Particle-Size-Dependent Delivery of Antitumoral miRNA Using Targeted Mesoporous Silica Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Synthesis of MSN-NH2in-Shout
2.3. Template Extraction
2.4. Characterization
2.5. Loading of Ctrl and miR200c for Gene Silencing
2.6. Loading of Fluorescent Dye for Cellular Internalization Studies
2.7. Capping with 454-GE11
2.8. miRNA Binding Assay
2.9. Cell Culture
2.10. Cell Viability Determined by MTT Assay
2.11. Cellular Adhesion Determined by Flow Cytometry
2.12. Confocal Fluorescence Microscopy
2.13. Luciferase Gene Silencing
2.14. Cell Cycle Analysis
2.15. Scratch Assay
3. Results
3.1. Particle Synthesis
3.2. Characterization
3.3. Polymer Capping with 454-PEG and 454-GE11 and Targeting
3.4. Cell Internalization
3.5. Loading of RNA
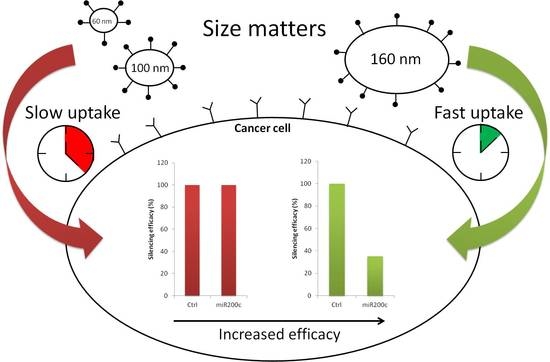
3.6. Gene Silencing
3.7. Antitumoral Effects
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Elbashir, S.M.; Harborth, J.; Lendeckel, W.; Yalcin, A.; Weber, K.; Tuschl, T. Duplexes of 21-Nucleotide Rnas Mediate RNA Interference in Cultured Mammalian Cells. Nature 2001, 411, 494. [Google Scholar] [CrossRef] [PubMed]
- McCaffrey, A.P.; Meuse, L.; Pham, T.-T.T.; Conklin, D.S.; Hannon, G.J.; Kay, M.A. RNA Interference in Adult Mice. Nature 2002, 418, 38. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Huang, M.; Guo, W.W.; Huang, Q.; Zhang, L.Z.; Jiang, G. Nano-Based Delivery of RNAi in Cancer Therapy. Mol. Cancer 2017, 16, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, R.C.; Farh, K.K.-H.; Burge, C.B.; Bartel, D.P. Most Mammalian mRNAs Are Conserved Targets of Micrornas. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.J.; Kim, M.J.; Kwon, I.C.; Roberts, T.M. Delivery Strategies and Potential Targets for siRNA in Major Cancer Types. Adv. Drug Deliv. Rev. 2016, 104, 2–15. [Google Scholar] [CrossRef] [Green Version]
- Kopp, F.; Wagner, E.; Roidl, A. The Proto-Oncogene KRAS Is Targeted by Mir-200c. Oncotarget 2013, 5, 185–195. [Google Scholar] [CrossRef]
- Hurteau, G.J.; Carlson, J.A.; Spivack, S.D.; Brock, G.J. Overexpression of the MicroRNA hsa-miR-200c Leads to Reduced Expression of Transcription Factor 8 and Increased Expression of E-Cadherin. Cancer Res. 2007, 67, 7972–7976. [Google Scholar] [CrossRef] [Green Version]
- Kopp, F.; Oak, P.S.; Wagner, E.; Roidl, A. Mir-200c Sensitizes Breast Cancer Cells to Doxorubicin Treatment by Decreasing Trkb and Bmi1 Expression. PLoS ONE 2012, 7, e50469. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Kim, A.; Miyata, K.; Kataoka, K. Recent Progress in Development of siRNA Delivery Vehicles for Cancer Therapy. Adv. Drug Deliv. Rev. 2016, 104, 61–77. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.; Park, J.-H.; Sailor, M.J. Rekindling RNAi Therapy: Materials Design Requirements for in Vivo siRNA Delivery. Adv. Mater. 2019, 31, 1903637. [Google Scholar] [CrossRef] [Green Version]
- Revia, R.A.; Stephen, Z.R.; Zhang, M. Theranostic Nanoparticles for Rna-Based Cancer Treatment. Acc. Chem. Res. 2019, 52, 1496–1506. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Gonzalez-Duarte, A.; O’Riordan, W.D.; Yang, C.C.; Ueda, M.; Kristen, A.V.; Tournev, I.; Schmidt, H.H.; Coelho, T.; Berk, J.L.; et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. New Engl. J. Med. 2018, 379, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gu, H.; Zhang, D.S.-Z.; Li, F.; Liu, T.; Xia, W. Highly Effective Inhibition of Lung Cancer Growth and Metastasis by Systemic Delivery of siRNA Via Multimodal Mesoporous Silica-Based Nanocarrier. Biomaterials 2014, 35, 10058–10069. [Google Scholar] [CrossRef] [PubMed]
- Hartono, S.B.; Gu, W.; Kleitz, F.; Liu, J.; He, L.; Middelberg, A.P.J.; Yu, C.; Lu, G.Q.; Qiao, S.Z. Poly-L-Lysine Functionalized Large Pore Cubic Mesostructured Silica Nanoparticles as Biocompatible Carriers for Gene Delivery. ACS Nano 2012, 6, 2104–2117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Möller, K.; Bein, T. Degradable Drug Carriers: Vanishing Mesoporous Silica Nanoparticles. Chem. Mater. 2019, 31, 4364–4378. [Google Scholar] [CrossRef]
- Braun, K.; Pochert, A.; Beck, M.; Fiedler, R.; Gruber, J.; Linden, M. Dissolution Kinetics of Mesoporous Silica Nanoparticles in Different Simulated Body Fluids. J. Sol.-Gel. Sci. Technol. 2016, 79, 319–327. [Google Scholar] [CrossRef]
- Kecht, J.; Schlossbauer, A.; Bein, T. Selective Functionalization of the Outer and Inner Surfaces in Mesoporous Silica Nanoparticles. Chem. Mater. 2008, 20, 7207–7214. [Google Scholar] [CrossRef]
- Möller, K.; Bein, T. Talented Mesoporous Silica Nanoparticles. Chem. Mater. 2017, 29, 371–388. [Google Scholar]
- Wang, J.; Lu, Z.; Wientjes, M.G.; Au, J.L.S. Delivery of siRNA Therapeutics: Barriers and Carriers. AAPS J. 2010, 12, 492–503. [Google Scholar] [CrossRef]
- Gao, F.; Botella, P.; Corma, A.; Blesa, J.; Dong, L. Monodispersed Mesoporous Silica Nanoparticles with Very Large Pores for Enhanced Adsorption and Release of DNA. J. Phys. Chem. B 2009, 113, 1796–1804. [Google Scholar] [CrossRef]
- Möller, K.; Müller, K.; Engelke, H.; Bräuchle, C.; Wagner, E.; Bein, T. Highly Efficient siRNA Delivery from Core–Shell Mesoporous Silica Nanoparticles with Multifunctional Polymer Caps. Nanoscale 2016, 8, 4007–4019. [Google Scholar]
- Jin, Y.; Lohstreter, S.; Pierce, D.T.; Parisien, J.; Wu, M.; Hall, C.; Zhao, J.X. Silica Nanoparticles with Continuously Tunable Sizes: Synthesis and Size Effects on Cellular Contrast Imaging. Chem. Mater. 2008, 20, 4411–4419. [Google Scholar] [CrossRef]
- Gan, Q.; Dai, D.; Yuan, Y.; Qian, J.; Sha, S.; Shi, J.; Liu, C. Effect of Size on the Cellular Endocytosis and Controlled Release of Mesoporous Silica Nanoparticles for Intracellular Delivery. Biomed. Microdevices 2012, 14, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Teng, X.; Chen, D.; Tang, F.; He, J. The Effect of the Shape of Mesoporous Silica Nanoparticles on Cellular Uptake and Cell Function. Biomaterials 2010, 31, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Tang, J.; Zhao, L.; Zhou, X.; Wang, Y.; Yu, C. Ultrasmall, Well-Dispersed, Hollow Siliceous Spheres with Enhanced Endocytosis Properties. Small 2010, 6, 276–282. [Google Scholar] [CrossRef]
- Chono, S.; Tanino, T.; Seki, T.; Morimoto, K. Uptake Characteristics of Liposomes by Rat Alveolar Macrophages: Influence of Particle Size and Surface Mannose Modification. J. Pharm. Pharmacol. 2007, 59, 75–80. [Google Scholar] [CrossRef]
- Osaki, F.; Kanamori, T.; Sando, S.; Sera, T.; Aoyama, Y. A Quantum Dot Conjugated Sugar Ball and Its Cellular Uptake. On the Size Effects of Endocytosis in the Subviral Region. J. Am. Chem. Soc. 2004, 126, 6520–6521. [Google Scholar] [CrossRef]
- Shang, L.; Nienhaus, K.; Nienhaus, G.U. Engineered Nanoparticles Interacting with Cells: Size Matters. J. Nanobiotechnology 2014, 12, 5. [Google Scholar] [CrossRef] [Green Version]
- Lu, F.; Wu, S.-H.; Hung, Y.; Mou, C.-Y. Size Effect on Cell Uptake in Well-Suspended, Uniform Mesoporous Silica Nanoparticles. Small 2009, 5, 1408–1413. [Google Scholar] [CrossRef]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C.W. Determining the Size and Shape Dependence of Gold Nanoparticle Uptake into Mammalian Cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef]
- Varela, J.A.; Bexiga, M.G.; Åberg, C.; Simpson, J.C.; Dawson, K.A. Quantifying Size-Dependent Interactions between Fluorescently Labeled Polystyrene Nanoparticles and Mammalian Cells. J. Nanobiotechnology 2012, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Bu, L.; Xie, J.; Chen, K.; Cheng, Z.; Li, X.; Chen, X. Effects of Nanoparticle Size on Cellular Uptake and Liver MRI with Polyvinylpyrrolidone-Coated Iron Oxide Nanoparticles. ACS Nano 2010, 4, 7151–7160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goel, S.; Chen, F.; Hong, H.; Valdovinos, H.; Hernandez, R.; Shi, S.; Barnhart, T.; Cai, W. Vegf121-Conjugated Mesoporous Silica Nanoparticle: A Tumor Targeted Drug Delivery System. ACS Appl. Mater. Interfaces 2014, 6, 21677–21685. [Google Scholar]
- Chen, F.; Nayak, T.R.; Goel, S.; Valdovinos, H.F.; Hong, H.; Theuer, C.P.; Barnhart, T.E.; Cai, W. In Vivo Tumor Vasculature Targeted Pet/Nirf Imaging with Trc105(Fab)-Conjugated, Dual-Labeled Mesoporous Silica Nanoparticles. Mol. Pharm. 2014, 11, 4007–4014. [Google Scholar] [CrossRef]
- Yu, M.; Niu, Y.; Zhang, J.; Zhang, H.; Yang, Y.; Taran, E.; Jambhrunkar, S.; Gu, W.; Thorn, P.; Yu, C. Size-Dependent Gene Delivery of Amine-Modified Silica Nanoparticles. Nano Res. 2016, 9, 291–305. [Google Scholar] [CrossRef]
- Nayak, T.R.; Krasteva, L.K.; Cai, W. Multimodality Imaging of RNA Interference. Curr. Med. chem. 2013, 20, 3664–3675. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Chen, Y.; Wang, M.; Ma, Y.; Xia, W.; Gu, H. A Mesoporous Silica Nanoparticle – Pei – Fusogenic Peptide System for siRNA Delivery in Cancer Therapy. Biomaterials 2013, 34, 1391–1401. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Liu, T.; Zhang, D.S.-Z.; Wang, Y.; Gu, H.; Di, W. Highly Effective Antiangiogenesis Via Magnetic Mesoporous Silica-Based siRNA Vehicle Targeting the Vegf Gene for Orthotopic Ovarian Cancer Therapy. Int. J. Nanomed. 2015, 10, 2579–2594. [Google Scholar]
- Wang, M.; Li, X.; Ma, Y.; Gu, H. Endosomal Escape Kinetics of Mesoporous Silica-Based System for Efficient siRNA Delivery. Int. J. Pharm. 2013, 448, 51–57. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, R.; Wu, X.; Sun, Y.; Yao, M.; Li, J.; Xu, Y.; Gu, J. Identification and Characterization of a Novel Peptide Ligand of Epidermal Growth Factor Receptor for Targeted Delivery of Therapeutics. Faseb. J. 2005, 19, 1978–1985. [Google Scholar] [CrossRef]
- Müller, K.; Klein, P.M.; Heissig, P.; Roidl, A.; Wagner, E. EGF Receptor Targeted Lipo-Oligocation Polyplexes for Antitumoral siRNA and Mirna Delivery. Nanotechnology 2016, 27, 464001. [Google Scholar] [CrossRef] [PubMed]
- Troiber, C.; Edinger, D.; Kos, P.; Schreiner, L.; Kläger, R.; Herrmann, A.; Wagner, E. Stabilizing Effect of Tyrosine Trimers on PDNA and siRNA Polyplexes. Biomaterials 2013, 34, 1624–1633. [Google Scholar] [CrossRef]
- Cauda, V.; Schlossbauer, A.; Kecht, J.; Zürner, A.; Bein, T. Multiple Core−Shell Functionalized Colloidal Mesoporous Silica Nanoparticles. J. Am. Chem. Soc. 2009, 131, 11361–11370. [Google Scholar] [CrossRef] [PubMed]
- Möller, K.; Kobler, J.; Bein, T. Colloidal Suspensions of Nanometer-Sized Mesoporous Silica. Adv. Funct. Mater. 2007, 17, 605–612. [Google Scholar] [CrossRef]
- Gu, J.; Huang, K.; Zhu, X.; Li, Y.; Wei, J.; Zhao, W.; Liu, C.; Shi, J. Sub-150nm Mesoporous Silica Nanoparticles with Tunable Pore Sizes and Well-Ordered Mesostructure for Protein Encapsulation. J. Colloid Interface Sci. 2013, 407, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Kobler, J.; Möller, K.; Bein, T. Colloidal Suspensions of Functionalized Mesoporous Silica Nanoparticles. ACS Nano 2008, 2, 791–799. [Google Scholar] [CrossRef]
- Schaffert, D.; Badgujar, N.; Wagner, E. Novel Fmoc-Polyamino Acids for Solid-Phase Synthesis of Defined Polyamidoamines. Org. Lett. 2011, 13, 1586–1589. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Muller, K.; Kessel, E.; Reinhard, S.; He, D.; Klein, P.M.; Hohn, M.; Rodl, W.; Kempter, S.; Wagner, E. Targeted siRNA Delivery Using a Lipo-Oligoaminoamide Nanocore with an Influenza Peptide and Transferrin Shell. Adv. Healthc. Mater. 2016, 5, 1493–1504. [Google Scholar] [CrossRef] [Green Version]
- Genta, I.; Chiesa, E.; Colzani, B.; Modena, T.; Conti, B.; Dorati, R. Ge11 Peptide as an Active Targeting Agent in Antitumor Therapy: A Minireview. Pharmaceutics 2017, 10, 2. [Google Scholar] [CrossRef] [Green Version]
- Biscaglia, F.; Rajendran, S.; Conflitti, P.; Benna, C.; Sommaggio, R.; Litti, L.; Mocellin, S.; Bocchinfuso, G.; Rosato, A.; Palleschi, A.; et al. Enhanced EGFR Targeting Activity of Plasmonic Nanostructures with Engineered Ge11 Peptide. Adv. Healthc. Mater. 2017, 6, 1700596. [Google Scholar] [CrossRef]
- Korpal, M.; Lee, E.S.; Hu, G.; Kang, Y. The miR-200 Family Inhibits Epithelial-Mesenchymal Transition and Cancer Cell Migration by Direct Targeting of E-Cadherin Transcriptional Repressors Zeb1 and Zeb2. J. Biol. Chem. 2008, 283, 14910–14914. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Name | Synthesis Method | Diameter TEM a [nm] | ABET [m2/g] | Pore Volume b [cc/g] | Pore Size c [nm] |
|---|---|---|---|---|---|
| MSN160 nm | CTAC Synthesis; TEOS:TEA = 1:10 | 159 ± 21 | 742 | 0.80 | 4.8 |
| MSN130 nm | CTAC Synthesis; TEOS:TEA = 1:5 | 128 ± 30 | 961 | 1.06 | 4.8 |
| MSN100 nm | CTAC Synthesis; TEOS:TEA = 1:3 | 100 ± 37 | 811 | 0.95 | 4.8 |
| MSN80 nm | F127 Synthesis; TEOS:TEA = 1:5 | 84 ± 23 | 685 | 0.95 | 6.0 |
| MSN60 nm | F127 Synthesis; TEOS:TEA = 1:3 | 59 ± 8 | 660 | 1.00 | 6.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haddick, L.; Zhang, W.; Reinhard, S.; Möller, K.; Engelke, H.; Wagner, E.; Bein, T. Particle-Size-Dependent Delivery of Antitumoral miRNA Using Targeted Mesoporous Silica Nanoparticles. Pharmaceutics 2020, 12, 505. https://doi.org/10.3390/pharmaceutics12060505
Haddick L, Zhang W, Reinhard S, Möller K, Engelke H, Wagner E, Bein T. Particle-Size-Dependent Delivery of Antitumoral miRNA Using Targeted Mesoporous Silica Nanoparticles. Pharmaceutics. 2020; 12(6):505. https://doi.org/10.3390/pharmaceutics12060505
Chicago/Turabian StyleHaddick, Lisa, Wei Zhang, Sören Reinhard, Karin Möller, Hanna Engelke, Ernst Wagner, and Thomas Bein. 2020. "Particle-Size-Dependent Delivery of Antitumoral miRNA Using Targeted Mesoporous Silica Nanoparticles" Pharmaceutics 12, no. 6: 505. https://doi.org/10.3390/pharmaceutics12060505
APA StyleHaddick, L., Zhang, W., Reinhard, S., Möller, K., Engelke, H., Wagner, E., & Bein, T. (2020). Particle-Size-Dependent Delivery of Antitumoral miRNA Using Targeted Mesoporous Silica Nanoparticles. Pharmaceutics, 12(6), 505. https://doi.org/10.3390/pharmaceutics12060505