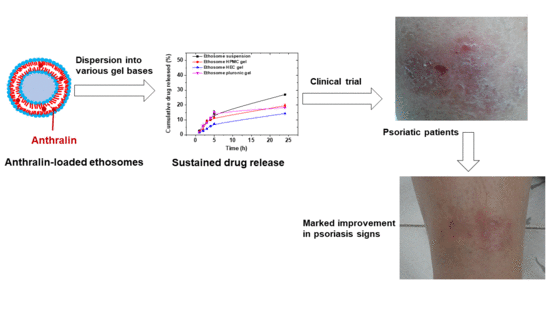
Liposomal and Ethosomal Gels for the Topical Delivery of Anthralin: Preparation, Comparative Evaluation and Clinical Assessment in Psoriatic Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Anthralin-Loaded Liposomes
2.3. Preparation of Anthralin-Loaded Ethosomes
2.4. Characterization of Anthralin-Loaded Vesicles
2.4.1. Determination of Drug Encapsulation Efficiency
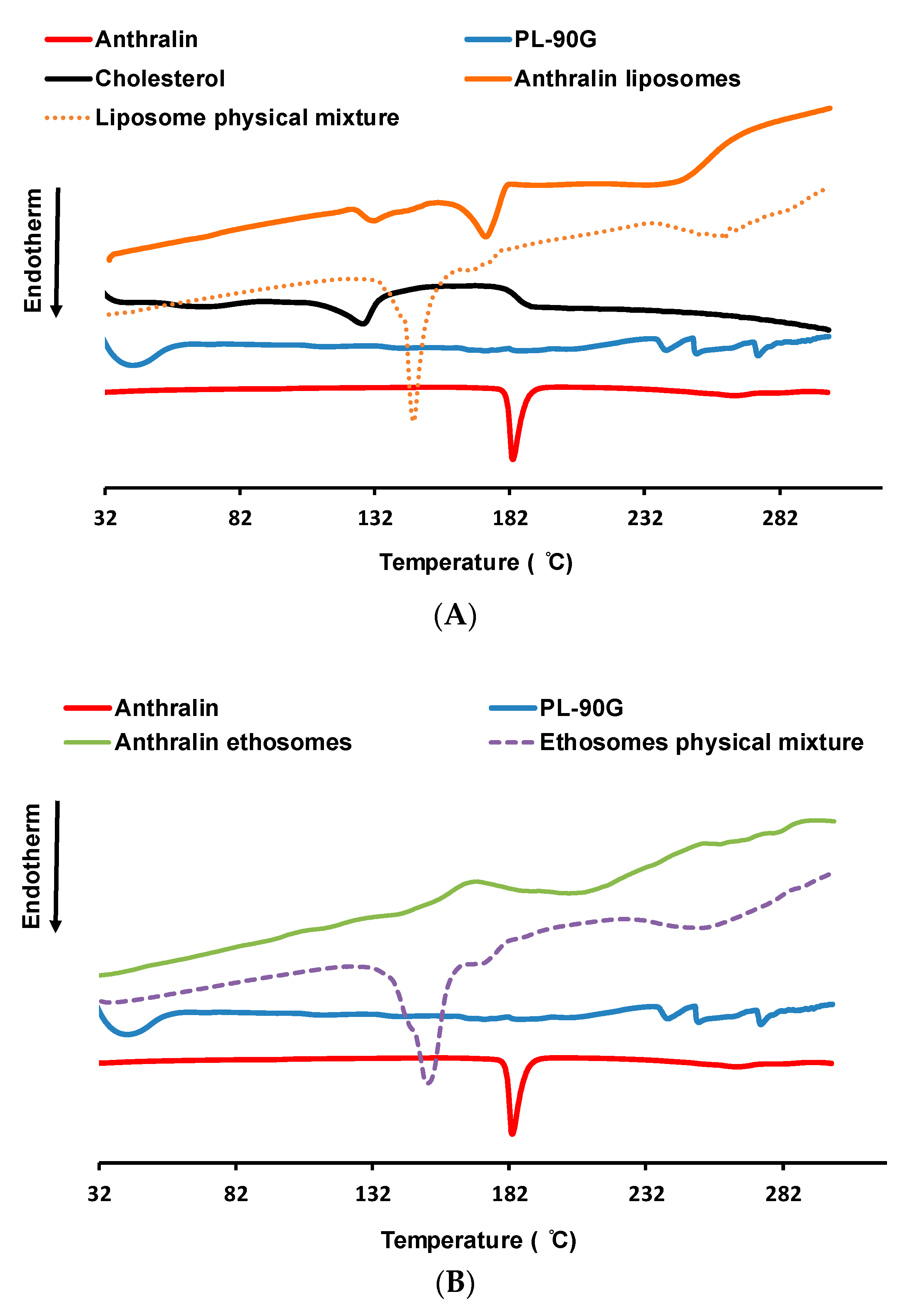
2.4.2. Thermal Analysis Studies
2.4.3. Fourier-Transform Infrared Spectroscopy (FT-IR) Studies
2.4.4. Measurement of Particle Size and Polydispersity Index
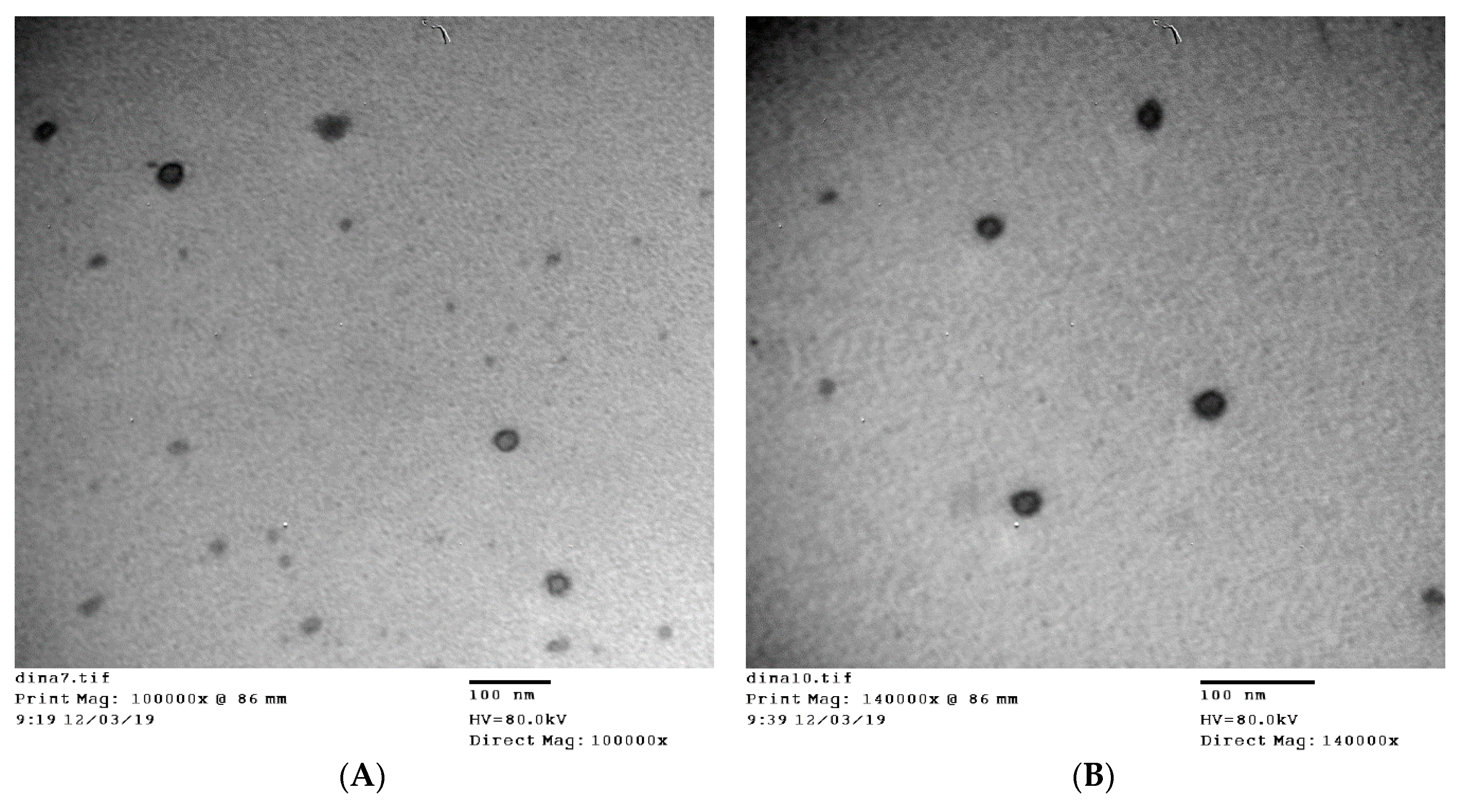
2.4.5. Transmission Electron Microscopy (TEM) Measurements
2.5. Incorporation of Anthralin-Loaded Vesicles into Different Gel Bases
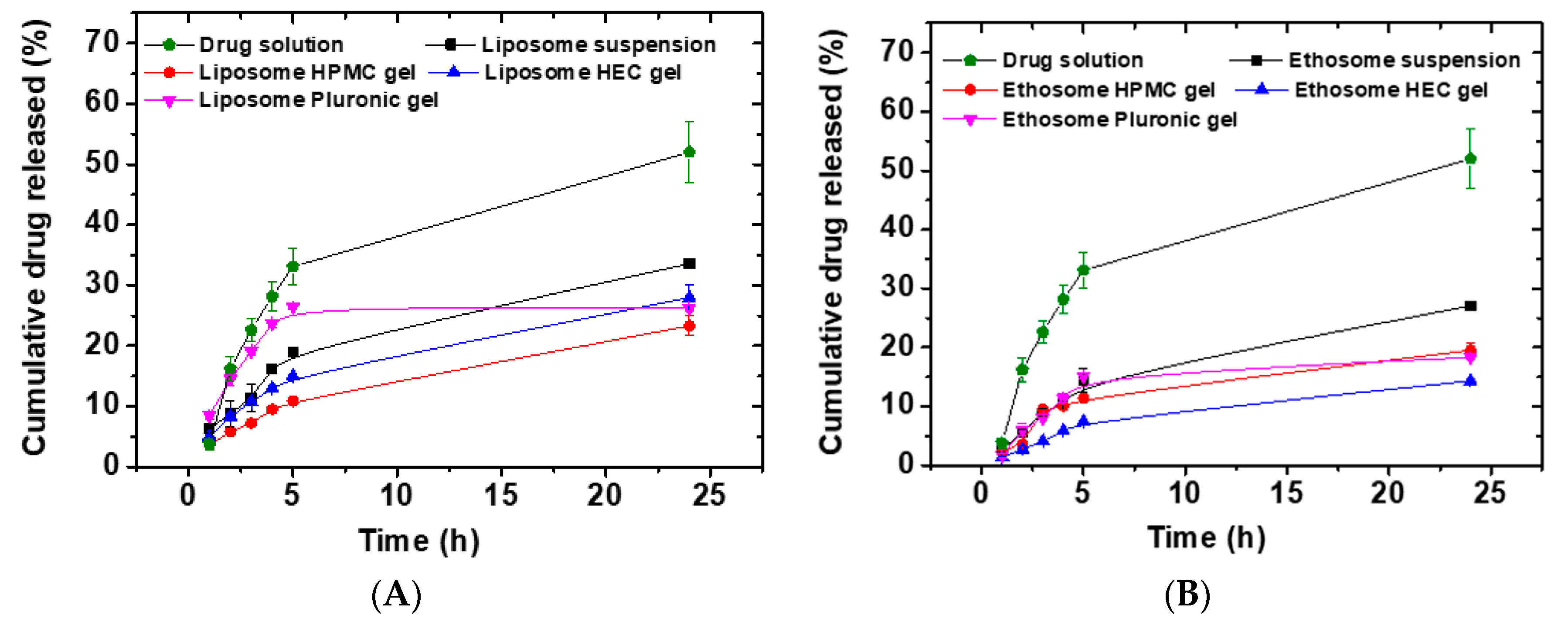
2.6. In Vitro Drug Release Studies
2.7. Kinetic Analysis of Drug Release Data
2.8. Ex Vivo Permeability Studies
2.9. Clinical Evaluation of Selected Anthralin Liposomal and Ethosomal Formulations
2.9.1. Study Design
2.9.2. Patients
2.9.3. The Application Protocol
2.9.4. Evaluation
2.10. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Evaluation of Anthralin-Loaded Liposomes
3.1.1. Effect of Drug/Lipid Ratio on Anthralin Encapsulation Efficiency
3.1.2. Effect of CH/PL-90G Ratio on Anthralin Encapsulation Efficiency
3.2. Preparation and Evaluation of Anthralin-Loaded Ethosomes
3.2.1. Effect of PL-90G Concentration on the Drug Encapsulation Efficiency
3.2.2. Effect of Ethanol Concentration on the Drug Encapsulation Efficiency
3.3. Particle Size and Polydispersity Index of Liposome and Ethosome Preparations
3.4. TEM Measurements
3.5. Thermal Analysis Studies
3.6. FT-IR Studies
3.7. In Vitro Drug Release Studies
3.8. Drug Release Kinetics
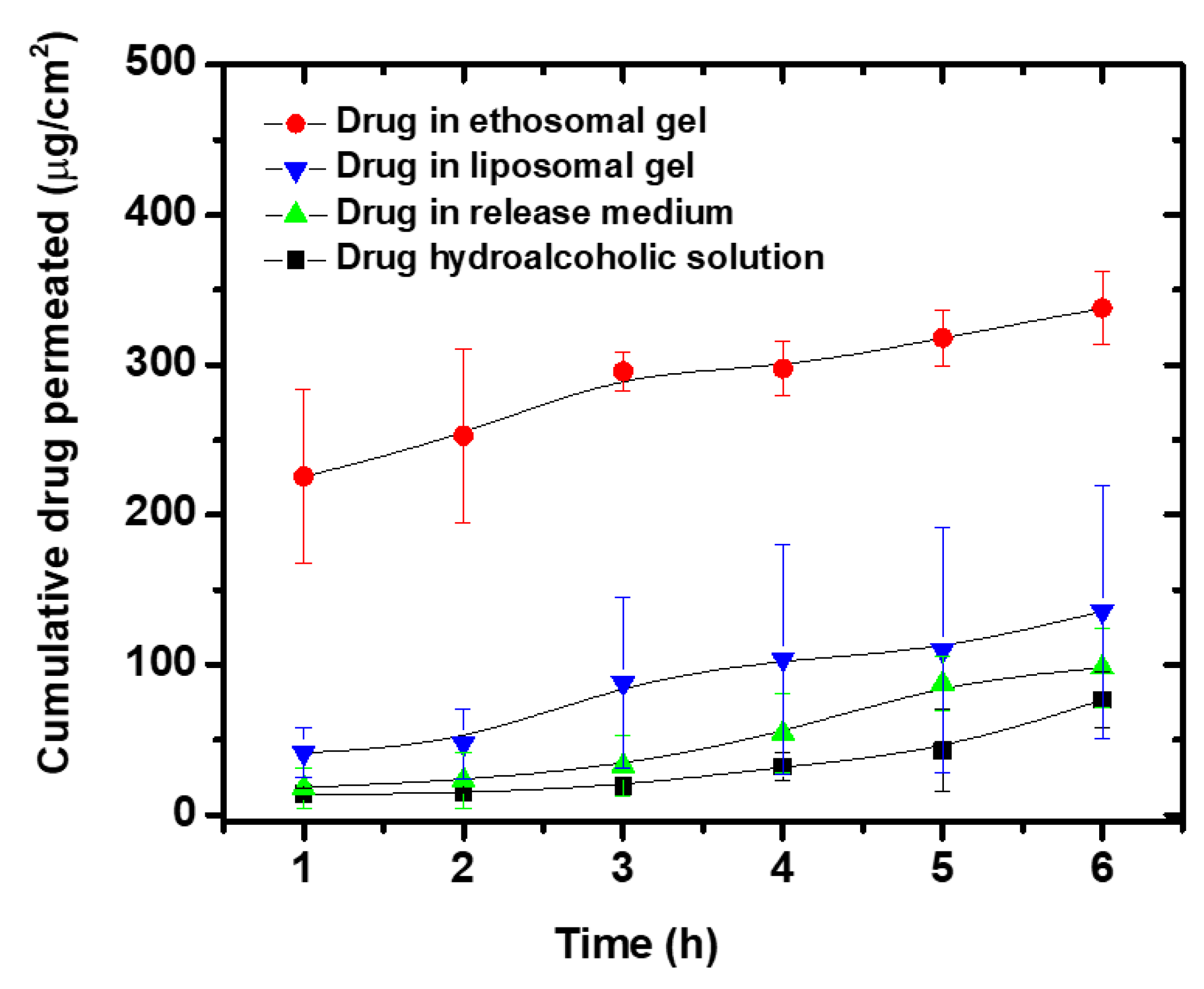
3.9. Ex Vivo Permeability Studies
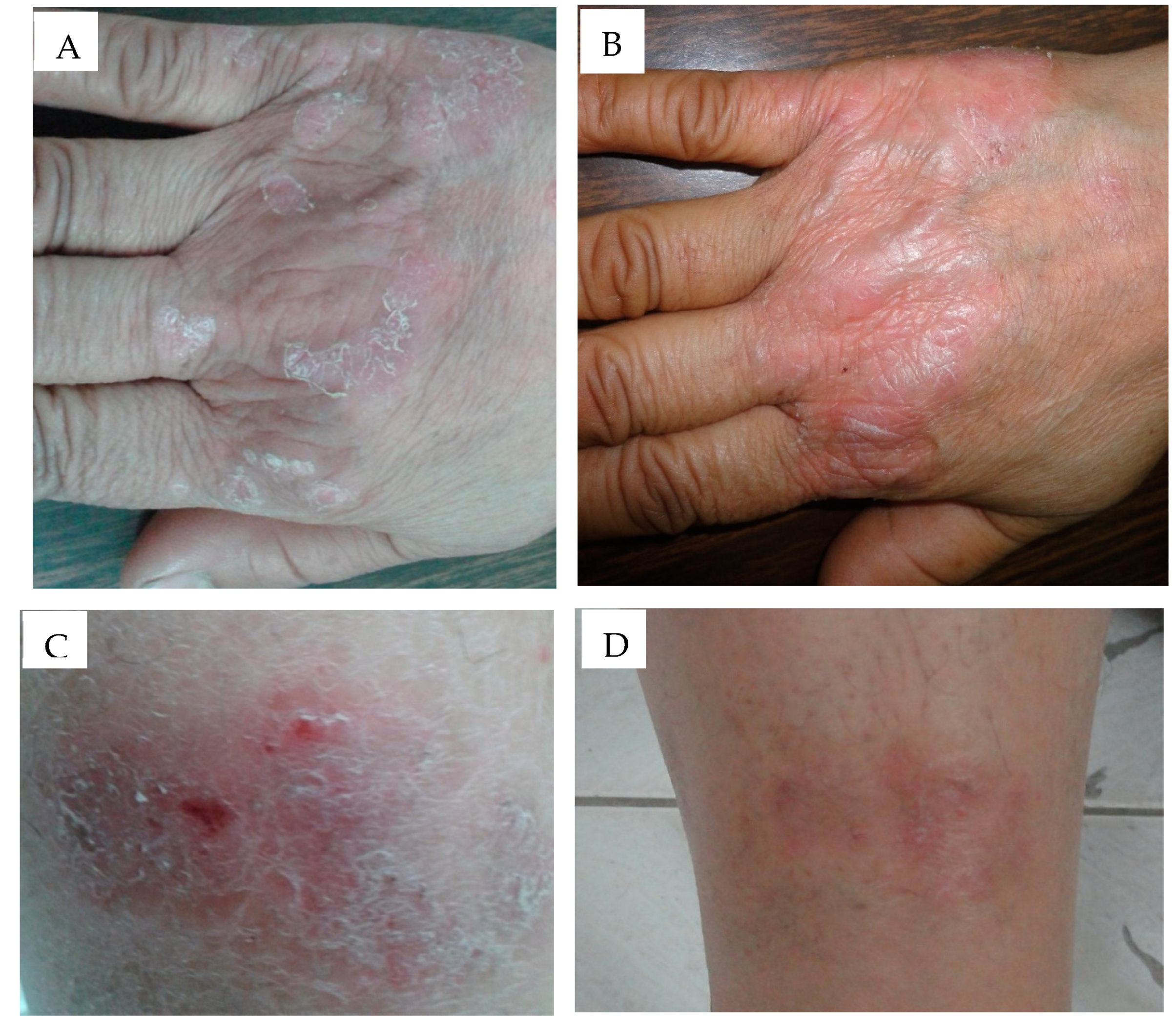
3.10. Clinical Evaluation of Selected Anthralin Liposomal and Ethosomal Formulations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tsuruta, D. NF-kappaB links keratinocytes and lymphocytes in the pathogenesis of psoriasis. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, M.; Singh, D.; Singh, M.R. Novel colloidal carriers for psoriasis: Current issues, mechanistic insight and novel delivery approaches. J. Control. Release 2013, 170, 380–395. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, W.H.; Schon, M.P. Psoriasis. Lancet 2015. [Google Scholar] [CrossRef]
- Krueger, J.G.; Bowcock, A. Psoriasis pathophysiology: Current concepts of pathogenesis. Ann. Rheum. Dis. 2005, 64 (Suppl. 2), ii30–ii36. [Google Scholar] [CrossRef]
- Koo, J. Population-based epidemiologic study of psoriasis with emphasis on quality of life assessment. Dermatol. Clin. 1996, 14, 485–496. [Google Scholar] [CrossRef]
- Peters, B.P.; Weissman, F.G.; Gill, M.A. Pathophysiology and treatment of psoriasis. Am. J. Health Syst. Pharm. 2000, 57, 645–659. [Google Scholar] [CrossRef]
- Ryan, S. Psoriasis: Characteristics, psychosocial effects and treatment options. Br. J. Nurs. 2008, 17, 284–290. [Google Scholar] [CrossRef]
- Traub, M.; Marshall, K. Psoriasis-pathophysiology, conventional, and alternative approaches to treatment. Altern. Med. Rev. 2007, 12, 319–330. [Google Scholar]
- Feely, M.A.; Smith, B.L.; Weinberg, J.M. Novel psoriasis therapies and patient outcomes, part 1: Topical medications. Cutis 2015, 95, 164–168, 170. [Google Scholar] [PubMed]
- Griffiths, C.E.; Taylor, H.; Collins, S.I.; Hobson, J.E.; Collier, P.A.; Chalmers, R.J.; Stewart, E.J.; Dey, P. The impact of psoriasis guidelines on appropriateness of referral from primary to secondary care: A randomized controlled trial. Br. J. Dermatol. 2006, 155, 393–400. [Google Scholar] [CrossRef]
- Feely, M.A.; Smith, B.L.; Weinberg, J.M. Novel psoriasis therapies and patient outcomes, part 2: Biologic treatments. Cutis 2015, 95, 282–290. [Google Scholar] [PubMed]
- Parish, L.C.; Millikan, L.E.; Witkowski, J.A. The modern story of anthralin. Int. J. Dermatol. 1989, 28, 373–374. [Google Scholar] [CrossRef] [PubMed]
- Saraswat, A.; Agarwal, R.; Katare, O.P.; Kaur, I.; Kumar, B. A randomized, double-blind, vehicle-controlled study of a novel liposomal dithranol formulation in psoriasis. J. Dermatol. Treat. 2007, 18, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Katare, O.P.; Vyas, S.P. Preparation and in vitro evaluation of liposomal/niosomal delivery systems for antipsoriatic drug dithranol. Int. J. Pharm. 2001, 228, 43–52. [Google Scholar] [CrossRef]
- Shahwal, V.K. Preformulation studies and preperation of dithranol loaded solid lipid nanoparticles. Int. J. Biomed. Res. 2012, 3, 343–350. [Google Scholar] [CrossRef] [Green Version]
- McGill, A.; Frank, A.; Emmett, N.; Turnbull, D.M.; Birch-Machin, M.A.; Reynolds, N.J. The anti-psoriatic drug anthralin accumulates in keratinocyte mitochondria, dissipates mitochondrial membrane potential, and induces apoptosis through a pathway dependent on respiratory competent mitochondria. FASEB J. 2005, 19, 1012–1014. [Google Scholar] [CrossRef] [Green Version]
- Mendonca, C.O.; Burden, A.D. Current concepts in psoriasis and its treatment. Pharmacol. Ther. 2003, 99, 133–147. [Google Scholar] [CrossRef]
- Sehgal, V.N.; Verma, P.; Khurana, A. Anthralin/dithranol in dermatology. Int. J. Dermatol. 2014, 53, e449–e460. [Google Scholar] [CrossRef]
- Gerritsen, M.J.; Boezeman, J.B.; Elbers, M.E.; van de Kerkhof, P.C. Dithranol embedded in crystalline monoglycerides combined with phototherapy (UVB): A new approach in the treatment of psoriasis. Skin Pharmacol. Appl. Skin Physiol. 1998, 11, 133–139. [Google Scholar] [CrossRef]
- Raza, K.; Negi, P.; Takyar, S.; Shukla, A.; Amarji, B.; Katare, O.P. Novel dithranol phospholipid microemulsion for topical application: Development, characterization and percutaneous absorption studies. J. Microencapsul. 2011, 28, 190–199. [Google Scholar] [CrossRef]
- Raza, K.; Katare, O.P.; Setia, A.; Bhatia, A.; Singh, B. Improved therapeutic performance of dithranol against psoriasis employing systematically optimized nanoemulsomes. J. Microencapsul. 2013, 30, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Savian, A.L.; Rodrigues, D.; Weber, J.; Ribeiro, R.F.; Motta, M.H.; Schaffazick, S.R.; Adams, A.I.; de Andrade, D.F.; Beck, R.C.; da Silva, C.B. Dithranol-loaded lipid-core nanocapsules improve the photostability and reduce the in vitro irritation potential of this drug. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 46, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, U.; Mehra, N.K.; Gupta, U.; Jain, N.K. Hyperbranched dendritic nano-carriers for topical delivery of dithranol. J. Drug Target. 2013, 21, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Dubey, V.; Mishra, D.; Dutta, T.; Nahar, M.; Saraf, D.K.; Jain, N.K. Dermal and transdermal delivery of an anti-psoriatic agent via ethanolic liposomes. J. Control. Release 2007, 123, 148–154. [Google Scholar] [CrossRef]
- Touitou, E.; Dayan, N.; Bergelson, L.; Godin, B.; Eliaz, M. Ethosomes—Novel vesicular carriers for enhanced delivery: Characterization and skin penetration properties. J. Control. Release 2000, 65, 403–418. [Google Scholar] [CrossRef]
- Dubey, V.; Mishra, D.; Jain, M.; Kumar, N. Novel methothrexate-based topical therapies for effective treatment of psoriasis. Clin. Dermatol. 2008, 24, 124–130. [Google Scholar]
- Li, G.; Fan, Y.; Fan, C.; Li, X.; Wang, X.; Li, M.; Liu, Y. Tacrolimus-loaded ethosomes: Physicochemical characterization and in vivo evaluation. Eur. J. Pharm. Biopharm. 2012, 82, 49–57. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Shen, L.N.; Wu, Z.H.; Zhao, J.H.; Feng, N.P. Comparison of ethosomes and liposomes for skin delivery of psoralen for psoriasis therapy. Int. J. Pharm. 2014, 471, 449–452. [Google Scholar] [CrossRef]
- Faisal, W.; Soliman, G.M.; Hamdan, A.M. Enhanced skin deposition and delivery of voriconazole using ethosomal preparations. J. Liposome Res. 2018, 28, 14–21. [Google Scholar] [CrossRef]
- Bhatia, A.; Kumar, R.; Katare, O.P. Tamoxifen in topical liposomes: Development, characterization and in-vitro evaluation. J. Pharm. Pharm. Sci. 2004, 7, 252–259. [Google Scholar]
- Fathalla, D.; Soliman, G.; Fouad, E. Development and in vitro/in vivo evaluation of liposomal gels for the sustained ocular delivery of latanoprost. J. Clin. Exp. Ophthalmol. 2015, 6, 2. [Google Scholar]
- Dayan, N.; Touitou, E. Carriers for skin delivery of trihexyphenidyl HCl: Ethosomes vs. liposomes. Biomaterials 2000, 21, 1879–1885. [Google Scholar] [CrossRef]
- Schmolka, I.R. Artificial skin. I. Preparation and properties of pluronic F-127 gels for treatment of burns. J. Biomed. Mater. Res. 1972, 6, 571–582. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Hixson, A.W.; Crowell, J.H. Dependence of reaction Vvelocity upon surface and agitation. Ind. Eng. Chem. 1931, 23, 923–931. [Google Scholar] [CrossRef]
- Baker, R.W.; Lonsdale, H. Controlled release: Mechanisms and rates. In Controlled Release of Biologically Active Agents; Springer: Boston, MA, USA, 1974; pp. 15–71. [Google Scholar]
- Jain, S.; Tiwary, A.K.; Sapra, B.; Jain, N.K. Formulation and evaluation of ethosomes for transdermal delivery of lamivudine. AAPS PharmSciTech 2007, 8, E111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sloan, K.B.; Beall, H.D.; Weimar, W.R.; Villanueva, R. The effect of receptor phase composition on the permeability of hairless mouse skin in diffusion cell experiments. Int. J. Pharm. 1991, 73, 97–104. [Google Scholar] [CrossRef]
- Zhang, H. Thin-film hydration followed by extrusion method for liposome preparation. Methods Mol. Biol. 2017, 1522, 17–22. [Google Scholar] [CrossRef]
- van Hoogevest, P. Review—An update on the use of oral phospholipid excipients. Eur. J. Pharm. Sci. 2017, 108, 1–12. [Google Scholar] [CrossRef]
- Rojanapanthu, P.; Sarisuta, N.; Chaturon, K.; Kraisintu, K. Physicochemical properties of amphotericin B liposomes prepared by reverse-phase evaporation method. Drug Dev. Ind. Pharm. 2003, 29, 31–37. [Google Scholar] [CrossRef]
- Kulkarni, S.B.; Betageri, G.V.; Singh, M. Factors affecting microencapsulation of drugs in liposomes. J. Microencapsul. 1995, 12, 229–246. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, S.S.; Avachat, A.M. Design and development of ethosomal transdermal drug delivery system of valsartan with preclinical assessment in Wistar albino rats. J. Liposome Res. 2013, 23, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, R.G.; Tekade, R.K.; Sharma, P.A.; Darwhekar, G.; Tyagi, A.; Patel, R.P.; Jain, D.K. Ethosomes and ultradeformable liposomes for transdermal delivery of clotrimazole: A comparative assessment. Saudi Pharm. J. 2012, 20, 161–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, P.; Pathak, K. Nanosized ethanolic vesicles loaded with econazole nitrate for the treatment of deep fungal infections through topical gel formulation. Nanomedicine 2012, 8, 489–496. [Google Scholar] [CrossRef]
- Paolino, D.; Lucania, G.; Mardente, D.; Alhaique, F.; Fresta, M. Ethosomes for skin delivery of ammonium glycyrrhizinate: In vitro percutaneous permeation through human skin and in vivo anti-inflammatory activity on human volunteers. J. Control. Release 2005, 106, 99–110. [Google Scholar] [CrossRef]
- Zhaowu, Z.; Xiaoli, W.; Yangde, Z.; Nianfeng, L. Preparation of matrine ethosome, its percutaneous permeation in vitro and anti-inflammatory activity in vivo in rats. J. Liposome Res. 2009, 19, 155–162. [Google Scholar] [CrossRef]
- Tosato, M.G.; Maya Girón, J.V.; Martin, A.A.; Krishna Tippavajhala, V.; Fernández Lorenzo de Mele, M.; Dicelio, L. Comparative study of transdermal drug delivery systems of resveratrol: High efficiency of deformable liposomes. Mater. Sci. Eng. C 2018, 90, 356–364. [Google Scholar] [CrossRef] [Green Version]
- Paliwal, S.; Tilak, A.; Sharma, J.; Dave, V.; Sharma, S.; Verma, K.; Tak, K.; Reddy, K.R.; Sadhu, V. Flurbiprofen-loaded ethanolic liposome particles for biomedical applications. J. Microbiol. Methods 2019, 161, 18–27. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Liu, X.; Fahr, A. Skin penetration and deposition of carboxyfluorescein and temoporfin from different lipid vesicular systems: In vitro study with finite and infinite dosage application. Int. J. Pharm. 2011, 408, 223–234. [Google Scholar] [CrossRef]
- Abdulbaqi, I.M.; Darwis, Y.; Khan, N.A.; Assi, R.A.; Khan, A.A. Ethosomal nanocarriers: The impact of constituents and formulation techniques on ethosomal properties, in vivo studies, and clinical trials. Int. J. Nanomed. 2016, 11, 2279–2304. [Google Scholar] [CrossRef] [Green Version]
- Hua, S. Lipid-based nano-delivery systems for skin delivery of drugs and bioactives. Front. Pharmacol. 2015, 6, 219. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.D.; Verma, S.; Blume, G.; Fahr, A. Particle size of liposomes influences dermal delivery of substances into skin. Int. J. Pharm. 2003, 258, 141–151. [Google Scholar] [CrossRef]
- Gambhire, M.S.; Bhalekar, M.R.; Shrivastava, B. Investigations in photostability of dithranol incorporated in solid lipid nanoparticles. Pharm. Chem. J. 2012, 46, 256–261. [Google Scholar] [CrossRef]
- Almeida, P.F.; Carter, F.E.; Kilgour, K.M.; Raymonda, M.H.; Tejada, E. Heat capacity of DPPC/cholesterol mixtures: Comparison of single bilayers with multibilayers and simulations. Langmuir 2018, 34, 9798–9809. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.; Badran, M.; Elmowafy, M.; Soliman, G.M. Spironolactone-loaded leciplexes as potential topical delivery systems for female acne: In vitro appraisal and ex vivo skin permeability studies. Pharmaceutics 2019, 12, 25. [Google Scholar] [CrossRef] [Green Version]
- Andersen, K.B.; Langgård, M.; Spanget-Larsen, J. Molecular and vibrational structure of anthralin. Infrared linear dichroism spectroscopy and quantum chemical calculations. J. Mol. Struct. 1999, 475, 131–140. [Google Scholar] [CrossRef]
- Gupta, U.; Singh, V.K.; Kumar, V.; Khajuria, Y. Spectroscopic studies of cholesterol: Fourier transform infra-red and vibrational frequency analysis. Mater. Focus 2014, 3, 211–217. [Google Scholar] [CrossRef]
- Bodratti, A.M.; Alexandridis, P. Formulation of poloxamers for drug delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef] [Green Version]
- Akash, M.S.; Rehman, K. Recent progress in biomedical applications of Pluronic (PF127): Pharmaceutical perspectives. J. Control. Release 2015, 209, 120–138. [Google Scholar] [CrossRef]
- El-Sayed, M.M.; Hussein, A.K.; Sarhan, H.A.; Mansour, H.F. Flurbiprofen-loaded niosomes-in-gel system improves the ocular bioavailability of flurbiprofen in the aqueous humor. Drug Dev. Ind. Pharm. 2017, 43, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, D.; Rani, M.J.; Haque, M.A.; Bakshi, V.; Jahangir, M.A.; Imam, S.S.; Gilani, S.J. Preparation and evaluation of transdermal naproxen niosomes: Formulation optimization to preclinical anti-inflammatory assessment on murine model. J. Liposome Res. 2019, 2019, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Faria, M.J.; Machado, R.; Ribeiro, A.; Goncalves, H.; Real Oliveira, M.; Viseu, T.; das Neves, J.; Lucio, M. Rational development of liposomal hydrogels: A strategy for topical vaginal antiretroviral drug delivery in the context of HIV prevention. Pharmaceutics 2019, 11, 485. [Google Scholar] [CrossRef] [Green Version]
- Ricci, E.J.; Lunardi, L.O.; Nanclares, D.M.; Marchetti, J.M. Sustained release of lidocaine from Poloxamer 407 gels. Int. J. Pharm. 2005, 288, 235–244. [Google Scholar] [CrossRef]
- Huang, W.; Tsui, C.P.; Tang, C.Y.; Gu, L. Effects of compositional tailoring on drug delivery behaviours of silica xerogel/polymer core-shell composite nanoparticles. Sci. Rep. 2018, 8, 13002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Badry, M.; Fetih, G.; Shakeel, F. Comparative topical delivery of antifungal drug croconazole using liposome and micro-emulsion-based gel formulations. Drug Deliv. 2014, 21, 34–43. [Google Scholar] [CrossRef]
- Glavas-Dodov, M.; Fredro-Kumbaradzi, E.; Goracinova, K.; Calis, S.; Simonoska, M.; Hincal, A.A. 5-Fluorouracil in topical liposome gels for anticancer treatment-formulation and evaluation. Acta Pharm. 2003, 53, 241–250. [Google Scholar]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Yucel, C.; Seker Karatoprak, G.; Degim, I.T. Anti-aging formulation of rosmarinic acid-loaded ethosomes and liposomes. J. Microencapsul. 2019, 36, 180–191. [Google Scholar] [CrossRef]
- Yang, L.; Wu, L.; Wu, D.; Shi, D.; Wang, T.; Zhu, X. Mechanism of transdermal permeation promotion of lipophilic drugs by ethosomes. Int. J. Nanomed. 2017, 12, 3357–3364. [Google Scholar] [CrossRef] [Green Version]
- Kirjavainen, M.; Urtti, A.; Valjakka-Koskela, R.; Kiesvaara, J.; Monkkonen, J. Liposome-skin interactions and their effects on the skin permeation of drugs. Eur. J. Pharm. Sci. 1999, 7, 279–286. [Google Scholar] [CrossRef]
- Van De Kerkhof, P.C.M.; Van Der Valk, P.G.M.; Swinkels, O.Q.J.; Kucharekova, M.; De Rie, M.A.; De Vries, H.J.C.; Damstra, R.; Oranje, A.P.; De Waard-van der Spek, F.B.; Van Neer, P.; et al. A comparison of twice-daily calcipotriol ointment with once-daily short-contact dithranol cream therapy: A randomized controlled trial of supervised treatment of psoriasis vulgaris in a day-care setting. Br. J. Dermatol. 2006, 155, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Sminkels, O.Q.; Prins, M.; Veeniiuis, R.T.; De Boo, T.; Gerritsen, M.J.; Van Der Wilt, G.J.; Van De Kerkhof, P.C.; Van Der Valk, P.G. Effectiveness and side effects of UVB-phototherapy, dithranol inpatient therapy and a care instruction programme of short contact dithranol in moderate to severe psoriasis. Eur. J. Dermatol. 2004, 14, 159–165. [Google Scholar]
- Ashton, R.E.; Andre, P.; Lowe, N.J.; Whitefield, M. Anthralin: Historical and current perspectives. J. Am. Acad. Dermatol. 1983, 9, 173–192. [Google Scholar] [CrossRef]
- Mbah, C.C.; Builders, P.F.; Attama, A.A. Nanovesicular carriers as alternative drug delivery systems: Ethosomes in focus. Expert Opin. Drug Deliv. 2014, 11, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.-P.; Tsai, Y.-H.; Wu, P.-C.; Huang, Y.-B. Comparison of 5-aminolevulinic acid-encapsulated liposome versus ethosome for skin delivery for photodynamic therapy. Int. J. Pharm. 2008, 356, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Bodade, S.S.; Shaikh, K.S.; Kamble, M.S.; Chaudhari, P.D. A study on ethosomes as mode for transdermal delivery of an antidiabetic drug. Drug Deliv. 2013, 20, 40–46. [Google Scholar] [CrossRef]


| Code | Drug/Lipid (wt%) | CH/PL-90G Ratio a | Particle Size (nm) b | PDI c | Encapsulation Efficiency (%) d |
|---|---|---|---|---|---|
| L1 | 10 | 0.3:0.7 | 198.9 ± 2.2 | 0.4 ± 0.01 | 97.2 ± 0.4 |
| L2 | 10 | 0.4:0.6 | 143.1 ± 5.7 | 0.9 ± 0.01 | 94.8 ± 0.1 |
| L3 | 10 | 0.5:0.5 | 231.5 ± 0.7 | 0.5 ± 0.01 | 91.8 ± 0.7 |
| L4 | 25 | 0.3:0.7 | 199.3 ± 4.2 | 0.7 ± 0.01 | 99.2 ± 0.2 |
| L5 | 50 | 0.3:0.7 | 116.1 ± 1.7 | 0.2 ± 0.01 | 99.5 ± 0.0 |
| L6 | 75 | 0.3:0.7 | 187.9 ± 4.6 | 0.3 ± 0.00 | 99.7 ± 0.0 |
| Code | Drug/Lipid (wt%) | PL-90G (%, w/v) | Ethanol (%, v/v) | Particle Size (nm) a | PDI b | Encapsulation Efficiency (%) c |
|---|---|---|---|---|---|---|
| E1 | 3.3 | 3 | 35 | 381.3 ± 3.2 | 0.1 ± 0.00 | 84.1 ± 3.1 |
| E2 | 2.5 | 4 | 35 | 320.5 ± 8.6 | 0.4 ± 0.01 | 81.3 ± 1.1 |
| E3 | 2 | 5 | 35 | 145.9 ± 0.8 | 0.3 ± 0.00 | 77.3 ± 0.7 |
| E4 | 3.3 | 3 | 40 | 201.5 ± 6.9 | 0.4 ± 0.00 | 85.0 ± 0.6 |
| E5 | 2.5 | 4 | 40 | 199.6 ± 5.7 | 0.4 ± 0.01 | 81.3 ± 0.7 |
| E6 | 2 | 5 | 40 | 233.5 ± 2.2 | 0.3 ± 0.01 | 80.8 ± 2.0 |
| E7 | 3.3 | 3 | 45 | 234.1 ± 6.9 | 0.7 ± 0.04 | 81.4 ± 0.3 |
| E8 | 2.5 | 4 | 45 | 225.5 ± 3.6 | 0.5 ± 0.00 | 79.2 ± 0.9 |
| E9 | 2 | 5 | 45 | 358.3 ± 5.8 | 1.0 ± 0.00 | 77.4 ± 3.5 |
| Formulation | Zeroth-Order | First-Order | Higuchi Model | Hixson Crowell Model | Baker–Lonsdale Model | Korsemeyer–Peppas Equation | |
|---|---|---|---|---|---|---|---|
| R2 | R2 | R2 | R2 | R2 | R2 | n | |
| Liposome | 0.9455 | −0.9604 | 0.9813 | 0.9557 | 0.9926 | 0.9779 | 0.54 |
| Liposome HPMC gel | 0.9766 | −0.9827 | 0.9976 | 0.9808 | 0.9996 | 0.9934 | 0.59 |
| Liposome HEC gel | 0.9585 | −0.9694 | 0.9913 | 0.9660 | 0.9973 | 0.9859 | 0.53 |
| Liposome PL-127 gel | 0.5847 | −0.5939 | 0.7044 | 0.5909 | 0.6262 | 0.8222 | 0.34 |
| Ethosome | 0.9483 | −0.9607 | 0.9843 | 0.9568 | 0.9954 | 0.9637 | 0.71 |
| Ethosome HPMC gel | 0.8830 | −0.8982 | 0.9404 | 0.8933 | 0.9794 | 0.9115 | 0.69 |
| Ethosome HEC gel | 0.9504 | −0.9566 | 0.9853 | 0.9545 | 0.9958 | 0.9680 | 0.73 |
| Ethosome PL-127 gel | 0.7717 | −0.7852 | 0.8593 | 0.7808 | 0.8712 | 0.8608 | 0.69 |
| Parameter | Qa | Jsb | Papp × 103 c | Q (skin) d |
|---|---|---|---|---|
| Drug solution in receptor medium | 98.06 ± 26.74 | 17.58 ± 3.88 | 14.56 ± 3.74 | 13.0 ± 4.9 |
| Liposome PL-127 gel | 135.7 ± 84.3 | 19.3 ± 15.8 | 17.4 ± 14.2 | 252.2 ± 106.9 |
| Drug hydroalcoholicsolution | 76.67 ± 19.06 | 11.79 ± 5.26 | 10.83 ± 4.84 | 31.4 ± 3.3 |
| Ethosome PL-127 gel | 337.6 ± 24.1 | 21.65 ± 8.0 | 19.9 ± 7.4 | 112.8 ± 21.9 |
| Parameter | Liposomal Preparations | Ethosomal Preparations | p Value |
|---|---|---|---|
| Number of patients | 10 | 10 | |
| Age (years), mean ± SD (range) | 36.3 ± 16.3 (11–58) | 43.1 ± 15.1 (13–55) | 0.34 |
| Sex Males/Females, n (%) | 9/1 (90/10) | 7/3 (70/30) | 0.28 |
| Residence% | |||
| Rural | 40% | 20% | |
| Urban | 60% | 80% | |
| Occupation % | |||
| Working | 90% | 70% | |
| Not working | 10% | 30% | |
| Family history | |||
| Positive | 30% | 0% | |
| Negative | 70% | 100% | |
| Age at onset (years), mean ± SD (range) | 31.9 ± 14.3 (10.9–53) | 37.3 ± 12.8 (12.6–48) | 0.39 |
| Duration of psoriasis (months), mean ± SD (range) | 52.8 ± 67.4 (3–228) | 70 ± 62.7 (6–204) | 0.56 |
| Provocation factors % | |||
| Stress | 60% | 40% | |
| Sun exposure | 0% | 10% | |
| Trauma including itching | 60% | 70% | |
| Infection | 10% | 0% | |
| Chemicals like detergent | 10% | 0% | |
| Effectiveness of preparations | |||
| Baseline PASI, median (IQR) | 3.4 (1.2–3.6) | 3.6 (1.2–4) | 0.53 |
| End of treatment PASI, median (IQR) | 0.6 (0.2–1.2) | 0.4 (0.2–1.2) | 0.59 |
| Percentage change in PASI (95%CI) | −68.66 (−77.44 to −59.88) | −81.84 (−87.02 to −76.67) | 0.009 * |
| Short contact therapy | |||
| Duration of treatment needed for improvement (days), mean ± SD | 21.7 ± 2.2 | 14.8 ± 4.8 | 0.0006 * |
| Adverse effects | No side effects | No side effects | |
| Patient satisfaction | |||
| Very satisfied (%) | 30% | 80% | |
| Satisfied (%) | 70% | 20% | |
| Recurrence after end of treatment up to 3 months | |||
| No recurrence | 70% | 80% | |
| Recurrence | 30% after 1 month | 20% after 2 month |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fathalla, D.; Youssef, E.M.K.; Soliman, G.M. Liposomal and Ethosomal Gels for the Topical Delivery of Anthralin: Preparation, Comparative Evaluation and Clinical Assessment in Psoriatic Patients. Pharmaceutics 2020, 12, 446. https://doi.org/10.3390/pharmaceutics12050446
Fathalla D, Youssef EMK, Soliman GM. Liposomal and Ethosomal Gels for the Topical Delivery of Anthralin: Preparation, Comparative Evaluation and Clinical Assessment in Psoriatic Patients. Pharmaceutics. 2020; 12(5):446. https://doi.org/10.3390/pharmaceutics12050446
Chicago/Turabian StyleFathalla, Dina, Eman M. K. Youssef, and Ghareb M. Soliman. 2020. "Liposomal and Ethosomal Gels for the Topical Delivery of Anthralin: Preparation, Comparative Evaluation and Clinical Assessment in Psoriatic Patients" Pharmaceutics 12, no. 5: 446. https://doi.org/10.3390/pharmaceutics12050446
APA StyleFathalla, D., Youssef, E. M. K., & Soliman, G. M. (2020). Liposomal and Ethosomal Gels for the Topical Delivery of Anthralin: Preparation, Comparative Evaluation and Clinical Assessment in Psoriatic Patients. Pharmaceutics, 12(5), 446. https://doi.org/10.3390/pharmaceutics12050446