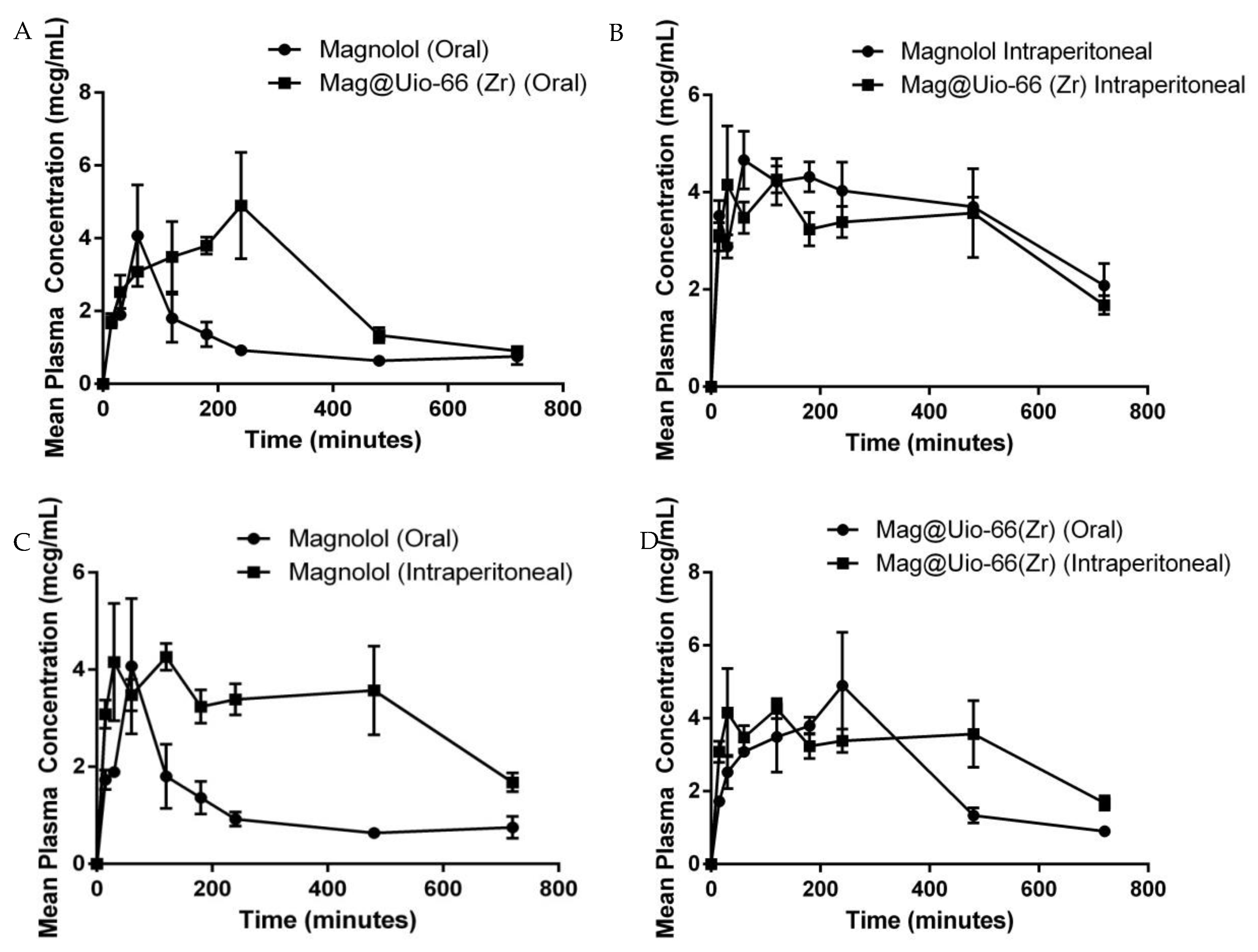
| Test Compound | Parameters | |||||
|---|---|---|---|---|---|---|
| AUC0–720 (µg/mL min) | AUC0–∞ (µg/mL min) | Tmax (min) | Cmax (µg/mL) | T1/2 (min) | Abs T1/2 (min) | |
| Magnolol PO | 823.3 ± 139.10 | 903.97 ± 140.09 | 55.77 ± 4.17 | 2.57 ± 0.26 | 100 ± 20.40 | 23.26 ± 3.16 |
| Mag@Uio-66(Zr) PO | 1823 ± 167.31 * | 2099.95 ± 148.48 * | 196.97 ± 17.38 * | 3.77 ± 0.33 * | 206.21 ± 27.95 * | 118.92 ± 6.22 * |
| Magnolol IP | 2582.67 ± 150.48 | 4016.90 ± 535.62 | 64.06 ± 6.88 | 5.10 ± 0.65 | 460.88 ± 37.41 | 17.14 ± 3.63 |
| Mag@Uio-66(Zr) IP | 2312.67 ± 253.76 | 3831.72 ± 451.57 | 114.27 ± 7.09 ** | 5.65 ± 2.41 | 606.35 ± 114.37 | 33.26 ± 4.09 ** |
| Test Compound | Organs | ||
|---|---|---|---|
| Brain (µg/g) | Liver (µg/g) | Kidneys (µg/g) | |
| Magnolol | 0.374 ± 0.022 | 0.79 ± 0.18 | 3.74 ± 0.89 |
| Mag@Uio-66(Zr) | 0.413 ± 0.034 | 1.07 ± 0.19 | 1.82 ± 0.21 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, J.H.; Quimque, M.T.J.; Macabeo, A.P.G.; Corpuz, M.J.-A.T.; Wang, Y.-M.; Lu, T.-T.; Lin, C.-H.; Villaflores, O.B. Enhanced Oral Bioavailability of the Pharmacologically Active Lignin Magnolol via Zr-Based Metal Organic Framework Impregnation. Pharmaceutics 2020, 12, 437. https://doi.org/10.3390/pharmaceutics12050437
Santos JH, Quimque MTJ, Macabeo APG, Corpuz MJ-AT, Wang Y-M, Lu T-T, Lin C-H, Villaflores OB. Enhanced Oral Bioavailability of the Pharmacologically Active Lignin Magnolol via Zr-Based Metal Organic Framework Impregnation. Pharmaceutics. 2020; 12(5):437. https://doi.org/10.3390/pharmaceutics12050437
Chicago/Turabian StyleSantos, Joshua H., Mark Tristan J. Quimque, Allan Patrick G. Macabeo, Mary Jho-Anne T. Corpuz, Yun-Ming Wang, Tsai-Te Lu, Chia-Her Lin, and Oliver B. Villaflores. 2020. "Enhanced Oral Bioavailability of the Pharmacologically Active Lignin Magnolol via Zr-Based Metal Organic Framework Impregnation" Pharmaceutics 12, no. 5: 437. https://doi.org/10.3390/pharmaceutics12050437
APA StyleSantos, J. H., Quimque, M. T. J., Macabeo, A. P. G., Corpuz, M. J.-A. T., Wang, Y.-M., Lu, T.-T., Lin, C.-H., & Villaflores, O. B. (2020). Enhanced Oral Bioavailability of the Pharmacologically Active Lignin Magnolol via Zr-Based Metal Organic Framework Impregnation. Pharmaceutics, 12(5), 437. https://doi.org/10.3390/pharmaceutics12050437