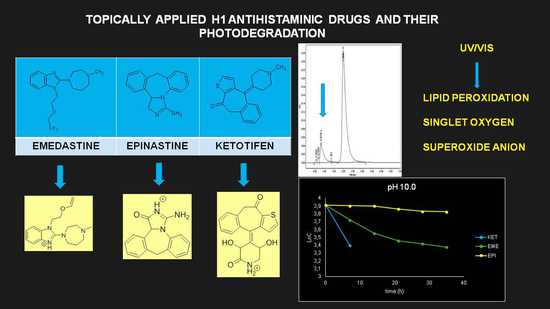
Photodegradation of the H1 Antihistaminic Topical Drugs Emedastine, Epinastine, and Ketotifen and ROS Tests for Estimations of Their Potent Phototoxicity
Abstract
:1. Introduction
2. Materials and Methods
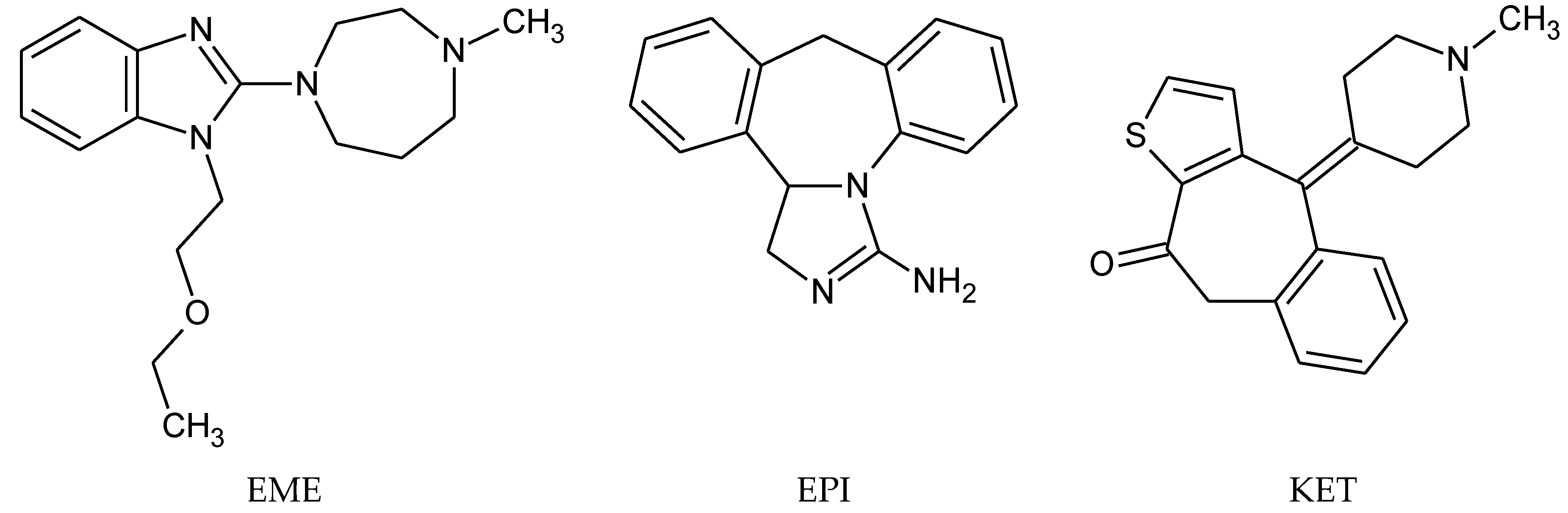
2.1. Materials
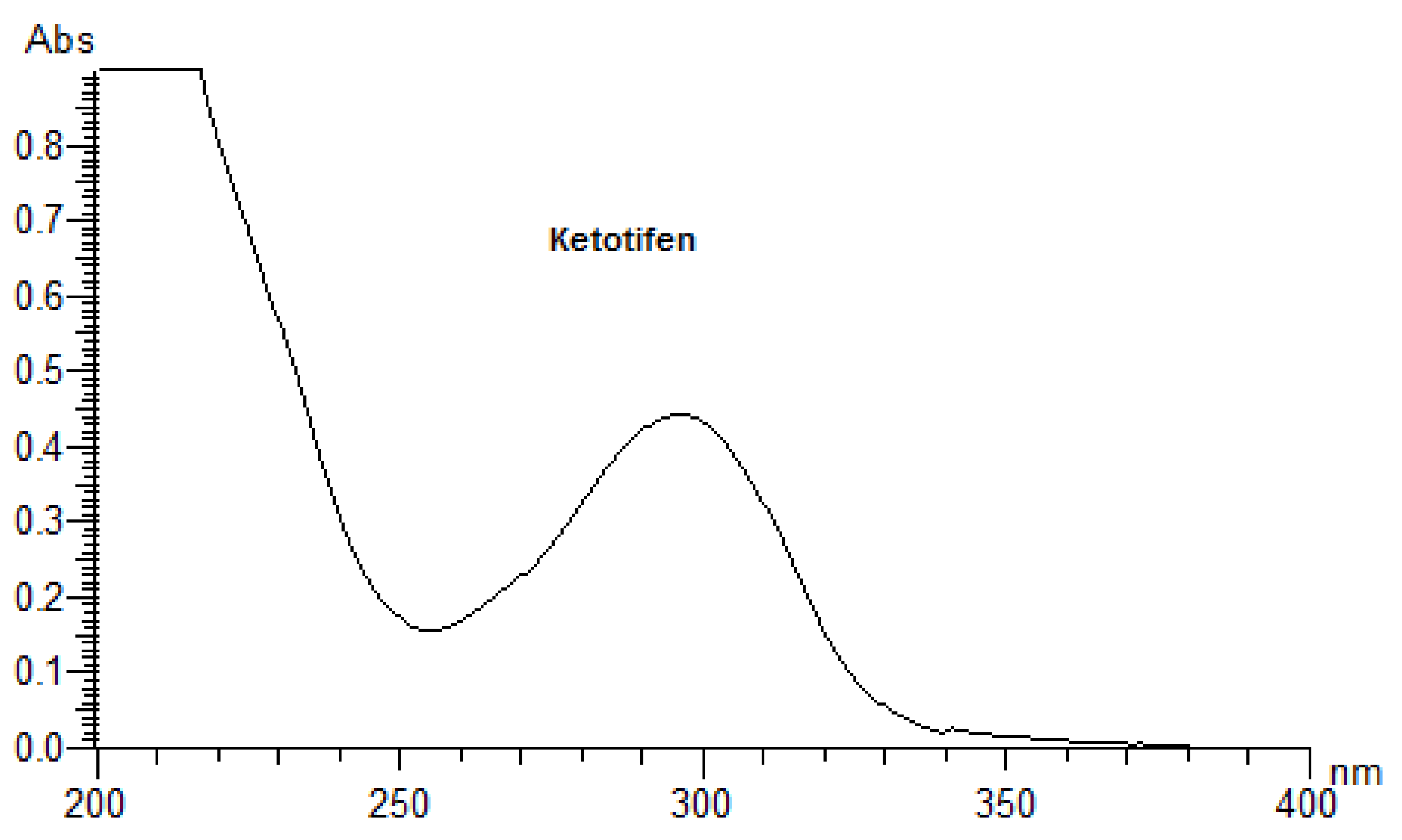
2.2. Preliminary Spectrophotometric Measurements
2.3. Solar Simulator
2.4. Photodegradation of EME, EPI, and KET in Solutions
Kinetics of Photodegradation
2.5. HPLC Method
2.5.1. Chromatography
2.5.2. Validation of the Methods
2.6. UPLC-MS/MS Analysis
2.7. ROS Assays
3. Results and Discussion
3.1. UV Measurements
3.2. Validation of the HPLC Method
3.3. Percentage of Degradation of EME, EPI, and KET
3.4. Kinetics of Degradation
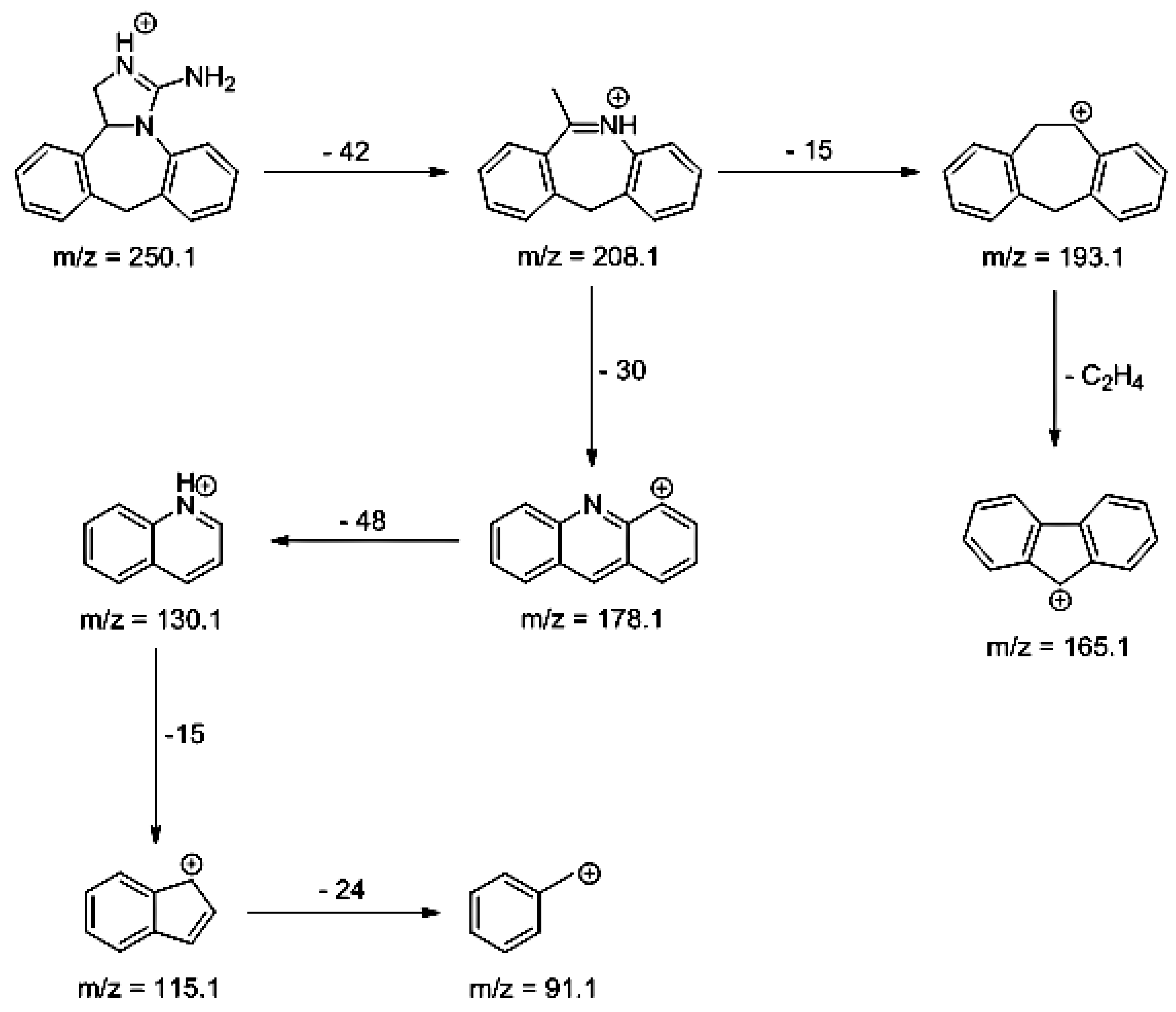
3.5. LC/MS
3.6. ROS Assays
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ahmad, I.; Ahmed, S.; Anwar, Z.; Ali Sheraz, M.; Sikorski, M. Photostability and photostabilization of drugs and drug products. Int. J. Photoenergy 2016, 7, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Bajaj, S.; Singla, D.; Sakhu, N. Stability testing of pharmaceutical Products. J. Appl. Pharm. Sci. 2012, 2, 129–138. [Google Scholar]
- Henry, B.; Foti, C.; Alsante, K. Can light absorption and photostability data be used to assess the photosafety risks in patients for a new drug molecule. J. Photochem. Photobiol. B Biol. 2009, 96, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Borges, M.; Ansotegui, J. Second generation antihistamines: An update. Curr. Opin. Allergy Immunol. 2019, 19, 358–364. [Google Scholar] [CrossRef]
- Ben-Eli, H.; Solomon, A. Topical antihistamines, mast cell stabilizers, and dual-action agents in ocular allergy: Current trends. Curr. Opin. Allergy Immunol. 2018, 18, 411–416. [Google Scholar] [CrossRef]
- Zgadzaj, A.; Kornacka, J.; Jastrzębska, A.; Parzonko, A.; Sommer, S.; Nałęcz-Jawecki, G. Development of photoprotective, antiphototoxic and antiphotogenotoxic formulations of ocular drugs with fluoroquinolones. J. Photochem. Photobiol. B Biol. 2018, 178, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Jamrógiewicz, M. Consequences of new approach to chemical stability tests to active pharmaceutical ingredients. Front. Pharmacol. 2016, 7, 17. [Google Scholar] [CrossRef] [Green Version]
- Kryczyk-Poprawa, A.; Kwiecień, A.; Opoka, W. Photostability of topical agents applied to the skin: A review. Pharmaceutics 2020, 12, 10. [Google Scholar] [CrossRef] [Green Version]
- Salama, N.N.; Abdel-Razeq, S.A.; Abdel-Atty, S.; El-Kosy, N. Development and validation of densitometry TLC stability indicating method for quantitative determination of azelastine hydrochloride and emedastine hydrochloride in their drug products. Br. J. Pharm. Res. 2014, 4, 79–92. [Google Scholar] [CrossRef]
- Dal Molim, D.; Steppe, M.; Schapoval, E. Development and validation of liquid chromatographic and ultraviolet derivative spectrophotometric methods for determination of epinastine hydrochloride in coated tablets. J. AOAC Int. 2007, 5, 1266–1271. [Google Scholar]
- El-Bagary, R.; Boshra, A.; El-Hakeem, M.; Abdelrao, A. Three analytical methods for determination of epinastine hydrochloride in bulk and in ophthalmic solutions. J. Chem. Pharm. Res. 2012, 4, 1361–1369. [Google Scholar]
- Malakar, A.; Bokshi, B. Development and validation of stability indicating RP-HPLC method for the determination of epinastine hydrochloride in pharmaceutical dosage form. Int. Curr. Pharm. J. 2012, 1, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Ahhirao, V.; Pawar, R. Stability-indicating LC method for the determination of epinastine in bulk drug and in pharmaceutical dosage form. Res. J. Recent Sci. 2012, 1, 281–288. [Google Scholar]
- Baburao Ubale, M. A validated stability-indicating HPLC assay method for epinastine HCl in bulk drug. J. Curr. Chem. Pharmaceut. Sci. 2012, 2, 107–112. [Google Scholar]
- Troche Concepción, Y.; Romero Diaz, J.A.; García Peña, C.M. Validation of analytical method to quality control and the stability study of 0.025% eye drops ketotifen. Rev. Cuba Farm. 2010, 44, 318–325. [Google Scholar]
- Nnane, I.P.; Damani, L.A.; Hutt, A.J. Development and validation of stability indicating high-performance liquid chromatographic assays for ketotifen in aqueous and silicon oil formulations. Chromatographia 1998, 48, 797–802. [Google Scholar] [CrossRef]
- Kabra, P.V.; Nargund, L.V.G.; Murthy, M.S. Development and validation of a stability-indicating LC-UV method for simultaneous determination of ketotifen and cetirizine in pharmaceutical dosage forms. Trop. J. Pharm. 2014, 13, 1141–1147. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Bary, A.A.; Salem, H.F.; Kharshoum, R.M. 2-Hydroxypropyl-β-cyclodextrin complex with ketotifen fumarate for eye drops preparations. Int. J. Drug Deliv. 2011, 3, 228–240. [Google Scholar]
- European Directorate for the Quality of Medicines & HealthCare. European Pharmacopoeia, 9th ed.; European Pharmacopoeia, Council of Europe: Strasbourg, France, 2016. [Google Scholar]
- Organization for Economic Co-Operation and Development. Guidelines for the Testing of Chemicals; Test Guideline No 495: Reactive Oxygen Species (ROS) Assay for Photoreactivity; OECD Publishing: Paris, France, 2019. [Google Scholar]
- ICH Harmonised Tripartite Guideline Q1B: Stability Testing: Photostability Testing of New Drug Substances and Products; EMEA: London, UK, 1996.
- Seto, Y.; Inoue, R.; Kato, M.; Yamada, S.; Onoue, S. Photosafety assessments on pirfenidone: Photochemical, photobiological and pharmacokinetic characterization. J. Photochem. Photobiol. B Biol. 2013, 120, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Onoue, S.; Suzuki, G.; Kato, M.; Hirota, M.; Nishida, H.; Kitagaki, M.; Kouzuki, H.; Yamada, S. Non-animal photosafety assessment approaches for cosmetics based on the photochemical and photobiochemical properties. Toxicol. In Vitro 2013, 27, 2316–2324. [Google Scholar] [CrossRef] [PubMed]
- International Conference on Harmonization. ICH Guideline S10: Guidance on Photosafety Evaluation of Pharmaceuticals; International Conference on Harmonization: EMA: London, UK, 2014. [Google Scholar]
- International Conference on Harmonization. ICH Harmonized Tripartite Guideline Q2(R1): Validation of Analytical Procedures: Methodology; International Conference on Harmonization: EMEA: London, UK, 1995. [Google Scholar]
- Ahmad, I.; Bano, R.; AliSheraz, M.; Ahmed, S.; Mirza, T. Photodegradation of levofloxacin in aqeous and organic solvents: A kinetic study. Acta Pharm. 2013, 63, 223–229. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, A.; Salama, A.; Nasser, W.; Uheida, A. Photodegradation of ibuprofen, cetirizine and naproxen by PAN-MWCNT/TiO2-NH2 nanofiber membrane under UV light irradiation. Environ. Sci. Eur. 2018, 30, 47. [Google Scholar] [CrossRef] [PubMed]
- Emedastine. Available online: https://www.drugbank.ca/drugs/DB01084 (accessed on 18 May 2020).
- Epinastine. Available online: https://www.drugbank.ca/drugs/DB00751 (accessed on 18 May 2020).
- Ketotifen. Available online: https://www.drugbank.ca/drugs/DB00920 (accessed on 18 May 2020).
- Abounassif, M.A.; El-Obeid, H.A.; Gadkariem, E.A. Stability studies on some benzocycloheptane antihistaminic agents. J. Pharm. Biomed. Anal. 2005, 36, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Shakouri, A.A.; Bahna, S.L. Hypersensitivity to antihistamines. Allergy Asthma Proc. 2013, 34, 488–496. [Google Scholar] [CrossRef]
- Rutkowski, K.; Li, P.H.; Wagner, A. The paradox of antihistamine hypersensitivity. J. Allergy Clin. Immunol. 2018, 6, 258–259. [Google Scholar] [CrossRef] [PubMed]
- Baranowski, P.; Karolewicz, B.; Gajda, M.; Pluta, J. Ophtalmic drug dosage forms: Characterization and research methods. Sci. World J. 2014, 1–14. [Google Scholar] [CrossRef] [Green Version]













| Parameter | Values | ||
|---|---|---|---|
| EME | EPI | KET | |
| Linearity range (µg/mL) | 10–100 | 10–100 | 10–100 |
| Slope | 0.01942 | 0.03020 | 0.0061 |
| SD of the slope | 0.00129 | 0.00028 | 0.00002 |
| Intercept | 0.06199 | 0.04752 | 0.0018 |
| SD of the intercept | 0.00913 | 0.00475 | 0.00012 |
| r2 | 0.9991 | 0.9962 | 0.9997 |
| SD of the r2 | 0.00011 | 0.00042 | 0.00018 |
| LOD (µg/mL) | 1.41 | 0.47 | 0.06 |
| LOQ (µg/mL) | 4.70 | 1.57 | 0.20 |
| Accuracy (% Recovery) | 97.95 | 104.65 | 102.69 |
| SD of the recovery | 1.73 | 1.14 | 2.10 |
| Inter day precision (% RSD) | 2.06 | 1.03 | 0.21 |
| Conditions | Degradation (%) | y = ax + b | r | K (min−1) | t0.1 (h) | t0.5 (h) |
|---|---|---|---|---|---|---|
| EME | ||||||
| Buffer pH 3.0 | 32.38 | y = −0.0107x + 3.8306 | 0.9136 | 4.11 × 10−4 | 4.26 | 28.12 |
| Buffer pH 7.0 | 38.34 | y = −0.0143x + 3.8604 | 0.9390 | 5.48 × 10−4 | 3.19 | 21.04 |
| Buffer pH 10.0 | 41.52 | y = −0.0151x + 3.8371 | 0.9543 | 5.79 × 10−4 | 3.02 | 19.92 |
| EPI | ||||||
| Buffer pH 3.0 | 19.10 | y = −0.0054x + 3.8728 | 0.9351 | 2.07 × 10−4 | 8.44 | 55.72 |
| Buffer pH 7.0 | 12.16 | y = −0.0044x + 3.9112 | 0.9860 | 1.69 × 10−4 | 10.4 | 68.39 |
| Buffer pH 10.0 | 8.10 | y = −0.0028x + 3.9214 | 0.9604 | 1.07 × 10−4 | 16.3 | 107.5 |
| KET | ||||||
| Buffer pH 3.0 | 14.64 | y = −0.0046x + 3.9004 | 0.9823 | 1.77 × 10−4 | 9.91 | 65.42 |
| Buffer pH 7.0 | 19.28 | y = −0.0049x + 3.8804 | 0.9303 | 8.87 × 10−4 | 1.97 | 13.03 |
| Buffer pH 10.0 | 100 | - | - | - | - | - |
| Compound | tR (min) | [M + H] + | Fragmentation Ions | Structure |
|---|---|---|---|---|
| EME | 2.20 | 303.2 | 134.1, 146.1, 174.1, 200.1, 218.1, 232.1, 246.2 | 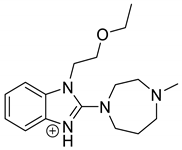 |
| EME-P1 pH 3.0 | 2.02 | 301.2 | 146.1, 218.1, 244.1 | 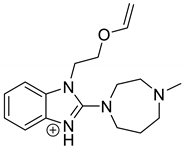 |
| EME-P2 = Impurity F [19] pH 3.0 pH 7.0 pH 10.0 | 2.49 | 277.2 | 134.1, 146.1, 174.1, 200.1, 218.1, 246.2 | 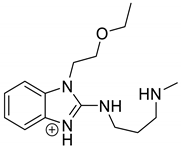 |
| Compound | tR (min) | [M + H] + | Fragmentation Ions | Structure |
|---|---|---|---|---|
| EPI | 3.79 | 250.1 | 91.1, 115.1, 130.1, 165.1, 178.1, 193.1, 208.1 | 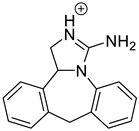 |
| EPI-P1 pH 3.0 | 3.30 | 266.1 | 127.1, 178.1, 204.1, 248.1 | 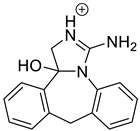 |
| EPI-P2 pH 3.0 | 3.43 | 264.1 | 178.1, 204.1, 246.1 | 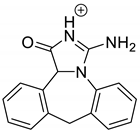 |
| EPI-P3 = Impurity A [19] pH 3.0 pH 10.0 | 4.06 | 248.1 | 127.1, 178.1, 204.1 |  |
| EPI-P4 pH 3.0 pH 10.0 | 3.35 | 270.1 | 192.1, 210.1, 237.1 |  |
| Compound | tR (min) | [M + H] + | Fragmentation Ions | Structure |
|---|---|---|---|---|
| KET | 3.79 | 310.1 | 96.1, 185.0, 199.0, 213.1, 221.0, 249.0 | 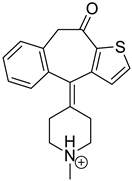 |
| KET-P1 pH 7.0 | 3.43 | 342.1 | 324.1, 306.1, 278.1, 221.0 | 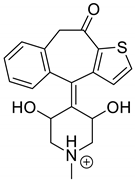 |
| KET-P2 pH 7.0 | 3.56 | 320.1 | 247.1, 275.1, 263.1, 292.1 | 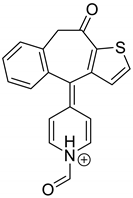 |
| KET-P3 pH 7.0 | 3.69 | 342.1 | 221.0, 250.1, 278.1, 306.1, 324.1 | 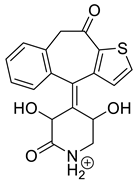 |
| Energy (kJ/m2) | EME | EPI | KET | Quinine | Benzocaine |
|---|---|---|---|---|---|
| TBARs | |||||
| 675 | 0.107 | 0.087 | 0.120 | 0.162 | 0.055 |
| 1350 | 0.118 | 0.089 | 0.121 | 0.170 | 0.057 |
| 2025 | 0.122 | 0.091 | 0.136 | 0.175 | 0.062 |
| 2700 | 0.131 | 0.093 | 0.141 | 0.189 | 0.063 |
| 8100 | 0.133 | 0.101 | 0.207 | 0.244 | 0.078 |
| 18,902 | 0.136 | 0.109 | 0.274 | 0.285 | 0.081 |
| SO | |||||
| 675 | 8 | 6 | 9 | 282 | −9 |
| 1350 | 10 | 8 | 11 | 319 | −8 |
| 2025 | 21 | 8 | 27 | 401 | −3 |
| 2700 | 37 | 23 | 55 | 493 | 10 |
| 8100 | 72 | 27 | 173 | 543 | 13 |
| 18,902 | 101 | 35 | 452 | 586 | 21 |
| SA | |||||
| 675 | 29 | 6 | 60 | 133 | −11 |
| 1350 | 34 | 21 | 86 | 148 | −8 |
| 2025 | 49 | 29 | 110 | 179 | −6 |
| 2700 | 110 | 34 | 151 | 221 | 2 |
| 8100 | 216 | 44 | 358 | 422 | 8 |
| 18,902 | 226 | 62 | 424 | 540 | 9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gumieniczek, A.; Berecka-Rycerz, A.; Hubicka, U.; Żmudzki, P.; Lejwoda, K.; Kozyra, P. Photodegradation of the H1 Antihistaminic Topical Drugs Emedastine, Epinastine, and Ketotifen and ROS Tests for Estimations of Their Potent Phototoxicity. Pharmaceutics 2020, 12, 560. https://doi.org/10.3390/pharmaceutics12060560
Gumieniczek A, Berecka-Rycerz A, Hubicka U, Żmudzki P, Lejwoda K, Kozyra P. Photodegradation of the H1 Antihistaminic Topical Drugs Emedastine, Epinastine, and Ketotifen and ROS Tests for Estimations of Their Potent Phototoxicity. Pharmaceutics. 2020; 12(6):560. https://doi.org/10.3390/pharmaceutics12060560
Chicago/Turabian StyleGumieniczek, Anna, Anna Berecka-Rycerz, Urszula Hubicka, Paweł Żmudzki, Karolina Lejwoda, and Paweł Kozyra. 2020. "Photodegradation of the H1 Antihistaminic Topical Drugs Emedastine, Epinastine, and Ketotifen and ROS Tests for Estimations of Their Potent Phototoxicity" Pharmaceutics 12, no. 6: 560. https://doi.org/10.3390/pharmaceutics12060560
APA StyleGumieniczek, A., Berecka-Rycerz, A., Hubicka, U., Żmudzki, P., Lejwoda, K., & Kozyra, P. (2020). Photodegradation of the H1 Antihistaminic Topical Drugs Emedastine, Epinastine, and Ketotifen and ROS Tests for Estimations of Their Potent Phototoxicity. Pharmaceutics, 12(6), 560. https://doi.org/10.3390/pharmaceutics12060560