Enhancement in Oral Absorption of Ceftriaxone by Highly Functionalized Magnetic Iron Oxide Nanoparticles
Abstract
:1. Introduction
2. Methods and Materials
2.1. Materials
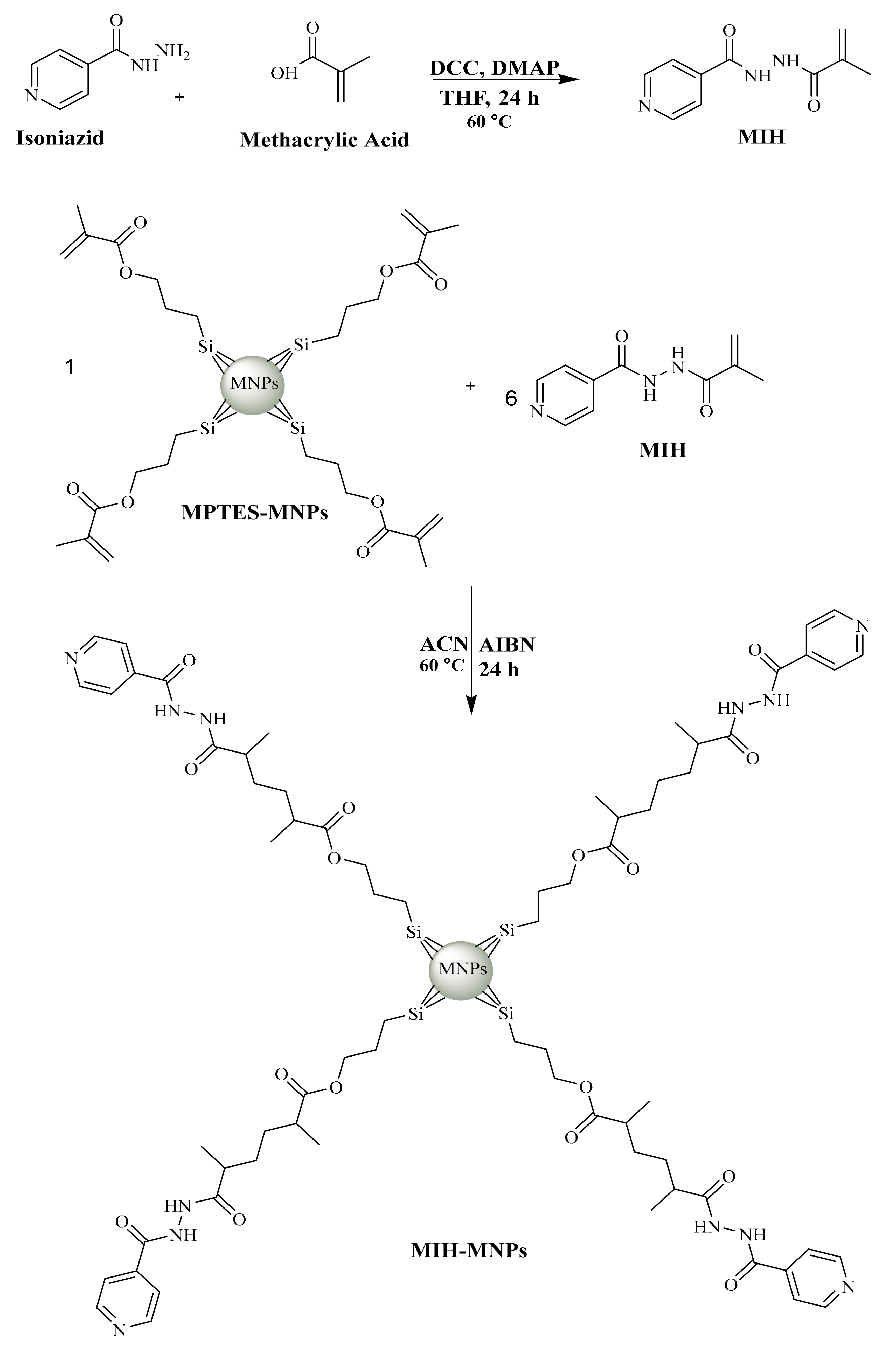
2.2. Synthesis of MIH
2.3. Preparation of MIH-MNPs and CFT-MIH-MNPs
2.4. Particle Size, Size Distribution, Zeta Potential and Morphology Studies
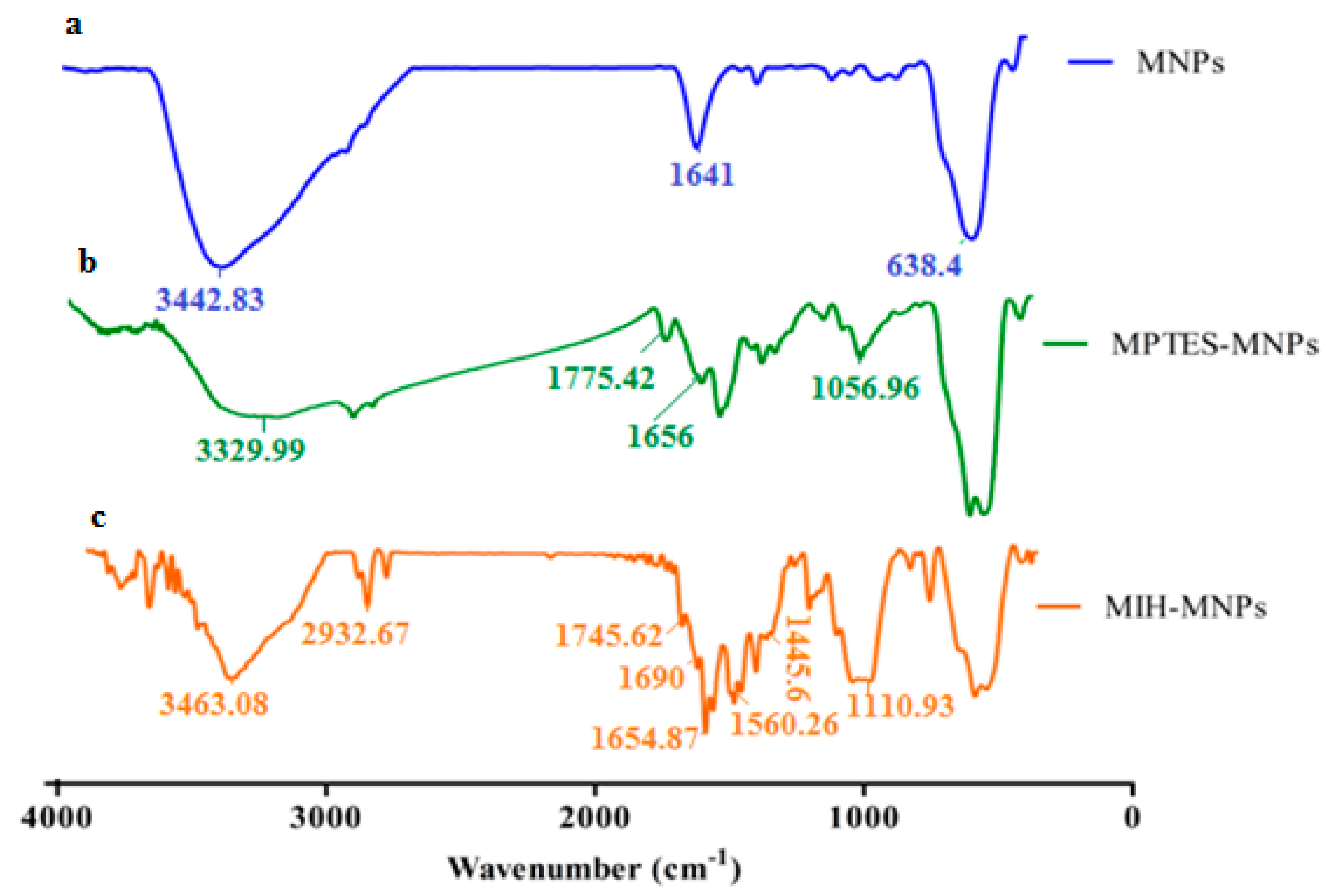
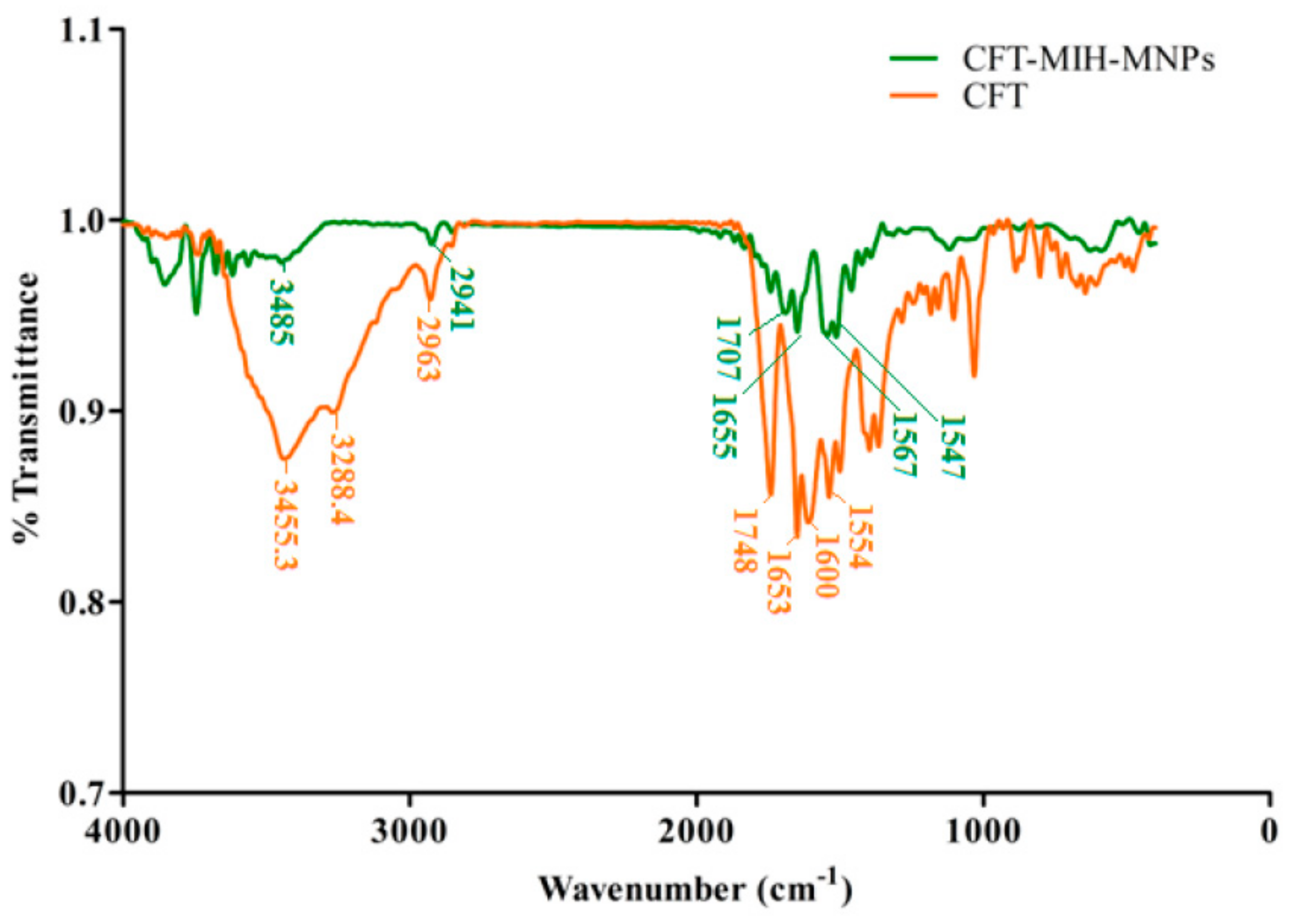
2.5. FTIR Spectroscopy
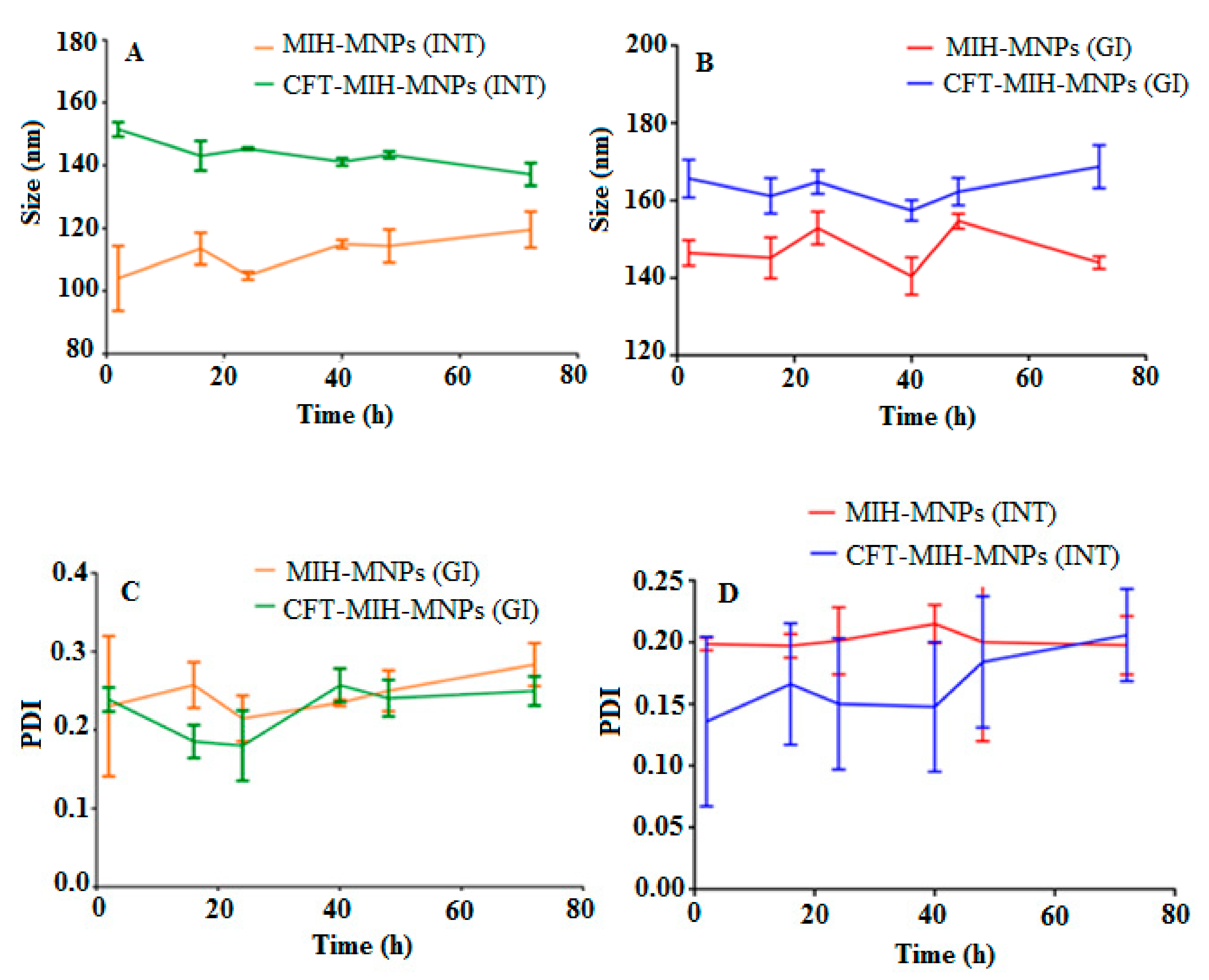
2.6. Stability of NPs in Simulated Gastric Fluids (SGF)
2.7. Development of HPLC Protocol for the Determination of CFT in Blood Plasma
2.8. Entrapment Efficiency Determination
2.9. In Vitro Release Study
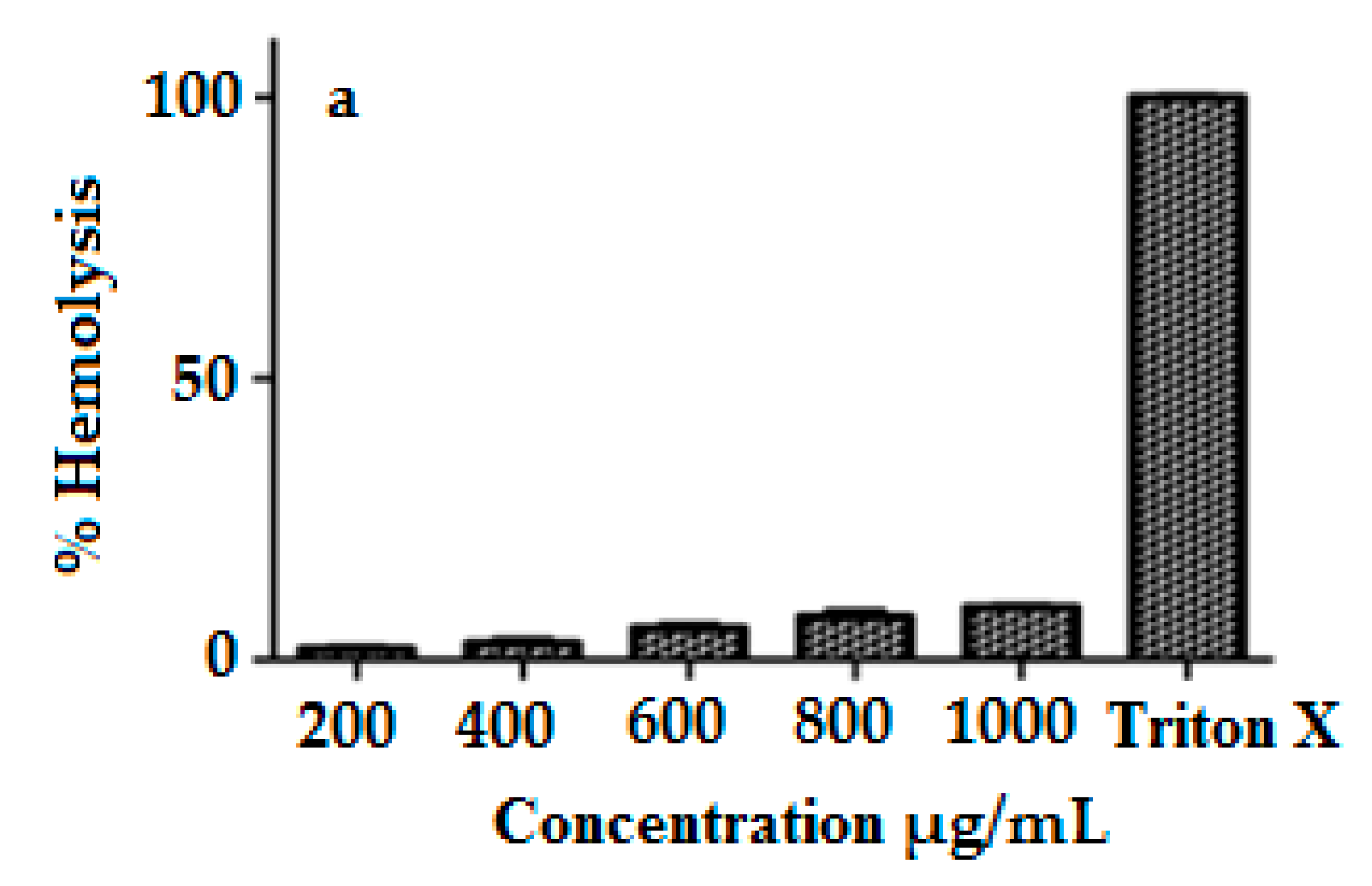
2.10. Hemocompatibility Study
2.11. In Vitro Cytotoxicity Study
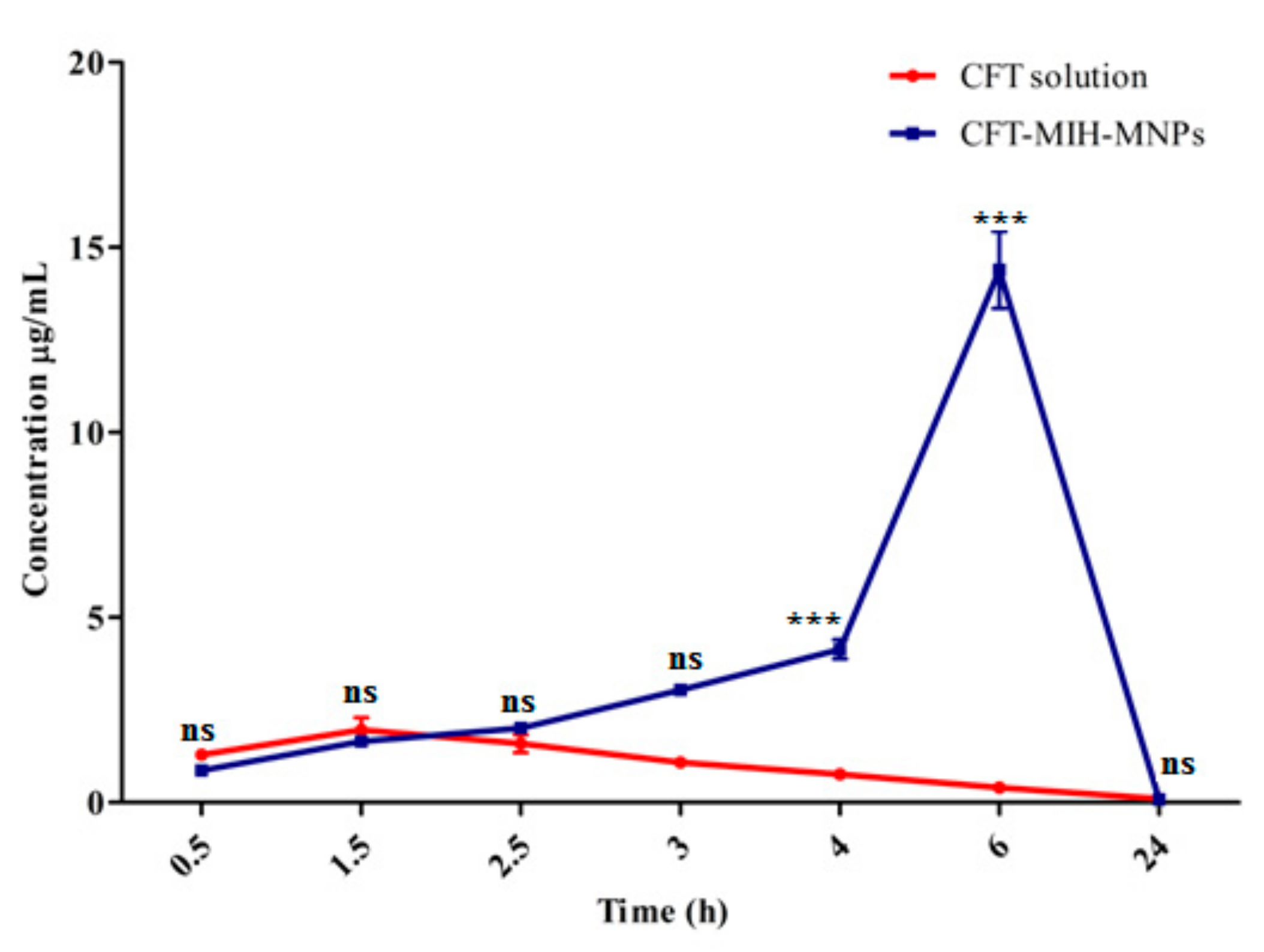
2.12. In Vivo Oral Pharmacokinetics Studies
2.13. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of MIH
3.2. Preparation of MIH-MNPs and CFT-MIH-MNPs
3.3. Particle Size, Size Distribution, Zeta Potential and Morphology Studies
3.4. Stability in Simulated Gastric Fluids (SGF)
3.5. Development of HPLC Protocol for the Determination of CFT in Blood Plasma
3.6. Drug Entrapment Efficiency
3.7. In Vitro Drug Release Study
3.8. Hemocompatibility
3.9. In Vitro Cytotoxicity
3.10. In Vivo Oral Pharmacokinetic Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, J.; Shu, Q.; Wang, L.; Wu, H.; Wang, A.Y.; Mao, H. Layer-by-layer assembled milk protein coated magnetic nanoparticle enabled oral drug delivery with high stability in stomach and enzyme-responsive release in small intestine. Biomaterials 2015, 39, 105–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thanki, K.; Gangwal, R.P.; Sangamwar, A.T.; Jain, S. Oral delivery of anticancer drugs: Challenges and opportunities. J. Control. Release 2013, 170, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Gamboa, J.M.; Leong, K.W. In vitro and in vivo models for the study of oral delivery of nanoparticles. Adv. Drug Deliv. Rev. 2013, 65, 800–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leong, K.W.; Sung, H.-W. Nanoparticle-and biomaterials-mediated oral delivery for drug, gene, and immunotherapy. Adv. Drug Deliv. Rev. 2013, 65, 757–758. [Google Scholar] [CrossRef]
- Chomoucka, J.; Drbohlavova, J.; Huska, D.; Adam, V.; Kizek, R.; Hubalek, J. Magnetic nanoparticles and targeted drug delivering. Pharmacol. Res. 2010, 62, 144–149. [Google Scholar] [CrossRef]
- Shete, H.K.; Vyas, S.S.; Patravale, V.B.; Disouza, J.I. Pulmonary multifunctional nano-oncological modules for lung cancer treatment and prevention. J. Biomed. Nanotechnol. 2014, 10, 1863–1893. [Google Scholar] [CrossRef]
- Santana, S.D.; Dhadge, V.L.; Roque, A.C. Dextran-coated magnetic supports modified with a biomimetic ligand for IgG purification. ACS Appl. Mater. Interfaces 2012, 4, 5907–5914. [Google Scholar] [CrossRef]
- Arruebo, M.; Fernández-Pacheco, R.; Ibarra, M.R.; Santamaría, J. Magnetic nanoparticles for drug delivery. Nano Today 2007, 2, 22–32. [Google Scholar] [CrossRef]
- Yuan, Q.; Venkatasubramanian, R.; Hein, S.; Misra, R. A stimulus-responsive magnetic nanoparticle drug carrier: Magnetite encapsulated by chitosan-grafted-copolymer. Acta Biomater. 2008, 4, 1024–1037. [Google Scholar] [CrossRef]
- Chen, J.-P.; Yang, P.-C.; Ma, Y.-H.; Wu, T. Characterization of chitosan magnetic nanoparticles for in situ delivery of tissue plasminogen activator. Carbohydr. Polym. 2011, 84, 364–372. [Google Scholar] [CrossRef]
- Zhang, J.; Misra, R. Magnetic drug-targeting carrier encapsulated with thermosensitive smart polymer: Core–shell nanoparticle carrier and drug release response. Acta Biomater. 2007, 3, 838–850. [Google Scholar] [CrossRef] [PubMed]
- Kebede, A.; Singh, A.K.; Rai, P.K.; Giri, N.K.; Rai, A.K.; Watal, G.; Gholap, A. Controlled synthesis, characterization, and application of iron oxide nanoparticles for oral delivery of insulin. Lasers Med Sci. 2013, 28, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Kansy, M.; Senner, F.; Gubernator, K. Physicochemical high throughput screening: Parallel artificial membrane permeation assay in the description of passive absorption processes. J. Med. Chem. 1998, 41, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, M.; Derle, N.D.; Bhamber, R. Permeability enhancement techniques for poorly permeable drugs: A review. J. Appl. Pharm. Sci. 2012, 2, 34–39. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Kim, S.K.; Lee, D.Y.; Park, K.; Kumar, T.S.; Chae, S.Y.; Byun, Y. Cationic analog of deoxycholate as an oral delivery carrier for ceftriaxone. J. Pharm. Sci. 2005, 94, 2541–2548. [Google Scholar] [CrossRef] [PubMed]
- Dresco, P.A.; Zaitsev, V.S.; Gambino, R.J.; Chu, B. Preparation and properties of magnetite and polymer magnetite nanoparticles. Langmuir 1999, 15, 1945–1951. [Google Scholar] [CrossRef]
- Saif, B.; Wang, C.; Chuan, D.; Shuang, S. Synthesis and characterization of Fe3O4 coated on APTES as carriers for morin-anticancer drug. J. Biomater. Nanobiotechnol. 2015, 6, 267. [Google Scholar] [CrossRef]
- Pan, Q.; Lv, Y.; Williams, G.R.; Tao, L.; Yang, H.; Li, H.; Zhu, L. Lactobionic acid and carboxymethyl chitosan functionalized graphene oxide nanocomposites as targeted anticancer drug delivery systems. Carbohydr. Polym. 2016, 151, 812–820. [Google Scholar] [CrossRef]
- Lazzari, S.; Moscatelli, D.; Codari, F.; Salmona, M.; Morbidelli, M.; Diomede, L. Colloidal stability of polymeric nanoparticles in biological fluids. J. Nanoparticle Res. 2012, 14, 920. [Google Scholar] [CrossRef] [Green Version]
- Date, A.A.; Nagarsenker, M.S.; Patere, S.; Dhawan, V.; Gude, R.; Hassan, P.; Aswal, V.; Steiniger, F.; Thamm, J.; Fahr, A. Lecithin-based novel cationic nanocarriers (Leciplex) II: Improving therapeutic efficacy of quercetin on oral administration. Mol. Pharm. 2011, 8, 716–726. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Firempong, C.K.; Zhang, H.; Wang, M.; Zhang, Y.; Zhu, Y.; Yu, J.; Xu, X. Enhanced solubility and bioavailability of naringenin via liposomal nanoformulation: Preparation and in vitro and in vivo evaluations. AAPS Pharmscitech 2017, 18, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.O.; Cantos, J.B.; D’Oca, C.R.M.; Soares, K.L.; Coelho, T.S.; Piovesan, L.A.; Russowsky, D.; da Silva, P.A.; D’Oca, M.G.M. Synthesis and antimycobacterial activity of isoniazid derivatives from renewable fatty acids. Bioorg. Med. Chem. 2013, 21, 6910–6914. [Google Scholar] [CrossRef] [PubMed]
- Sutar, Y.B.; Mali, J.K.; Telvekar, V.N.; Rajmani, R.S.; Singh, A. Transferrin conjugates of antitubercular drug isoniazid: Synthesis and in vitro efficacy. Eur. J. Med. Chem. 2019, 183, 111713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valeur, E.; Bradley, M. Amide bond formation: Beyond the myth of coupling reagents. Chem. Soc. Rev. 2009, 38, 606–631. [Google Scholar] [CrossRef]
- Santos, M.; Seabra, A.; Pelegrino, M.; Haddad, P. Synthesis, characterization and cytotoxicity of glutathione-and PEG-glutathione-superparamagnetic iron oxide nanoparticles for nitric oxide delivery. Appl. Surf. Sci. 2016, 367, 26–35. [Google Scholar] [CrossRef]
- Raliya, R. Nanoscale Engineering in Agricultural Management; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Jiao, F.; Gao, F.; Wang, H.; Deng, Y.; Zhang, Y.; Qian, X.; Zhang, Y. Polymeric hydrophilic ionic liquids used to modify magnetic nanoparticles for the highly selective enrichment of N-linked glycopeptides. Sci. Rep. 2017, 7, 6984. [Google Scholar] [CrossRef] [Green Version]
- Petcharoen, K.; Sirivat, A. Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B 2012, 177, 421–427. [Google Scholar] [CrossRef]
- Arsalani, N.; Fattahi, H.; Nazarpoor, M. Synthesis and characterization of PVP-functionalized superparamagnetic Fe3O4 nanoparticles as an MRI contrast agent. Express Polym. Lett. 2010, 4, 329–338. [Google Scholar] [CrossRef]
- Zaki, N.M.; Hafez, M.M. Enhanced antibacterial effect of ceftriaxone sodium-loaded chitosan nanoparticles against intracellular Salmonella typhimurium. AAPS Pharmscitechnol. 2012, 13, 411–421. [Google Scholar] [CrossRef] [Green Version]
- Chandra, D.; Kohli, G.; Prasad, K.; Bisht, G.; Punetha, V.D.; Khetwal, K.; Devrani, M.K.; Pandey, H. Phytochemical and Ethnomedicinal Uses of Family Violaceae. Curr. Res. Chem. 2015, 7, 44–52. [Google Scholar] [CrossRef] [Green Version]
- Hasanova, U.; Ramazanov, M.; Maharramov, A.; Gakhramanova, Z.; Hajiyeva, S.; Eyvazova, Q.; Vezirova, L.; Hajiyeva, F.; Hasanova, M.; Guliyeva, N. Synthesis of Macrocycle (MC)–Mimics the Properties of Natural Siderophores and Preparation the Nanostructures on the Basis of MC and Magnetite Nanoparticles. Chem. Eng. Trans. 2016, 47, 109–114. [Google Scholar]
- Caddeo, C.; Teskač, K.; Sinico, C.; Kristl, J. Effect of resveratrol incorporated in liposomes on proliferation and UV-B protection of cells. Int. J. Pharm. 2008, 363, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-C.; Paik, S.-Y.-R.; Ryu, J.; Choi, K.-O.; Kang, T.S.; Lee, J.K.; Song, C.W.; Ko, S. Dynamic light scattering-based method to determine primary particle size of iron oxide nanoparticles in simulated gastrointestinal fluid. Food Chem. 2014, 161, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, H.; Ge, X. Simultaneous determination of the combined drugs of ceftriaxone sodium, metronidazole, and levofloxacin in human urine by high-performance liquid chromatography. J. Clin. Lab. Anal. 2012, 26, 486–492. [Google Scholar] [CrossRef]
- Gaihre, B.; Khil, M.S.; Lee, D.R.; Kim, H.Y. Gelatin-coated magnetic iron oxide nanoparticles as carrier system: Drug loading and in vitro drug release study. Int. J. Pharm. 2009, 365, 180–189. [Google Scholar] [CrossRef]
- Beskid, G.; Unowsky, J.; Behl, C.R.; Siebelist, J.A.; Tossounian, J.L.; McGarry, C.M.; Shah, N.H.; Cleeland, R. Enteral, oral, and rectal absorption of ceftriaxone using glyceride enhancers. Chemotherapy 1988, 34, 77–84. [Google Scholar] [CrossRef]
- Fischer, D.; Li, Y.; Ahlemeyer, B.; Krieglstein, J.; Kissel, T. In vitro cytotoxicity testing of polycations: Influence of polymer structure on cell viability and hemolysis. Biomaterials 2003, 24, 1121–1131. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Clogston, J.D.; Neun, B.W.; Hall, J.B.; Patri, A.K.; McNeil, S.E. Method for Analysis of Nanoparticle Hemolytic Properties in Vitro. Nano Lett. 2008, 8, 2180–2187. [Google Scholar] [CrossRef]
- Seabra, A.B.; Pasquoto, T.; Ferrarini, A.C.F.; Santos, M.d.C.; Haddad, P.S.; de Lima, R. Preparation, characterization, cytotoxicity, and genotoxicity evaluations of thiolated-and S-nitrosated superparamagnetic iron oxide nanoparticles: Implications for cancer treatment. Chem. Res. Toxicol. 2014, 27, 1207–1218. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Jeon, O.-C.; Hwang, S.R.; Al-Hilal, T.A.; Park, J.W.; Moon, H.T.; Lee, S.; Park, J.H.; Byun, Y. Oral delivery of ionic complex of ceftriaxone with bile acid derivative in non-human primates. Pharm. Res. 2013, 30, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-W.; Lee, J.S.; Choi, S.-H. Enhanced oral bioavailability of poorly absorbed drugs. I. Screening of absorption carrier for the ceftriaxone complex. J. Pharm. Sci. 2004, 93, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Siu, F.Y.; Ye, S.; Lin, H.; Li, S. Galactosylated PLGA nanoparticles for the oral delivery of resveratrol: Enhanced bioavailability and in vitro anti-inflammatory activity. Int. J. Nanomed. 2018, 13, 4133–4144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bora, C.; Prabhu, R.; Patravale, V. Lymphatic Delivery: Concept, Challenges and Applications (INDIAN DRUGS Best Review Article Award 2017). Indian Drugs 2017, 54, 5–22. [Google Scholar]
- Lombardo, D.; Kiselev, M.A.; Caccamo, M.T. Smart Nanoparticles for Drug Delivery Application: Development of Versatile Nanocarrier Platforms in Biotechnology and Nanomedicine. J. Nanomater. 2019, 2019, 26. [Google Scholar] [CrossRef]
- Shabestari Khiabani, S.; Farshbaf, M.; Akbarzadeh, A.; Davaran, S. Magnetic nanoparticles: Preparation methods, applications in cancer diagnosis and cancer therapy. Artif. Cells Nanomed. Biotechnol. 2017, 45, 6–17. [Google Scholar] [CrossRef]
- Elsheikh, M.A.; Elnaggar, Y.S.; Gohar, E.Y.; Abdallah, O.Y. Nanoemulsion liquid preconcentrates for raloxifene hydrochloride: Optimization and in vivo appraisal. Int. J. Nanomed. 2012, 7, 3787. [Google Scholar]
- Tokuoka, T.; Tobe, H. Phylogenetic analyses of Malpighiales using plastid and nuclear DNA sequences, with particular reference to the embryology of Euphorbiaceae sens. str. J. Plant Res. 2006, 119, 599–616. [Google Scholar] [CrossRef]
- Vinogradova, T.I.; Aleksandrova, A.E.; Tschegoleva, R.A. Cephalosporins as possible methods of etiotropic therapy of tuberculosis. Probl. Tuberk. 1993, 45–48. [Google Scholar]
- Jeevarathinam, A.S.; Lemaster, J.E.; Chen, F.; Zhao, E.; Jokerst, J. Photoacoustic Imaging Quantifies Drug Release from Nanocarriers via Redox Chemistry of Dye-Labeled Cargo. Angew. Chem. Int. Ed. 2019, 59, 4678–4683. [Google Scholar] [CrossRef]




| Sample | Average Size (nm) | Polydispersity Index (PDI) | Zeta Potential (mV) | Drug Entrapment Efficiency (%) |
|---|---|---|---|---|
| MPTES-MNPs | 124 ± 3.4 | 0.232 | –11.4 ± 1.3 | - |
| CFT-MPTES-MNPs | 143 ± 5.9 | 0.252 | –18.3 ± 0.2 | 73.1 ± 2.4 |
| MIH-MNPs | 168 ± 7.3 | 0.237 | –17.7 ± 0.4 | - |
| CFT-MIH-MNPs | 184 ± 2.7 | 0.265 | –20.2 ± 0.4 | 79.4 ± 1.5 |
| Pharmacokinetic Parameters | CFT Solution | CFT-MIH-MNPs Formulation |
|---|---|---|
| Dose (mg/kg) | 50.0 ± 0.3 | 25.0 ± 0.3 |
| Cmax (µg/mL) | 2.0 ± 0.6 | 14.4 ± 1.8 *** |
| AUC0-24 (µg.h/mL) | 16.1 ± 3.7 | 156.8 ± 0.3 *** |
| MRT (h) | 8.4 ± 0.1 | 12.2 ± 0.2 *** |
| Tmax (h) | 1.5 | 6 |
| Clearance (L/h) | 3.9 ± 0.4 | 1.6 ± 0.2 *** |
| Volume distribution (L) | 33.0 ± 1.94 | 10.9 ± 2.4 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawish, M.; Elhissi, A.; Jabri, T.; Muhammad Iqbal, K.; Zahid, H.; Shah, M.R. Enhancement in Oral Absorption of Ceftriaxone by Highly Functionalized Magnetic Iron Oxide Nanoparticles. Pharmaceutics 2020, 12, 492. https://doi.org/10.3390/pharmaceutics12060492
Kawish M, Elhissi A, Jabri T, Muhammad Iqbal K, Zahid H, Shah MR. Enhancement in Oral Absorption of Ceftriaxone by Highly Functionalized Magnetic Iron Oxide Nanoparticles. Pharmaceutics. 2020; 12(6):492. https://doi.org/10.3390/pharmaceutics12060492
Chicago/Turabian StyleKawish, Muhammad, Abdelbary Elhissi, Tooba Jabri, Kanwal Muhammad Iqbal, Hina Zahid, and Muhammad Raza Shah. 2020. "Enhancement in Oral Absorption of Ceftriaxone by Highly Functionalized Magnetic Iron Oxide Nanoparticles" Pharmaceutics 12, no. 6: 492. https://doi.org/10.3390/pharmaceutics12060492
APA StyleKawish, M., Elhissi, A., Jabri, T., Muhammad Iqbal, K., Zahid, H., & Shah, M. R. (2020). Enhancement in Oral Absorption of Ceftriaxone by Highly Functionalized Magnetic Iron Oxide Nanoparticles. Pharmaceutics, 12(6), 492. https://doi.org/10.3390/pharmaceutics12060492






