A Naturally Derived Carrier for Photodynamic Treatment of Squamous Cell Carcinoma: In Vitro and In Vivo Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Formulation of Chlorin-E6-Loaded Ethosomes (Ce6 Ethosomes)
2.3. Physicochemical Characterization of Ce6 Ethosomes
2.4. Evaluation of Singlet Oxygen (1O2) Production
2.5. Analysis of Cell Viability
2.6. Determination of Reactive Oxygen Species (ROS) Production
2.7. Analysis of Apoptosis by Active Caspase 3/7
2.8. 3D Spheroid Culture
2.9. Evaluation of PDT Effect of Ce6 Ethosomes Using Light Microscopy and Acid Phosphatase Assay
2.10. Histology and Immunohistochemistry
2.11. Analysis of Squamous Cell Carcinoma Xenografts in CAM Assay
2.12. Statistical Analysis
3. Results
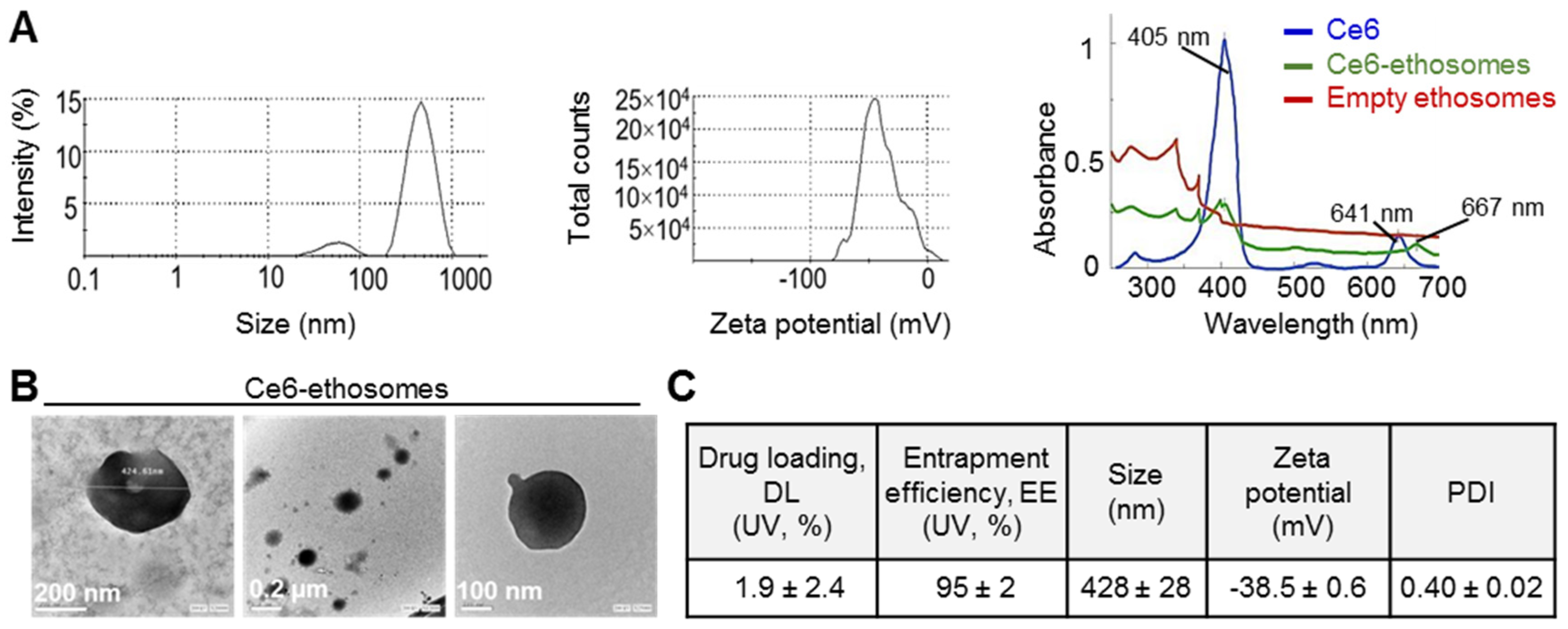
3.1. Characterization of Ce6 Ethosomes
3.2. Analysis of Kinetics of Ce6-Induced Singlet Oxygen (1O2) and ROS Production
3.3. Photodynamic Effect of Ce6 Ethosomes on Squamous Cell Carcinoma Monolayer
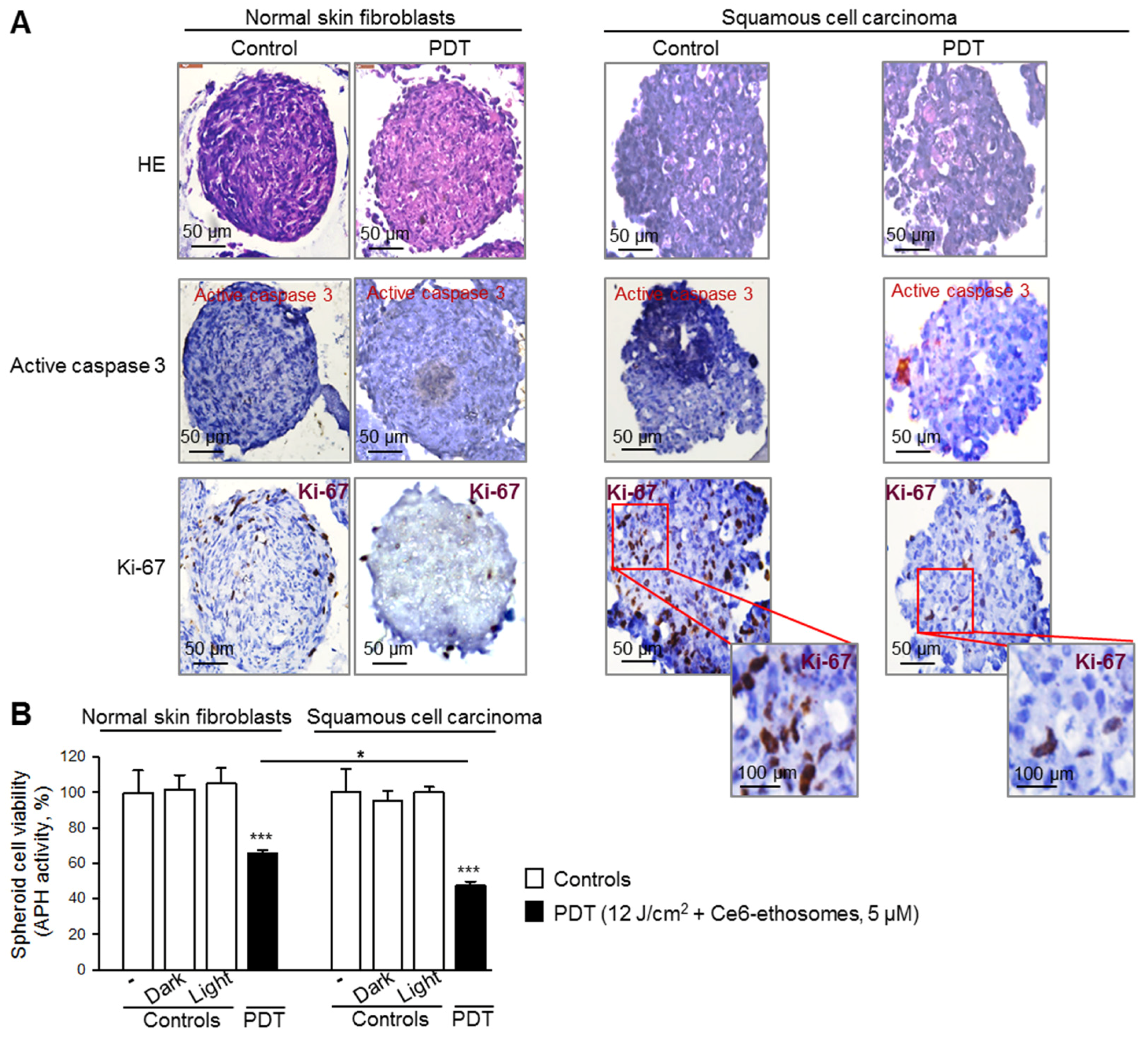
3.4. Photodynamic Effect of Ce6 ethosomes on Squamous Cell Carcinoma 3D Spheroids
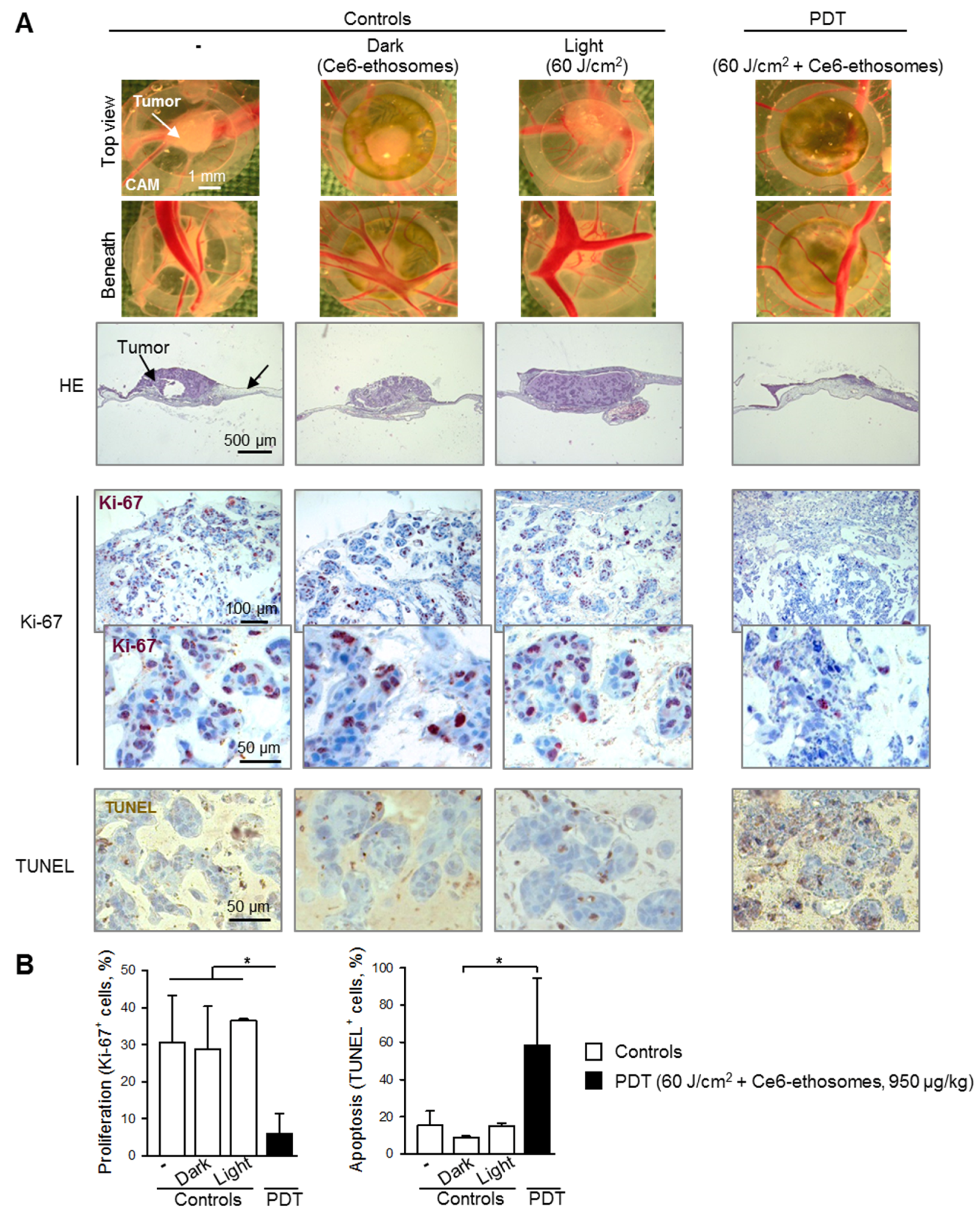
3.5. Photodynamic Effect of Ce6 Ethosomes on Squamous Cell Carcinoma Xenografts
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Samarasinghe, V.; Madan, V. Nonmelanoma skin cancer. J. Cutan. Aesthet. Surg. 2012, 5, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Veness, M.J. High-risk cutaneous squamous cell carcinoma of the head and neck. J. Biomed. Biotechnol. 2007, 2007, 80572. [Google Scholar] [CrossRef] [PubMed]
- Waldman, A.; Schmults, C. Cutaneous squamous cell carcinoma. Hematol. Oncol. Clin. N. Am. 2019, 33, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.B.; Brown, E.A.; Walker, I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004, 5, 497–508. [Google Scholar] [CrossRef]
- Golombek, S.K.; May, J.N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor targeting via EPR: Strategies to enhance patient responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Chin, W.W.; Heng, P.W.; Thong, P.S.; Bhuvaneswari, R.; Hirt, W.; Kuenzel, S.; Soo, K.C.; Olivo, M. Improved formulation of photosensitizer chlorin e6 polyvinylpyrrolidone for fluorescence diagnostic imaging and photodynamic therapy of human cancer. Eur. J. Pharm. Biopharm. 2008, 69, 1083–1093. [Google Scholar] [CrossRef]
- Sebak, A.A.; Gomaa, I.E.O.; ElMeshad, A.N.; AbdelKader, M.H. Targeted photodynamic-induced singlet oxygen production by peptide-conjugated biodegradable nanoparticles for treatment of skin melanoma. Photodiagnosis Photodyn. Ther. 2018, 23, 181–189. [Google Scholar] [CrossRef]
- Mehraban, N.; Freeman, H.S. Developments in PDT sensitizers for increased selectivity and singlet oxygen production. Materials 2015, 8, 4421–4456. [Google Scholar] [CrossRef]
- Ormond, A.B.; Freeman, H.S. Dye sensitizers for photodynamic therapy. Materials 2013, 6, 817–840. [Google Scholar] [CrossRef]
- Cunderlikova, B.; Gangeskar, L.; Moan, J. Acid-base properties of chlorin e6: Relation to cellular uptake. J. Photochem. Photobiol. B 1999, 53, 81–90. [Google Scholar] [CrossRef]
- Ding, Y.F.; Li, S.; Liang, L.; Huang, Q.; Yuwen, L.; Yang, W.; Wang, R.; Wang, L.H. Highly biocompatible chlorin e6-loaded chitosan nanoparticles for improved photodynamic cancer therapy. ACS Appl. Mater. Interfaces 2018, 10, 9980–9987. [Google Scholar] [CrossRef] [PubMed]
- Kostryukova, L.V.; Prozorovskiy, V.N.; Medvedeva, N.V.; Ipatova, O.M. Comparison of a new nanoform of the photosensitizer chlorin e6, based on plant phospholipids, with its free form. FEBS Open Biol. 2018, 8, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Sadanala, K.C.; Chaturvedi, P.K.; Seo, Y.M.; Kim, J.M.; Jo, Y.S.; Lee, Y.K.; Ahn, W.S. Sono-photodynamic combination therapy: A review on sensitizers. Anticancer Res. 2014, 34, 4657–4664. [Google Scholar] [PubMed]
- Elsayed, M.M.; Abdallah, O.Y.; Naggar, V.F.; Khalafallah, N.M. Deformable liposomes and ethosomes as carriers for skin delivery of ketotifen. Pharmazie 2007, 62, 133–137. [Google Scholar]
- Lopez-Pinto, J.M.; Gonzalez-Rodriguez, M.L.; Rabasco, A.M. Effect of cholesterol and ethanol on dermal delivery from DPPC liposomes. Int. J. Pharm. 2005, 298, 1–12. [Google Scholar] [CrossRef]
- Touitou, E.; Dayan, N.; Bergelson, L.; Godin, B.; Eliaz, M. Ethosomes-novel vesicular carriers for enhanced delivery: Characterization and skin penetration properties. J. Control. Release 2000, 65, 403–418. [Google Scholar] [CrossRef]
- Nasr, S.; Rady, M.; Gomaa, I.; Syrovets, T.; Simmet, T.; Fayad, W.; Abdel-Kader, M. Ethosomes and lipid-coated chitosan nanocarriers for skin delivery of a chlorophyll derivative: A potential treatment of squamous cell carcinoma by photodynamic therapy. Int. J. Pharm. 2019, 568, 118528. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-dimensional in vitro cell culture models in drug discovery and drug repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef]
- Liu, M.; Scanlon, C.S.; Banerjee, R.; Russo, N.; Inglehart, R.C.; Willis, A.L.; Weiss, S.J.; D’Silva, N.J. The histone methyltransferase EZH2 mediates tumor progression on the chick chorioallantoic membrane assay, a novel model of head and neck squamous cell carcinoma. Transl. Oncol. 2013, 6, 273–281. [Google Scholar] [CrossRef]
- Busch, C.; Krochmann, J.; Drews, U. The chick embryo as an experimental system for melanoma cell invasion. PLoS ONE 2013, 8, e53970. [Google Scholar] [CrossRef]
- Schmiech, M.; Lang, S.J.; Ulrich, J.; Werner, K.; Rashan, L.J.; Syrovets, T.; Simmet, T. Comparative investigation of frankincense nutraceuticals: Correlation of boswellic and lupeolic acid contents with cytokine release inhibition and toxicity against triple-negative breast cancer cells. Nutrients 2019, 11, 2341. [Google Scholar] [CrossRef] [PubMed]
- El Gaafary, M.; Hafner, S.; Lang, S.J.; Jin, L.; Sabry, O.M.; Vogel, C.V.; Vanderwal, C.D.; Syrovets, T.; Simmet, T. A polyhalogenated monoterpene induces cell cycle arrest and apoptosis in breast cancer cells. Mar. Drugs 2019, 17, 437. [Google Scholar] [CrossRef] [PubMed]
- Estrada, A.C.; Syrovets, T.; Pitterle, K.; Lunov, O.; Büchele, B.; Schimana-Pfeifer, J.; Schmidt, T.; Morad, S.A.; Simmet, T. Tirucallic acids are novel pleckstrin homology domain-dependent Akt inhibitors inducing apoptosis in prostate cancer cells. Mol. Pharmacol. 2010, 77, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Syrovets, T.; Gschwend, J.E.; Büchele, B.; Laumonnier, Y.; Zugmaier, W.; Genze, F.; Simmet, T. Inhibition of IκB kinase activity by acetyl-boswellic acids promotes apoptosis in androgen-independent PC-3 prostate cancer cells in vitro and in vivo. J. Biol. Chem. 2005, 280, 6170–6180. [Google Scholar] [CrossRef] [PubMed]
- Halayqa, M.; Domanska, U. PLGA biodegradable nanoparticles containing perphenazine or chlorpromazine hydrochloride: Effect of formulation and release. Int. J. Mol. Sci. 2014, 15, 23909–23923. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Steelant, W.; Kumar, M.; Scholfield, M. Versatile photosensitizers for photodynamic therapy at infrared excitation. J. Am. Chem. Soc. 2007, 129, 4526–4527. [Google Scholar] [CrossRef] [PubMed]
- El Gaafary, M.; Büchele, B.; Syrovets, T.; Agnolet, S.; Schneider, B.; Schmidt, C.Q.; Simmet, T. An α-acetoxy-tirucallic acid isomer inhibits Akt/mTOR signaling and induces oxidative stress in prostate cancer cells. J. Pharmacol. Exp. Ther. 2015, 352, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fryknas, M.; Hernlund, E.; Fayad, W.; De Milito, A.; Olofsson, M.H.; Gogvadze, V.; Dang, L.; Pahlman, S.; Schughart, L.A.; et al. Induction of mitochondrial dysfunction as a strategy for targeting tumour cells in metabolically compromised microenvironments. Nat. Commun. 2014, 5, 3295. [Google Scholar] [CrossRef] [PubMed]
- Abu-Sinna, E.; Hasan, M.Y.; El-Deftar, M.M.; Amer, S.A.; Abdelsalam, L.O.; Nakhla, J.A. Galectin-3 and HBME-1 expression on agarose cell blocks from fine-needle aspirates of follicular cell-derived thyroid tumors. J. Cytol. 2018, 35, 27–32. [Google Scholar] [CrossRef]
- Wang, C.; Youle, R.J. The role of mitochondria in apoptosis. Annu. Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef]
- Sims, L.B.; Huss, M.K.; Frieboes, H.B.; Steinbach-Rankins, J.M. Distribution of PLGA-modified nanoparticles in 3D cell culture models of hypo-vascularized tumor tissue. J. Nanobiotechnol. 2017, 15, 67. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gomaa, I.; Sebak, A.; Afifi, N.; Abdel-Kader, M. Liposomal delivery of ferrous chlorophyllin: A novel third generation photosensitizer for in vitro PDT of melanoma. Photodiagnosis Photodyn. Ther. 2017, 18, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.T.; Shen, L.N.; Wu, Z.H.; Zhao, J.H.; Feng, N.P. Evaluation of skin viability effect on ethosome and liposome-mediated psoralen delivery via cell uptake. J. Pharm. Sci. 2014, 103, 3120–3126. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Chakraborty, S.; Roy, C.; Rajabalaya, R.; Mohaimin, A.W.; Khanam, J.; Nanda, A.; David, S.R. Ethosomes as novel vesicular carrier: An overview of the principle, preparation and its applications. Curr. Drug Deliv. 2018, 15, 795–817. [Google Scholar] [CrossRef]
- Rady, M.; Gomaa, I.; Afifi, N.; Abdel-Kader, M. Dermal delivery of Fe-chlorophyllin via ultradeformable nanovesicles for photodynamic therapy in melanoma animal model. Int. J. Pharm. 2018, 548, 480–490. [Google Scholar] [CrossRef]
- Ricci, J.E.; Gottlieb, R.A.; Green, D.R. Caspase-mediated loss of mitochondrial function and generation of reactive oxygen species during apoptosis. J. Cell Biol. 2003, 160, 65–75. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef]
- Ippolito, L.; Giannoni, E.; Chiarugi, P.; Parri, M. Mitochondrial redox hubs as promising targets for anticancer therapy. Front. Oncol. 2020, 10, 256. [Google Scholar] [CrossRef]
- Wang, W.; Moriyama, L.T.; Bagnato, V.S. Photodynamic therapy induced vascular damage: An overview of experimental PDT. Laser Phys. Lett. 2012, 10, 023001. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasr, S.; Rady, M.; Sebak, A.; Gomaa, I.; Fayad, W.; El Gaafary, M.; Abdel-Kader, M.; Syrovets, T.; Simmet, T. A Naturally Derived Carrier for Photodynamic Treatment of Squamous Cell Carcinoma: In Vitro and In Vivo Models. Pharmaceutics 2020, 12, 494. https://doi.org/10.3390/pharmaceutics12060494
Nasr S, Rady M, Sebak A, Gomaa I, Fayad W, El Gaafary M, Abdel-Kader M, Syrovets T, Simmet T. A Naturally Derived Carrier for Photodynamic Treatment of Squamous Cell Carcinoma: In Vitro and In Vivo Models. Pharmaceutics. 2020; 12(6):494. https://doi.org/10.3390/pharmaceutics12060494
Chicago/Turabian StyleNasr, Soad, Mai Rady, Aya Sebak, Iman Gomaa, Walid Fayad, Menna El Gaafary, Mahmoud Abdel-Kader, Tatiana Syrovets, and Thomas Simmet. 2020. "A Naturally Derived Carrier for Photodynamic Treatment of Squamous Cell Carcinoma: In Vitro and In Vivo Models" Pharmaceutics 12, no. 6: 494. https://doi.org/10.3390/pharmaceutics12060494
APA StyleNasr, S., Rady, M., Sebak, A., Gomaa, I., Fayad, W., El Gaafary, M., Abdel-Kader, M., Syrovets, T., & Simmet, T. (2020). A Naturally Derived Carrier for Photodynamic Treatment of Squamous Cell Carcinoma: In Vitro and In Vivo Models. Pharmaceutics, 12(6), 494. https://doi.org/10.3390/pharmaceutics12060494





