MERS-CoV Spike Protein Vaccine and Inactivated Influenza Vaccine Formulated with Single Strand RNA Adjuvant Induce T-Cell Activation through Intranasal Immunization in Mice
Abstract
:1. Introduction
2. Materials and Methods
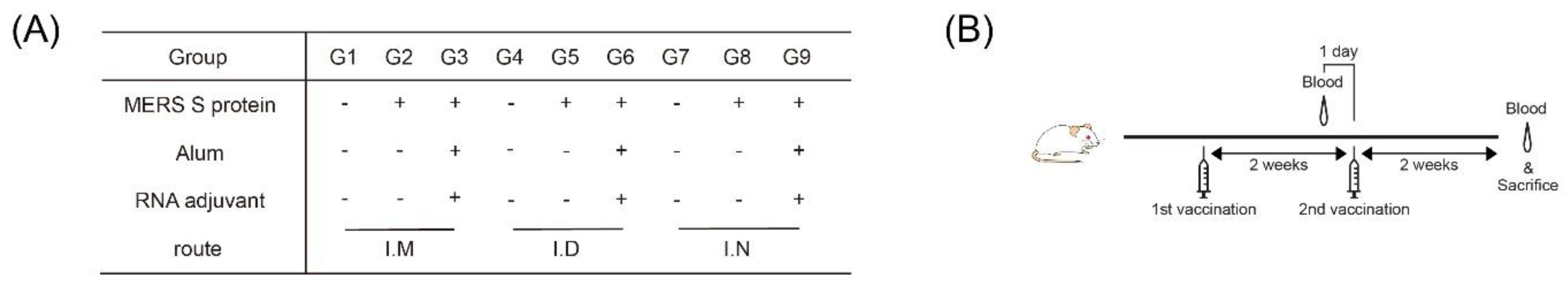
2.1. Mice
2.2. Vaccines
2.3. In Vitro Transcription and RNA Purification
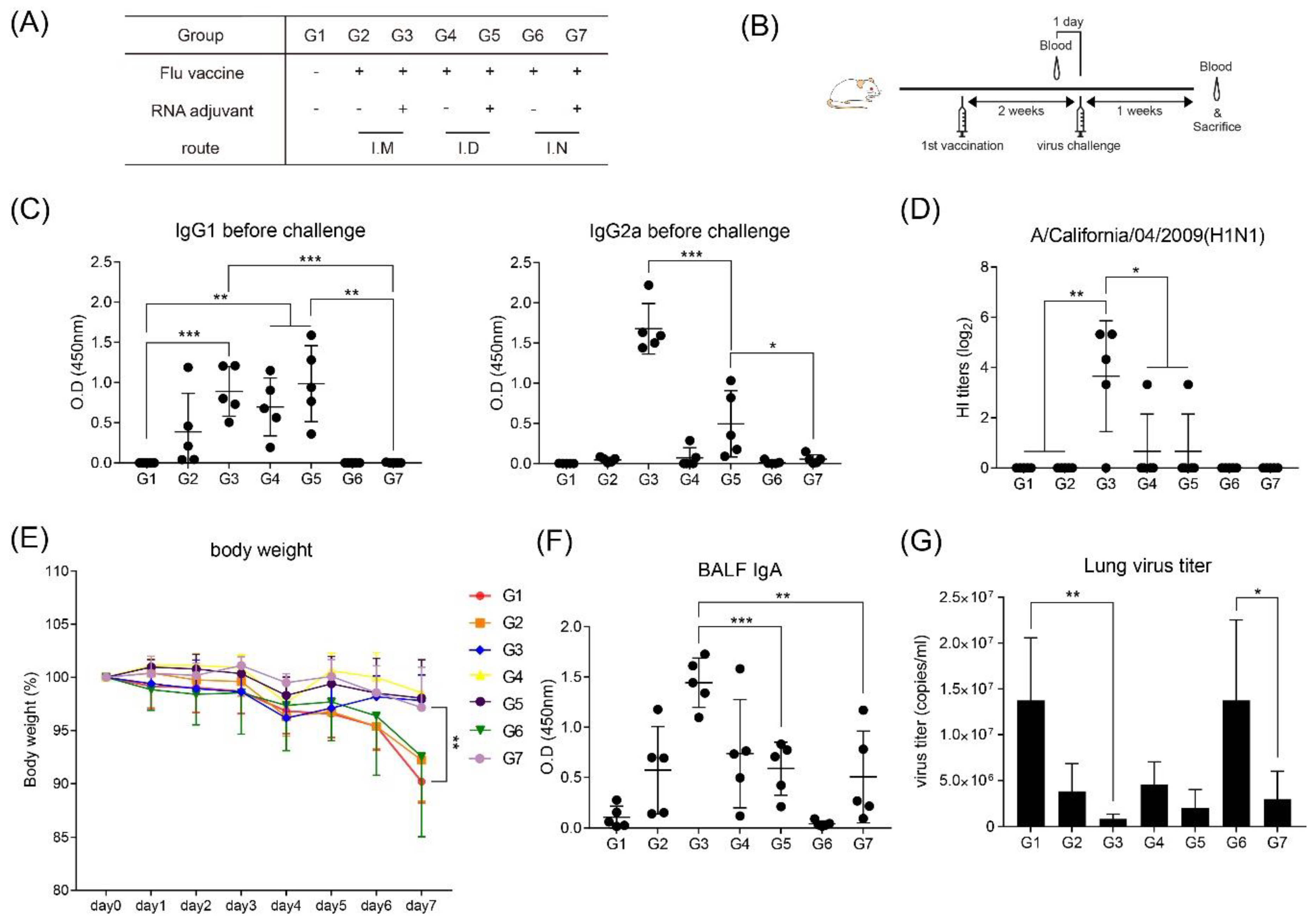
2.4. Immunization
2.5. Challenge with Influenza Virus
2.6. Serum Collection
2.7. Bronchoalveolar Lavage Fluid (BALF) Collection
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Plaque-Reduction Neutralization Test for Middle East Respiratory Syndrome Coronavirus (MERS-CoV)
2.10. Enzyme-Linked Immunospot (ELISPOT)
2.11. Flow Cytometry
2.12. Real-Time PCR for Virus Titration
2.13. Statistical Analysis
3. Results
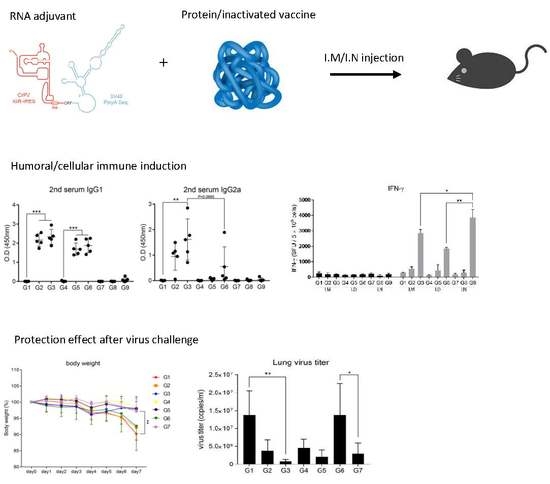
3.1. Intramuscular Inoculation with RNA Adjuvant Formulated Protein Vaccine is Effective to Induce the Humoral Immune Response
3.2. Intramuscular Inoculation is the Most Effective to Induce NAb, Regardless of Vaccine Components
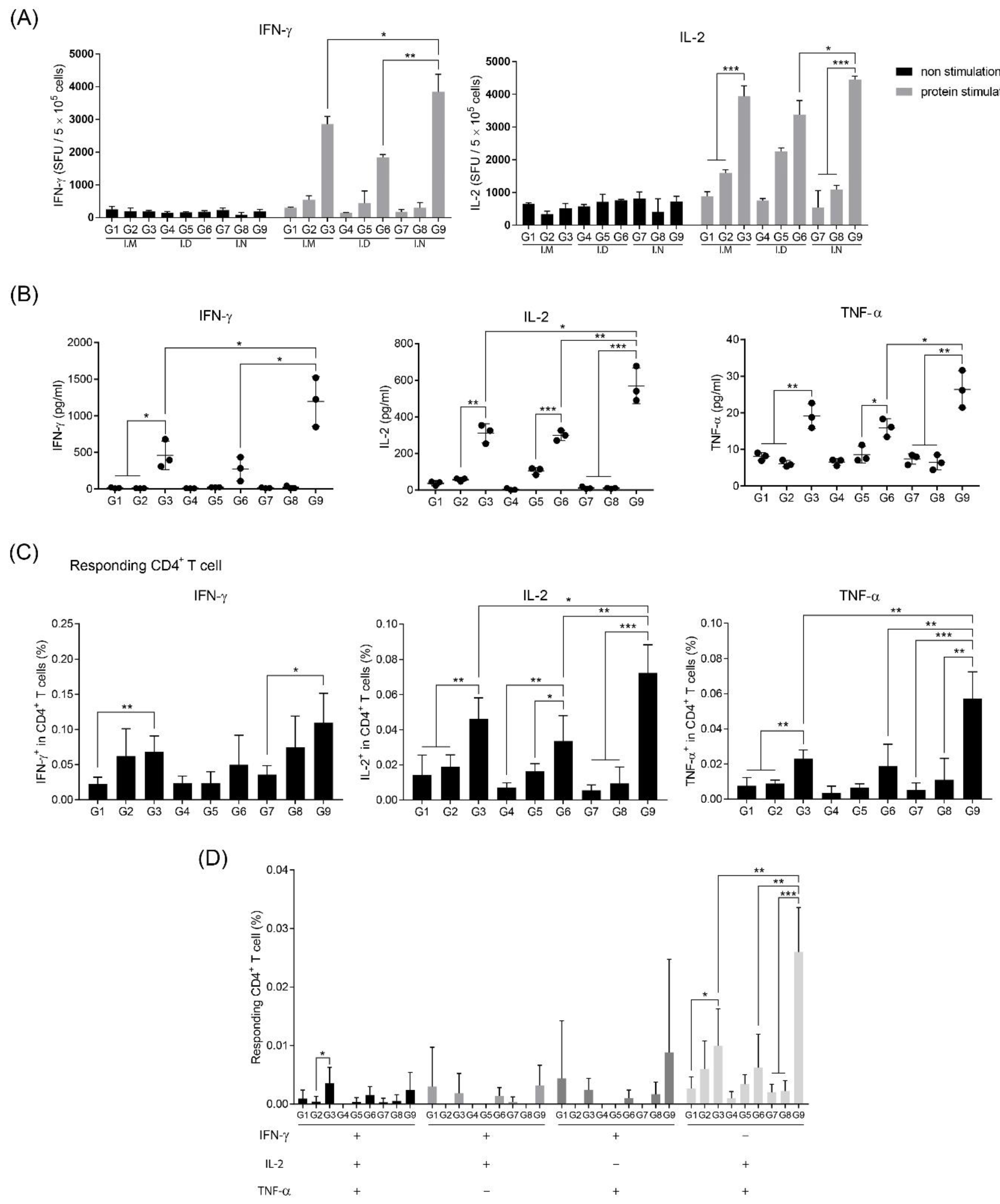
3.3. Intranasal Immunization of MERS S Protein Enhances CD4+ T-Cell Function
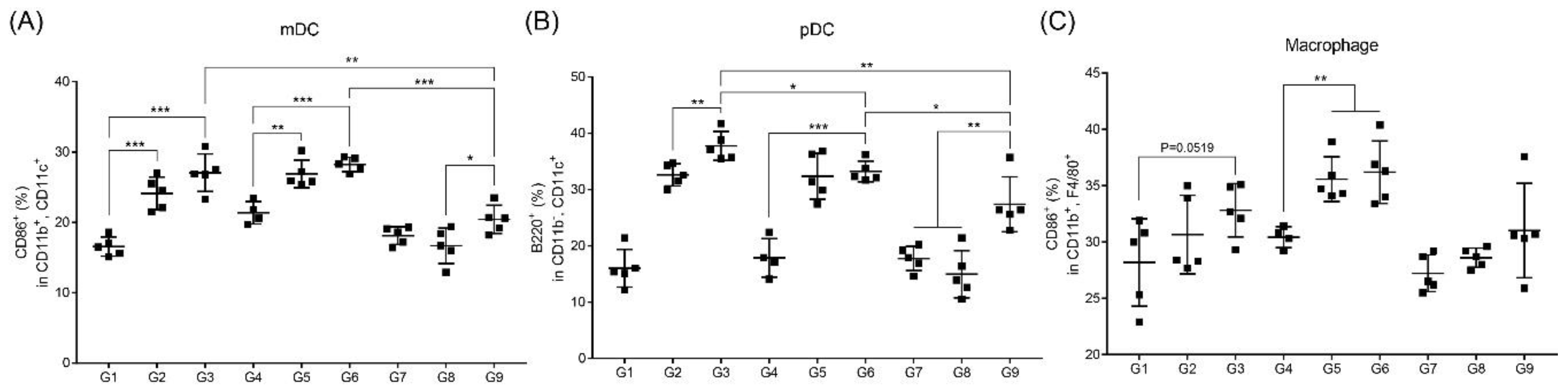
3.4. RNA Adjuvant Induces Antigen-Presenting Cells, Especially Dendritic Cells
3.5. RNA Adjuvant Protects Against Viruses
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Delany, I.; Rappuoli, R.; De Gregorio, E. Vaccines for the 21st century. EMBO Mol. Med. 2014, 6, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Rappuoli, R.; Pizza, M.; Del Giudice, G.; De Gregorio, E. Vaccines, new opportunities for a new society. Proc. Natl. Acad. Sci. USA 2014, 111, 12288–12293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pulendran, B.; Ahmed, R. Immunological mechanisms of vaccination. Nat. Immunol. 2011, 12, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Hardt, K.; Bonanni, P.; King, S.; Santos-Preciado, J.I.; El-Hodhod, M.; Zimet, G.D.; Preiss, S. Vaccine strategies: Optimising outcomes. Vaccine 2016, 34, 6691–6699. [Google Scholar] [CrossRef] [Green Version]
- Criscuolo, E.; Caputo, V.; Diotti, R.A.; Sautto, G.A.; Kirchenbaum, G.A.; Clementi, N. Alternative Methods of Vaccine Delivery: An Overview of Edible and Intradermal Vaccines. J. Immunol. Res. 2019, 2019, 8303648. [Google Scholar] [CrossRef] [Green Version]
- Ensign, L.M.; Cone, R.; Hanes, J. Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barriers. Adv. Drug Deliv. Rev. 2011, 64, 557–570. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization: Vaccine Safety Basics. Available online: https://vaccine-safety-training.org/route-of-administration.html (accessed on 14 September 2019).
- Van Aalst, S.; Jansen, M.A.; Ludwig, I.S.; Van Der Zee, R.; Van Eden, W.; Broere, F. Routing dependent immune responses after experimental R848-adjuvated vaccination. Vaccine 2018, 36, 1405–1413. [Google Scholar] [CrossRef]
- Nascimento, I.; Leite, L.C. Recombinant vaccines and the development of new vaccine strategies. Braz. J. Med. Boil. Res. 2012, 45, 1102–1111. [Google Scholar] [CrossRef] [Green Version]
- Del Giudice, G.; Rappuoli, R.; Didierlaurent, A.M. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin. Immunol. 2018, 39, 14–21. [Google Scholar] [CrossRef]
- Kool, M.; Fierens, K.; Lambrecht, B.N. Alum adjuvant: Some of the tricks of the oldest adjuvant. J. Med. Microbiol. 2012, 61, 927–934. [Google Scholar] [CrossRef] [Green Version]
- He, P.; Zou, Y.; Hu, Z. Advances in aluminum hydroxide-based adjuvant research and its mechanism. Hum. Vaccines Immunother. 2015, 11, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Lofano, G.; Mancini, F.; Salvatore, G.; Cantisani, R.; Monaci, E.; Carrisi, C.; Tavarini, S.; Sammicheli, C.; Paccani, S.R.; Soldaini, E.; et al. Oil-in-Water Emulsion MF59 Increases Germinal Center B Cell Differentiation and Persistence in Response to Vaccination. J. Immunol. 2015, 195, 1617–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, S.G.; Bertholet, S.; Coler, R.N.; Friede, M. New horizons in adjuvants for vaccine development. Trends Immunol. 2009, 30, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Xia, P.; Li, S.; Zhang, T.; Wang, T.T.; Zhu, J. RNA sensors of the innate immune system and their detection of pathogens. IUBMB Life 2017, 69, 297–304. [Google Scholar] [CrossRef]
- Jensen, S.; Thomsen, A.R. Sensing of RNA Viruses: A Review of Innate Immune Receptors Involved in Recognizing RNA Virus Invasion. J. Virol. 2012, 86, 2900–2910. [Google Scholar] [CrossRef] [Green Version]
- Kwak, H.W.; Park, H.-J.; Ko, H.L.; Park, H.; Cha, M.H.; Lee, S.-M.; Kang, K.W.; Kim, R.-H.; Ryu, S.R.; Kim, H.-J.; et al. Cricket paralysis virus internal ribosome entry site-derived RNA promotes conventional vaccine efficacy by enhancing a balanced Th1/Th2 response. Vaccine 2019, 37, 5191–5202. [Google Scholar] [CrossRef]
- Park, H.-J.; Ko, H.L.; Won, D.-H.; Hwang, D.-B.; Shin, Y.-S.; Kwak, H.-W.; Kim, H.-J.; Yun, J.-W.; Nam, J.-H. Comprehensive Analysis of the Safety Profile of a Single-Stranded RNA Nano-Structure Adjuvant. Pharmaceutics 2019, 11, 464. [Google Scholar] [CrossRef] [Green Version]
- Ko, H.L.; Park, H.-J.; Kim, J.; Kim, H.; Youn, H.; Nam, J.-H. Development of an RNA Expression Platform Controlled by Viral Internal Ribosome Entry Sites. J. Microbiol. Biotechnol. 2019, 29, 127–140. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, S. Routes of administration. In The Laboratory Mouse; Elsevier: Hannover, Germany, 2004; pp. 527–541. [Google Scholar]
- Zhu, J.; Paul, W.E. CD4 T cells: Fates, functions, and faults. Blood 2008, 112, 1557–1569. [Google Scholar] [CrossRef] [Green Version]
- Brode, S.; Macary, P. Cross-presentation: Dendritic cells and macrophages bite off more than they can chew! Immunology 2004, 112, 345–351. [Google Scholar] [CrossRef]
- Rescigno, M.; Winzler, C.; Delia, M.; Mutini, C.; Lutz, M.; Ricciardi-Castagnoli, P. Dendritic cell maturation is required for initiation of the immune response. J. Leukoc. Boil. 1997, 61, 415–421. [Google Scholar] [CrossRef] [Green Version]
- Mubarak, A.; Alturaiki, W.H.; Hemida, M.G. Middle East Respiratory Syndrome Coronavirus (MERS-CoV): Infection, Immunological Response, and Vaccine Development. J. Immunol. Res. 2019, 2019, 6491738. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Nguyen, M.T. Recent Advances of Vaccine Adjuvants for Infectious Diseases. Immune Netw. 2015, 15, 51–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baxter, D. Active and passive immunity, vaccine types, excipients and licensing. Occup. Med. 2007, 57, 552–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tregoning, J.; Russell, R.F.; Kinnear, E. Adjuvanted influenza vaccines. Hum. Vaccines Immunother. 2018, 14, 550–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, N.-H.; Lee, J.-A.; Park, S.-Y.; Song, C.-S.; Choi, I.-S.; Lee, J.-B. A review of vaccine development and research for industry animals in Korea. Clin. Exp. Vaccine Res. 2012, 1, 18–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, S.-S.; Webby, R. Traditional and New Influenza Vaccines. Clin. Microbiol. Rev. 2013, 26, 476–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perricone, C.; Colafrancesco, S.; Mazor, R.D.; Soriano, A.; Agmon-Levin, N.; Shoenfeld, Y. Autoimmune/inflammatory syndrome induced by adjuvants (ASIA) 2013: Unveiling the pathogenic, clinical and diagnostic aspects. J. Autoimmun. 2013, 47, 1–16. [Google Scholar] [CrossRef]
- Tomljenovic, L.; Shaw, C.A. Aluminum vaccine adjuvants: Are they safe? Curr. Med. Chem. 2011, 18, 2630–2637. [Google Scholar] [CrossRef]
- Ma, C.; Li, Y.; Wang, L.; Zhao, G.; Tao, X.; Tseng, C.-T.K.; Zhou, Y.; Du, L.; Jiang, S. Intranasal vaccination with recombinant receptor-binding domain of MERS-CoV spike protein induces much stronger local mucosal immune responses than subcutaneous immunization: Implication for designing novel mucosal MERS vaccines. Vaccine 2014, 32, 2100–2108. [Google Scholar] [CrossRef]
- Zuercher, A.W.; Coffin, S.E.; Thurnheer, M.C.; Fundova, P.; Cebra, J.J. Nasal-associated lymphoid tissue is a mucosal inductive site for virus-specific humoral and cellular immune responses. J. Immunol. 2002, 168, 1796–1803. [Google Scholar] [CrossRef]
- Porgador, A.; Staats, H.F.; Itoh, Y.; Kelsall, B.L. Intranasal Immunization with Cytotoxic T-Lymphocyte Epitope Peptide and Mucosal Adjuvant Cholera Toxin: Selective Augmentation of Peptide-Presenting Dendritic Cells in Nasal Mucosa-Associated Lymphoid Tissue. Infect. Immun. 1998, 66, 5876–5881. [Google Scholar] [CrossRef] [Green Version]
- Lycke, N.Y.; Bemark, M. The regulation of gut mucosal IgA B-cell responses: Recent developments. Mucosal Immunol. 2017, 10, 1361–1374. [Google Scholar] [CrossRef] [PubMed]
- Takaki, H.; Ichimiya, S.; Matsumoto, M.; Seya, T. Mucosal Immune Response in Nasal-Associated Lymphoid Tissue upon Intranasal Administration by Adjuvants. J. Innate Immun. 2018, 10, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Ogra, P.L.; Faden, H.; Welliver, R.C. Vaccination Strategies for Mucosal Immune Responses. Clin. Microbiol. Rev. 2001, 14, 430–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyaka, P.N. Inducing Mucosal IgA: A Challenge for Vaccine Adjuvants and Delivery Systems. J. Immunol. 2017, 199, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jang, Y.-S. The development of mucosal vaccines for both mucosal and systemic immune induction and the roles played by adjuvants. Clin. Exp. Vaccine Res. 2017, 6, 15–21. [Google Scholar] [CrossRef]
- Holmgren, J.; Czerkinsky, C. Mucosal immunity and vaccines. Nat. Med. 2005, 11, S45–S53. [Google Scholar] [CrossRef]
- Kang, S.; Brown, H.; Hwang, S. Direct Antiviral Mechanisms of Interferon-Gamma. Immune Netw. 2018, 18, 18. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-J.; Kwak, H.W.; Kang, K.W.; Bang, Y.-J.; Lee, Y.-S.; Park, H.-J.; Kim, J.-Y.; Park, H.-J.; Hwang, K.-A.; Lee, S.-M.; et al. MERS-CoV Spike Protein Vaccine and Inactivated Influenza Vaccine Formulated with Single Strand RNA Adjuvant Induce T-Cell Activation through Intranasal Immunization in Mice. Pharmaceutics 2020, 12, 441. https://doi.org/10.3390/pharmaceutics12050441
Kim H-J, Kwak HW, Kang KW, Bang Y-J, Lee Y-S, Park H-J, Kim J-Y, Park H-J, Hwang K-A, Lee S-M, et al. MERS-CoV Spike Protein Vaccine and Inactivated Influenza Vaccine Formulated with Single Strand RNA Adjuvant Induce T-Cell Activation through Intranasal Immunization in Mice. Pharmaceutics. 2020; 12(5):441. https://doi.org/10.3390/pharmaceutics12050441
Chicago/Turabian StyleKim, Hye-Jung, Hye Won Kwak, Kyung Won Kang, Yoo-Jin Bang, Yu-Sun Lee, Hyeong-Jun Park, Jae-Yong Kim, Hyo-Jung Park, Kyung-Ah Hwang, Sang-Myeong Lee, and et al. 2020. "MERS-CoV Spike Protein Vaccine and Inactivated Influenza Vaccine Formulated with Single Strand RNA Adjuvant Induce T-Cell Activation through Intranasal Immunization in Mice" Pharmaceutics 12, no. 5: 441. https://doi.org/10.3390/pharmaceutics12050441
APA StyleKim, H.-J., Kwak, H. W., Kang, K. W., Bang, Y.-J., Lee, Y.-S., Park, H.-J., Kim, J.-Y., Park, H.-J., Hwang, K.-A., Lee, S.-M., & Nam, J.-H. (2020). MERS-CoV Spike Protein Vaccine and Inactivated Influenza Vaccine Formulated with Single Strand RNA Adjuvant Induce T-Cell Activation through Intranasal Immunization in Mice. Pharmaceutics, 12(5), 441. https://doi.org/10.3390/pharmaceutics12050441