Preserving the Integrity of Liposomes Prepared by Ethanol Injection upon Freeze-Drying: Insights from Combined Molecular Dynamics Simulations and Experimental Data
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Molecular Dynamics Simulations
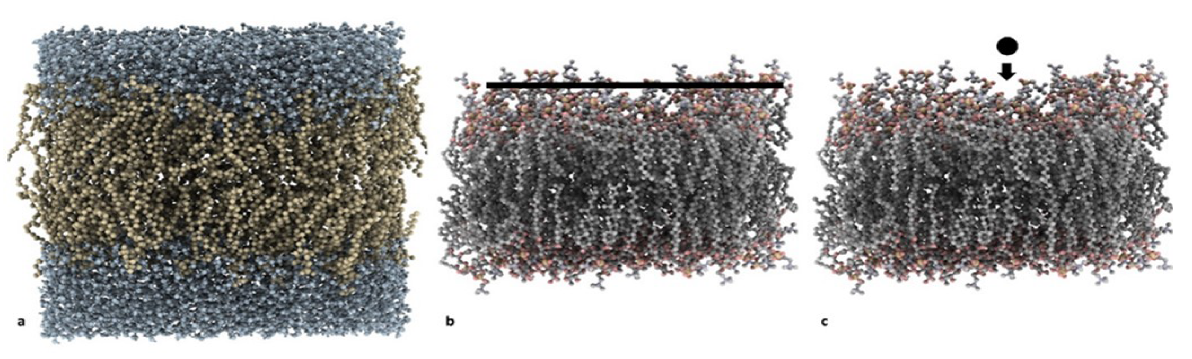
- MD set: MD simulations, where a layer of 48 sugar (e.g., sucrose or trehalose) molecules was placed on top of the membrane and the change in its physicochemical properties during the simulation was analyzed (Figure 1b);
- SMD set 1: SMD simulations, where a single sugar molecule was pulled from bulk water into the bilayer (Figure 1c); and
- SMD set 2: SMD simulations, where DPPC molecules were pulled from a PVP/trehalose cluster or from a PVP layer.
- Periodic boundary conditions were applied to stabilize the simulation space.
- Newton’s equation was integrated using the r-RESPA method (every 4 fs for long-range electrostatic forces, 2 fs for short-range non-bonded forces, and 1 fs for bonded forces).
- The long-range electrostatic potential was computed by the Particle Mesh Ewald summation method, and the chosen cutoff length was 12 Å for both the Van der Waals and electrostatic potentials, with a switching function starting at 8 Å.
- The temperature was maintained at 300 ± 10 K by Langevin’s algorithm.
- Lennard–Jones (L.-J.) interactions were calculated with a cutoff of 10 Å, and the pair list was updated every 20 iterations.
- A frame was memorized every 1 ps.
- No constraints were imposed to the systems.
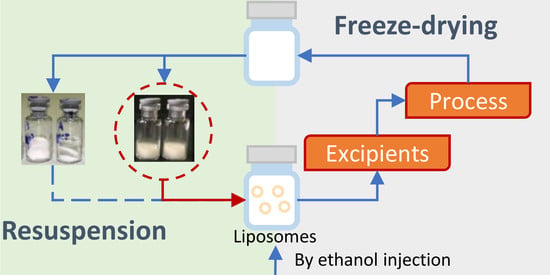
2.3. Liposome Preparation
2.3.1. Ethanol Injection
2.3.2. Thin-Film Hydration Method
2.4. Liposome Characterization
2.5. Residual Ethanol Content
2.6. Compatibility Study
2.7. Freeze-Thawing
2.8. Evaluation of the Fluidity of the Membrane in the Presence of Ethanol
2.9. Thermal Study on DPPC-Excipient Interactions
2.10. Freeze-Drying
2.11. Residual Water Content
2.12. Reconstitution
3. Results
3.1. Molecular Dynamics Simulations
- a set of MD simulations on system comprised of a layer of disaccharides and a DPPC bilayer to investigate the cryoprotectant effect of the disaccharides (MD set);
- a first set of Steered MD simulations to compare the energy of the trehalose-bilayer and sucrose-bilayer interactions (SMD set 1); and
- a second set of Steered MD simulations to compare the energy of the PVP-bilayer and trehalose-PVP-bilayer interactions (SMD set 2).
3.2. Liposome Characterization
3.3. Compatibility and Thawing Study
3.4. Thermotropic Behavior of DPPC in Presence of Protectant
3.5. Impact of Protectants on Freeze-Dried Product Quality
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Charcosset, C.; Juban, A.; Valour, J.P.; Urbaniak, S.; Fessi, H. Preparation of Liposomes at Large Scale Using the Ethanol Injection Method: Effect of Scale-up and Injection Devices. Chem. Eng. Res. Des. 2015, 94, 508–515. [Google Scholar] [CrossRef]
- Calvar, N.; González, B.; Gómez, E.; Domínguez, A. Study of the Behaviour of the Azeotropic Mixture Ethanol-Water with Imidazolium-Based Ionic Liquids. Fluid Phase Equilib. 2007, 259, 51–56. [Google Scholar] [CrossRef]
- Franzé, S.; Selmin, F.; Samaritani, E.; Minghetti, P.; Cilurzo, F. Lyophilization of Liposomal Formulations: Still Necessary, Still Challenging. Pharmaceutics 2018, 10, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Grainger, D.W. Lyophilized Liposome-Based Parenteral Drug Development: Reviewing Complex Product Design Strategies and Current Regulatory Environments. Adv. Drug Deliv. Rev. 2019, 151–152, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Arsiccio, A.; Pisano, R. Stability of Proteins in Carbohydrates and Other Additives during Freezing: The Human Growth Hormone as a Case Study. J. Phys. Chem. B 2017, 121, 8652–8660. [Google Scholar] [CrossRef]
- Arsiccio, A.; Pisano, R. Clarifying the Role of Cryo- and Lyo-Protectants in the Biopreservation of Proteins. Phys. Chem. Chem. Phys. 2018, 20, 8267–8277. [Google Scholar] [CrossRef]
- Arsiccio, A.; Pisano, R. The Preservation of Lyophilized Human Growth Hormone Activity: How Do Buffers and Sugars Interact? Pharm. Res. 2018, 35, 131. [Google Scholar] [CrossRef]
- Arsiccio, A.; Barresi, A.A.; Pisano, R. Prediction of Ice Crystal Size Distribution after Freezing of Pharmaceutical Solutions. Cryst. Growth Des. 2017, 17, 4573–4581. [Google Scholar] [CrossRef]
- Searles, J.A.; Carpenter, J.F.; Randolph, T.W. The Ice Nucleation Temperature Determines the Primary Drying Rate of Lyophilization for Samples Frozen on a Temperature-Controlled Shelf. J. Pharm. Sci. 2001, 90, 860–871. [Google Scholar] [CrossRef]
- Searles, J.A.; Carpenter, J.F.; Randolph, T.W. Annealing to Optimize the Primary Drying Rate, Reduce Freezing-Induced Drying Rate Heterogeneity, and Determine T(g)′ in Pharmaceutical Lyophilization. J. Pharm. Sci. 2001, 90, 872–887. [Google Scholar] [CrossRef]
- Pisano, R.; Capozzi, L.C. Prediction of Product Morphology of Lyophilized Drugs in the Case of Vacuum Induced Surface Freezing. Chem. Eng. Res. Des. 2017, 125, 119–129. [Google Scholar] [CrossRef]
- Oddone, I.; Barresi, A.A.; Pisano, R. Influence of Controlled Ice Nucleation on the Freeze-Drying of Pharmaceutical Products: The Secondary Drying Step. Int. J. Pharm. 2017, 524, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Bogner, R.H.; Nail, S.L.; Pikal, M.J. Stability of Freeze-Dried Protein Formulations: Contributions of Ice Nucleation Temperature and Residence Time in the Freeze-Concentrate. J. Pharm. Sci. 2020, 1–9. [Google Scholar] [CrossRef]
- Kunz, C.; Gieseler, H. Factors Influencing the Retention of Organic Solvents in Products Freeze-Dried From Co-Solvent Systems. J. Pharm. Sci. 2018, 2005–2012. [Google Scholar] [CrossRef] [PubMed]
- Teagarden, D.L.; Baker, D.S. Practical Aspects of Lyophilization Using Non-Aqueous Co-Solvent Systems. Eur. J. Pharm. Sci. 2002, 115–133. [Google Scholar] [CrossRef]
- Patel, S.M.; Nail, S.L.; Pikal, M.J.; Geidobler, R.; Winter, G.; Hawe, A.; Davagnino, J.; Rambhatla Gupta, S. Lyophilized Drug Product Cake Appearance: What Is Acceptable? J. Pharm. Sci. 2017, 1706–1721. [Google Scholar] [CrossRef]
- Abdelwahed, W.; Degobert, G.; Stainmesse, S.; Fessi, H. Freeze-Drying of Nanoparticles: Formulation, Process and Storage Considerations. Adv. Drug Deliv. Rev. 2006, 1688–1713. [Google Scholar] [CrossRef]
- Rocco, P.; Cilurzo, F.; Minghetti, P.; Vistoli, G.; Pedretti, A. Molecular Dynamics as a Tool for in Silico Screening of Skin Permeability. Eur. J. Pharm. Sci. 2017, 106, 328–335. [Google Scholar] [CrossRef]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kalé, L.; Schulten, K. Scalable Molecular Dynamics with NAMD. J. Comput. Chem. 2005, 1782–1802. [Google Scholar] [CrossRef] [Green Version]
- MacKerell, A.D.; Bashford, D.; Bellott, M.; Dunbrack, R.L.; Evanseck, J.D.; Field, M.J.; Fischer, S.; Gao, J.; Guo, H.; Ha, S.; et al. All-Atom Empirical Potential for Molecular Modeling and Dynamics Studies of Proteins. J. Phys. Chem. B 1998, 102, 3586–3616. [Google Scholar] [CrossRef]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. CHARMM General Force Field: A Force Field for Drug-like Molecules Compatible with the CHARMM All-Atom Additive Biological Force Fields. J. Comput. Chem. 2010, 31, 671–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedretti, A.; Villa, L.; Vistoli, G. VEGA: A Versatile Program to Convert, Handle and Visualize Molecular Structure on Windows-Based PCs. J. Mol. Graph. Model. 2002, 47–49. [Google Scholar] [CrossRef]
- Franzé, S.; Donadoni, G.; Podestà, A.; Procacci, P.; Orioli, M.; Carini, M.; Minghetti, P.; Cilurzo, F. Tuning the Extent and Depth of Penetration of Flexible Liposomes in Human Skin. Mol. Pharm. 2017, 14, 1998–2009. [Google Scholar] [CrossRef] [PubMed]
- Sauvage, F.; Franzè, S.; Bruneau, A.; Alami, M.; Denis, S.; Nicolas, V.; Lesieur, S.; Legrand, F.X.; Barratt, G.; Messaoudi, S.; et al. Formulation and in Vitro Efficacy of Liposomes Containing the Hsp90 Inhibitor 6BrCaQ in Prostate Cancer Cells. Int. J. Pharm. 2016, 499, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Pikal, M.J. Design of Freeze-Drying Processes for Pharmaceuticals: Practical Advice. Pharm. Res. 2004, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Magri, G.; Franzé, S.; Musazzi, U.M.; Selmin, F.; Cilurzo, F. Maltodextrins as Drying Auxiliary Agent for the Preparation of Easily Resuspendable Nanoparticles. J. Drug Deliv. Sci. Technol. 2019, 50, 181–187. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods. V. Modification of NDDO approximations and application to 70 elements. J. Mol. Model. 2007, 13, 1173–1213. [Google Scholar] [CrossRef] [Green Version]
- Jerez, A.T.; Ming-Liang, T.; Toshiko, I. Solvation of Biomolecules by the Soft Sticky Dipole-Quadrupole-Octupole Water Model. Chem. Phys. Lett. 2010, 486, 70–73. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.L.; Debenedetti, P.G.; Errington, J.R. A Computational Study of Hydration, Solution Structure, and Dynamics in Dilute Carbohydrate Solutions. J. Chem. Phys. 2005, 122. [Google Scholar] [CrossRef]
- Jarzynski, C. Nonequilibrium Equality for Free Energy Differences. Phys. Rev. Lett. 1997, 78, 2690–2693. [Google Scholar] [CrossRef] [Green Version]
- Wagner, A.; Vorauer-Uhl, K.; Kreismayr, G.; Katinger, H. Ehhanced Protein Loadind into Liposomes by the Multiple Crossflow Injection Technique. J. Liposome Res. 2002, 12, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Stetter, F.W.S.; Hugel, T. The Nanomechanical Properties of Lipid Membranes Are Significantly Influenced by the Presence of Ethanol. Biophys. J. 2013, 104, 1049–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohtake, S.; Schebor, C.; de Pablo, J.J. Effects of Trehalose on the Phase Behavior of DPPC-Cholesterol Unilamellar Vesicles. Biochim. Biophys. Acta Biomembr. 2006, 1758, 65–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savva, M.; Torchilin, V.P.; Huang, L. Effect of Polyvinyl Pyrrolidone on the Thermal Phase Transition of 1,2 Dipalmitoyl-Sn-Glycero-3-Phosphocholine Bilayer. J. Colloid Interface Sci. 1999, 217, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Fang, N.; Chan, V.; Mao, H.Q.; Leong, K.W. Interactions of Phospholipid Bilayer with Chitosan: Effect of Molecular Weight and PH. Biomacromolecules 2001, 2, 1161–1168. [Google Scholar] [CrossRef]
- Kunz, C.; Gieseler, H. Merits and Limitations of Dynamic Vapor Sorption Studies on the Morphology and Physicochemical State of Freeze-Dried Products. J. Pharm. Sci. 2018, 107, 2179–2191. [Google Scholar] [CrossRef]
- Schersch, K.; Betz, O.; Garidel, P.; Muehlau, S.; Bassarab, S.; Winter, G. Systematic Investigation of the Effect of Lyophilizate Collapse on Pharmaceutically Relevant Proteins I: Stability after Freeze-Drying. J. Pharm. Sci. 2010, 99, 2256–2278. [Google Scholar] [CrossRef]
- Depaz, R.A.; Pansare, S.; Patel, S.M. Freeze-Drying above the Glass Transition Temperature in Amorphous Protein Formulations While Maintaining Product Quality and Improving Process Efficiency. J. Pharm. Sci. 2016, 105, 40–49. [Google Scholar] [CrossRef]




| Lyo-Cycle | Freezing | Primary Drying | Secondary Drying |
|---|---|---|---|
| A | −40 °C 8 h | −40 °C | 25 °C |
| 0.2 mbar | 0.2 mbar | ||
| 48 h | 10 h | ||
| B | −40 °C 8 h | −48 °C | 25 °C |
| 0.2 mbar | 0.2 mbar | ||
| 48 h | 10 h | ||
| C | −48 °C 8 h | −48 °C for 12 h | 25 °C |
| −40 °C for 13 h | 0.1 mbar | ||
| 0.1 mbar | 6 h |
| Form ID | Composition | Before Freezing | After Thawing | |||
|---|---|---|---|---|---|---|
| Disaccharide | PVP | Dh (nm) | PDI | Dh (nm) | PDI | |
| 1 | Trehalose | - | 170 ± 1 | 0.04 ± 0.02 | 169 ± 9 | 0.14 ± 0.06 |
| 2 | Sucrose | - | 171 ± 2 | 0.05 ± 0.03 | 200 ± 18 | 0.29 ± 0.03 |
| 3 | - | 0.50 | 172 ± 1 | 0.03 ± 0.01 | - * | - * |
| 4 | - | 0.75 | 171 ± 3 | 0.03 ± 0.02 | - * | - * |
| 5 | Trehalose | 0.50 | 179 ± 2 | 0.10 ± 0.02 | 205 ± 9 | 0.15 ± 0.01 |
| 6 | Trehalose | 0.75 | 176 ± 2 | 0.12 ± 0.01 | >400 | >0.4 |
| 7 | Sucrose | 0.50 | 180 ± 3 | 0.11 ± 0.01 | 209 ± 0 | 0.19 ± 0.04 |
| Inner Phase (Liposome Core) | Outer Phase (Dispersant) | Tp | Tm | ||||
|---|---|---|---|---|---|---|---|
| ∆H (J/g) | Tonset (°C) | Tpeak (°C) | ∆H (J/g) | Tonset (°C) | Tpeak (°C) | ||
| - | - | 3.21 ± 0.96 | 34.16 ± 0.40 | 35.39 ± 0.19 | 55.54 ± 0.33 | 41.39 ± 0.02 | 41.82 ± 0.03 |
| Trehalose | Trehalose | 4.88 ± 0.23 | 33.94 ± 0.47 | 35.69 ± 0.47 | 62.55 ± 1.41 | 40.89 ± 0.32 | 41.59 ± 0.16 |
| PVP | PVP | - | - | - | 36.36 ± 0.44 | 41.51 ± 0.08 | 41.95 ± 0.09 |
| Water | PVP/trehalose | 1.94 ± 0.21 | 35.23 ± 0.40 | 36.26 ± 0.04 | 40.92 ± 0.46 | 41.40 ± 0.02 | 41.96 ± 0.15 |
| Trehalose | PVP/trehalose | 4.63 ± 0.06 | 35.01 ± 0.22 | 36.47 ± 0.31 | 36.48 ± 1.86 | 41.59 ± 0.04 | 41.90 ± 0.19 |
| PVP/trehalose | PVP/trehalose | 4.65 ± 0.79 | 35.83 ± 0.15 | 37.35 ± 0.19 | 42.69 ± 0.97 | 41.87 ± 0.10 | 42.12 ± 0.12 |
| % Ethanol | Tg′ (°C) | ||
|---|---|---|---|
| Trehalose | PVP | Mixture | |
| 0 | −28.53 ± 1.44 | −28.32 ± 0.68 | −27.63 ± 0.06 |
| 0.1 | −29.35 ± 1.52 | −32.48 ± 0.48 | −30.17 ± 1.10 |
| 1 | −35.42 ± 1.53 | −47.98 ± 3.16 | −45.04 ± 0.86 |
| 5 | −45.47 ± 1.61 | - 1 | - 1 |
| Ethanol (%) | Trehalose: DPPC (mol/mol) | PVP (%, m/v) | Pre-Lyophilization | Post-Lyophilization | ||
|---|---|---|---|---|---|---|
| DH (nm) | PDI | DH (nm) | PDI | |||
| 0 | 5:1 | - | 170 ± 1 | 0.04 ± 0.02 | 286 ± 24 | 0.38 ± 0.03 |
| 5:1 | 0.5 | 185 ± 1 | 0.12 ± 0.03 | 261 ± 4 | 0.20 ± 0.02 | |
| 0.1 | 5:1 | - | 112 ± 0 | 0.14 ± 0.12 | 213 ± 14 | 0.39 ± 0.00 |
| 5:1 | 0.5 | 173 ± 2 | 0.28 ± 0.00 | 194 ± 16 | 0.30 ± 0.01 | |
| 1.0 | 5:1 | - | 118 ± 1 | 0.16 ± 0.00 | 216 ± 51 | 0.48 ± 0.20 |
| 5:1 | 0.5 | 125 ± 0 | 0.22 ± 0.00 | 266 ± 22 | 0.39 ± 0.06 | |
| 6.0 | 5:1 | - | 158 ± 2 | 0.25 ± 0.01 | 512 ± 133 | 0.53 ± 0.14 |
| 5:1 | 0.5 | 158 ± 2 | 0.23 ± 0.02 | 484 ± 368 | 0.48 ± 0.26 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franzè, S.; Selmin, F.; Rocco, P.; Colombo, G.; Casiraghi, A.; Cilurzo, F. Preserving the Integrity of Liposomes Prepared by Ethanol Injection upon Freeze-Drying: Insights from Combined Molecular Dynamics Simulations and Experimental Data. Pharmaceutics 2020, 12, 530. https://doi.org/10.3390/pharmaceutics12060530
Franzè S, Selmin F, Rocco P, Colombo G, Casiraghi A, Cilurzo F. Preserving the Integrity of Liposomes Prepared by Ethanol Injection upon Freeze-Drying: Insights from Combined Molecular Dynamics Simulations and Experimental Data. Pharmaceutics. 2020; 12(6):530. https://doi.org/10.3390/pharmaceutics12060530
Chicago/Turabian StyleFranzè, Silvia, Francesca Selmin, Paolo Rocco, Giuseppe Colombo, Antonella Casiraghi, and Francesco Cilurzo. 2020. "Preserving the Integrity of Liposomes Prepared by Ethanol Injection upon Freeze-Drying: Insights from Combined Molecular Dynamics Simulations and Experimental Data" Pharmaceutics 12, no. 6: 530. https://doi.org/10.3390/pharmaceutics12060530