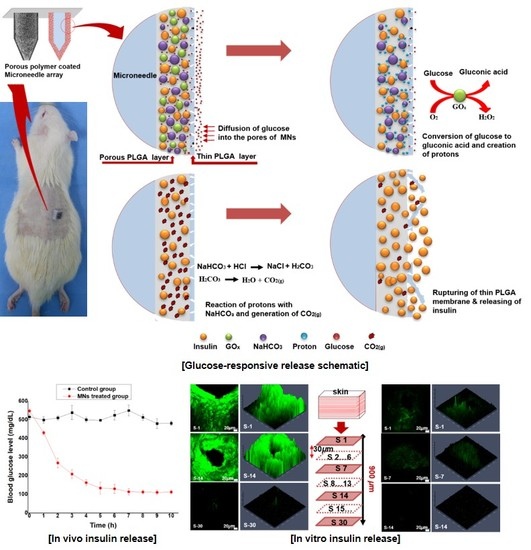
Smart Microneedles with Porous Polymer Layer for Glucose-Responsive Insulin Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
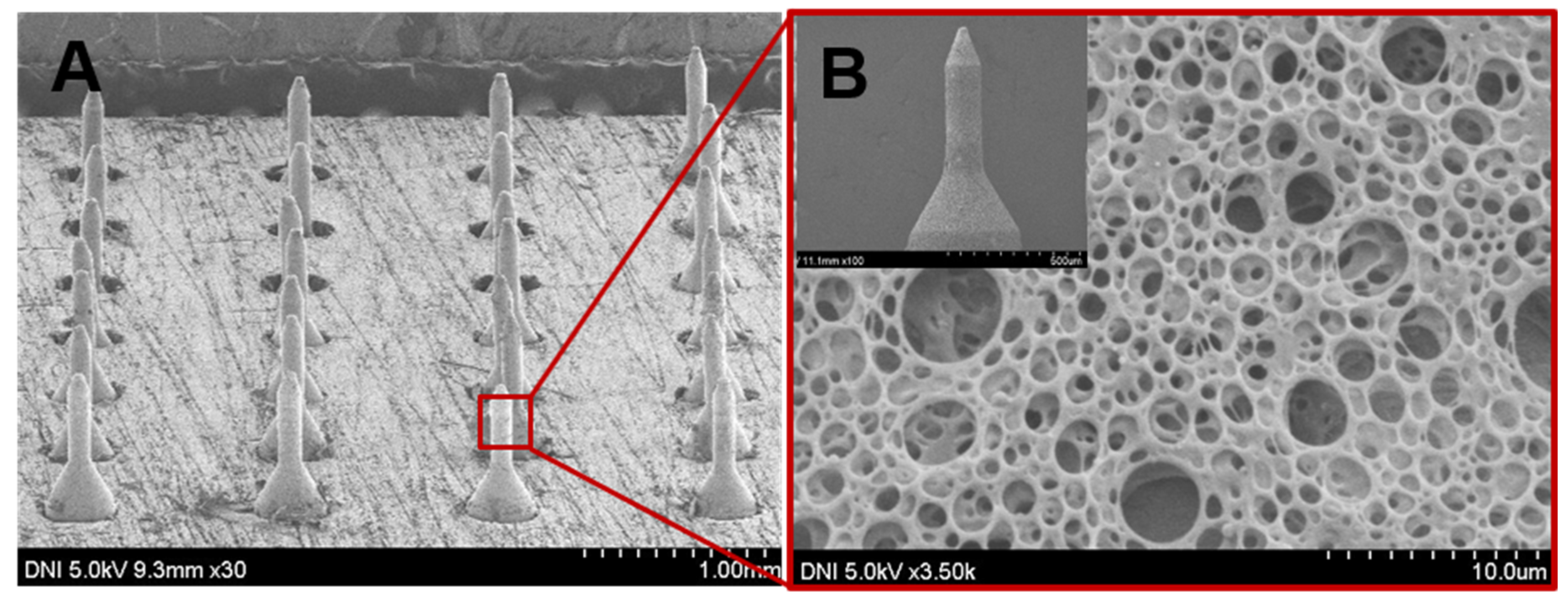
2.2. Fabrication of Porous-Coated MNs and Preparation of Coating Solution
2.3. Characterization
2.4. In Vitro Glucose-Responsive Insulin Release of Porous Coated MNs
2.5. Pulsatile Release and Parametric Study
2.6. Insulin Diffusion and Insertion Effectiveness of Porous-Coated MNs in Porcine Skin
2.7. Confocal Laser Scanning Microscope (CLSM)
2.8. In Vivo Glucose-Responsive Insulin Release in Diabetic Rats
3. Results and Discussion
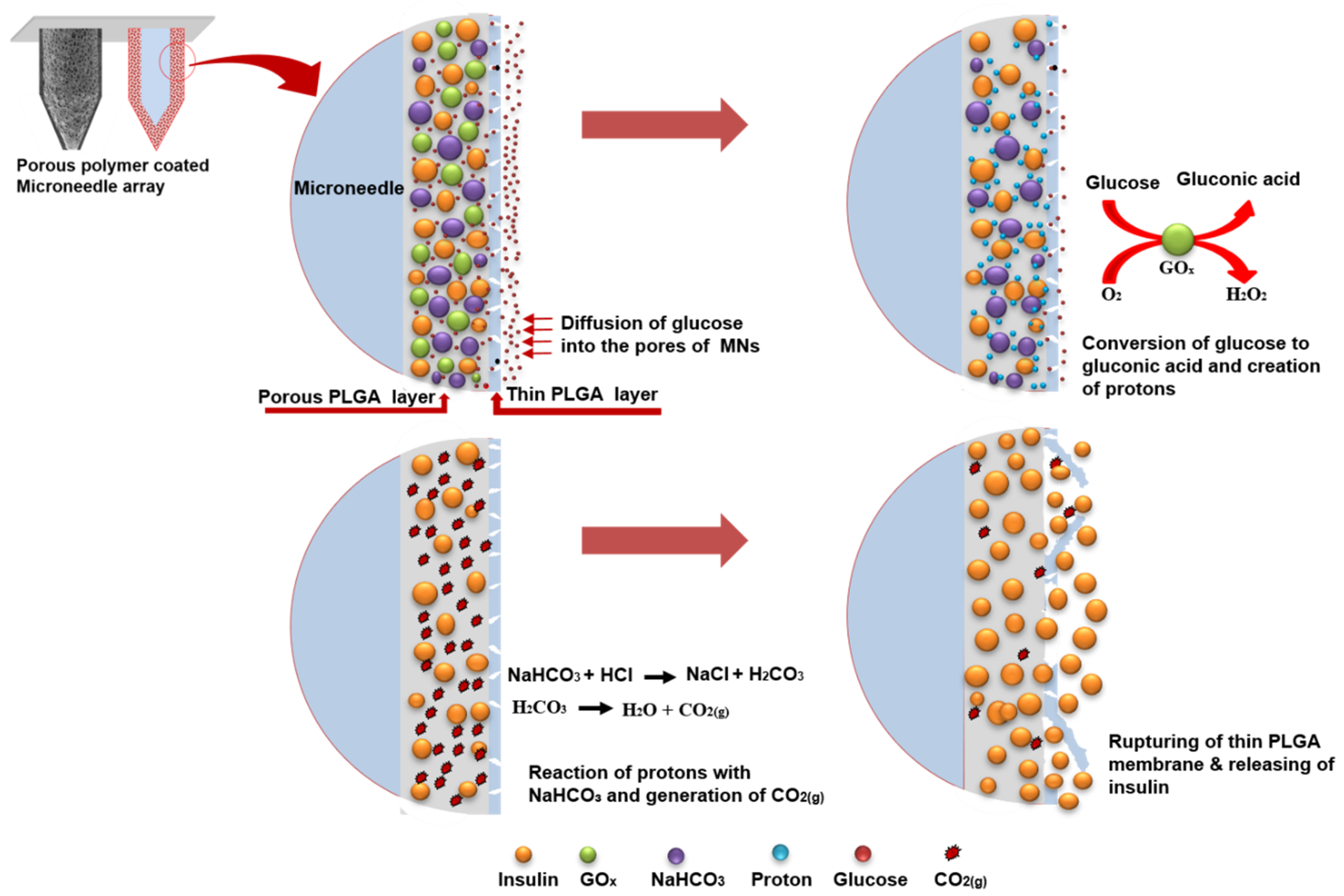
3.1. Fabrication of Glucose-Responsive MNs Array
3.2. In Vitro Insulin Release of Porous Coated MNs in Response to Different Glucose Levels
3.3. Insulin Diffusion and Insertion Effectiveness of Porous-Coated MNs in Porcine Skin
3.4. In Vivo Glucose-Responsive Insulin Release in Diabetic Rats
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mo, R.; Jiang, T.; Di, J.; Tai, W.; Gu, Z. Emerging micro-and nanotechnology based synthetic approaches for insulin delivery. Chem. Soc. Rev. 2014, 43, 3595–3629. [Google Scholar] [CrossRef]
- Oulahiane, A.; Anaddam, S.; Ouleghzal, H.; Elhaddad, N.; Moussaoui, S.; Yaagoubi, N.; Boufares, F.; Belmejdoub, G. Diabetes management issues for patients with chronic kidney disease. J. Nephrol. Ther. 2012, 8, 135–140. [Google Scholar] [CrossRef]
- Owens, D.R.; Zinman, B.; Bolli, G.B. Insulins today and beyond. Lancet 2001, 358, 739–746. [Google Scholar] [CrossRef]
- Morales, J.O.; Fathe, K.R.; Brunaugh, A.; Ferrati, S.; Li, S.; Montenegro-Nicolini, M.; Mousavikhamene, Z.; McConville, J.T.; Prausnitz, M.R.; Smyth, H.D.C. Challenges and future prospects for the delivery of biologics: Oral mucosal, pulmonary, and transdermal routes. AAPS J. 2017, 19, 652–668. [Google Scholar] [CrossRef]
- Giudice, E.L.; Campbell, J.D. Needle-free vaccine delivery. Adv. Drug Deliv. Rev. 2006, 58, 68–89. [Google Scholar] [CrossRef] [PubMed]
- Zelikin, A.N.; Ehrhardt, C.; Healy, A.M. Materials and methods for delivery of biological drugs. Nat. Chem. 2016, 8, 997. [Google Scholar] [CrossRef] [PubMed]
- DeMuth, P.C.; Garcia-Beltran, W.F.; Ai-Ling, M.L.; Hammond, P.T.; Irvine, D.J. Composite dissolving microneedles for coordinated control of antigen and adjuvant delivery kinetics in transcutaneous vaccination. Adv. Funct. Mater. 2013, 23, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Mitragotri, S.; Langer, R. Current status and future potential of transdermal drug delivery. Nat. Rev. Drug Discov. 2004, 3, 115–124. [Google Scholar] [CrossRef]
- Fukushima, K.; Ise, A.; Morita, H.; Hasegawa, R.; Ito, Y.; Sugioka, N.; Takada, K. Two-layered dissolving microneedles for percutaneous delivery of peptide/protein drugs in rats. Pharm. Res. 2011, 28, 7–21. [Google Scholar] [CrossRef]
- Chu, L.Y.; Choi, S.-O.; Prausnitz, M.R. Fabrication of dissolving polymer microneedles for controlled drug encapsulation and delivery: Bubble and pedestal microneedle designs. J. Pharm. Sci. 2010, 99, 4228–4238. [Google Scholar] [CrossRef]
- Matsumoto, A.; Matsukawa, Y.; Suzuki, T.; Yoshino, H. Drug release characteristics of multi-reservoir type microspheres with poly (dl-lactide-co-glycolide) and poly (dl-lactide). J. Control. Release 2005, 106, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, S.; Iyer, L.K.; Panchal, J.P.; Topp, E.M.; Cannon, J.B.; Ranade, V.V. Microarrays and microneedle arrays for delivery of peptides, proteins, vaccines and other applications. Expert Opin. Drug Deliv. 2013, 10, 1155–1170. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Broaders, K.E.; Grandhe, S.; Fréchet, J.M.J. A biocompatible oxidation-triggered carrier polymer with potential in therapeutics. J. Am. Chem. Soc. 2011, 133, 756–758. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Chen, F. pH-responsive drug-delivery systems. J Chem. Asian J. 2015, 10, 284–305. [Google Scholar] [CrossRef]
- Roointan, A.; Farzanfar, J.; Mohammadi-Samani, S.; Behzad-Behbahani, A.; Farjadian, F. Smart pH responsive drug delivery system based on poly (HEMA-co-DMAEMA) nanohydrogel. Int. J. Pharm. 2018, 552, 301–311. [Google Scholar] [CrossRef]
- Tai, W.; Mo, R.; Di, J.; Subramanian, V.; Gu, X.; Buse, J.B.; Gu, Z. Bio-inspired synthetic nanovesicles for glucose-responsive release of insulin. Biomacromolecules 2014, 15, 3495–3502. [Google Scholar] [CrossRef]
- Ke, C.J.; Su, T.Y.; Chen, H.L.; Liu, H.L.; Chiang, W.L.; Chu, P.C.; Xia, Y.; Sung, H.W. Smart multifunctional hollow microspheres for the quick release of drugs in intracellular lysosomal compartments. J. Angew. Chem. Int. Ed. 2011, 50, 8086–8089. [Google Scholar] [CrossRef]
- Xu, B.; Cao, Q.; Zhang, Y.; Yu, W.; Zhu, J.; Liu, D.; Jiang, G. Microneedles integrated with ZnO quantum-dot-capped mesoporous bioactive glasses for glucose-mediated insulin delivery. ACS Biomater. Sci. Eng. 2018, 4, 2473–2483. [Google Scholar] [CrossRef]
- Lee, H.; Choi, T.K.; Lee, Y.B.; Cho, H.R.; Ghaffari, R.; Wang, L.; Choi, H.J.; Chung, T.D.; Lu, N.; Hyeon, T. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol. 2016, 11, 566. [Google Scholar] [CrossRef]
- Manouras, T.; Vamvakaki, M. Field responsive materials: Photo-, electro-, magnetic-and ultrasound-sensitive polymers. Polym. Chem. 2017, 8, 74–96. [Google Scholar] [CrossRef]
- Ter Schiphorst, J.; Saez, J.; Diamond, D.; Benito-Lopez, F.; Schenning, A.P.H.J. Light-responsive polymers for microfluidic applications. Lab Chip 2018, 18, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Mandracchia, D.; Pitarresi, G.; Palumbo, F.S.; Carlisi, B.; Giammona, G. pH-sensitive hydrogel based on a novel photocross-linkable copolymer. Biomacromolecules 2004, 5, 1973–1982. [Google Scholar] [CrossRef] [PubMed]
- Zuliani, C.; Ng, F.S.; Alenda, A.; Eftekhar, A.; Peters, N.S.; Toumazou, C. An array of individually addressable micro-needles for mapping pH distributions. Analyst 2016, 141, 4659–4666. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yu, J.; Qian, C.; Lu, Y.; Kahkoska, A.R.; Xie, Z.; Jing, X.; Buse, J.B.; Gu, Z. H2O2-responsive vesicles integrated with transcutaneous patches for glucose-mediated insulin delivery. ACS Nano 2017, 11, 613–620. [Google Scholar] [CrossRef]
- Ke, C.-J.; Lin, Y.-J.; Hu, Y.-C.; Chiang, W.-L.; Chen, K.-J.; Yang, W.-C.; Liu, H.-L.; Fu, C.-C.; Sung, H.-W. Multidrug release based on microneedle arrays filled with pH-responsive PLGA hollow microspheres. Biomaterials 2012, 33, 5156–5165. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, Y.; Ye, Y.; Di Santo, R.; Sun, W.; Ranson, D.; Ligler, F.S.; Buse, J.B.; Gu, Z. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc. Natl. Acad. Sci. USA 2015, 112, 8260–8265. [Google Scholar] [CrossRef]
- Xu, B.; Jiang, G.; Yu, W.; Liu, D.; Zhang, Y.; Zhou, J.; Sun, S.; Liu, Y. H2O2-responsive mesoporous silica nanoparticles integrated with microneedle patches for the glucose-monitored transdermal delivery of insulin. J. Mater. Chem. B 2017, 5, 8200–8208. [Google Scholar] [CrossRef]
- Ullah, A.; Kim, C.M.; Kim, G.M. Solvent effects on the porosity and size of porous PLGA microspheres using gelatin and PBS as porogens in a microfluidic flow-focusing device. J. Nanosci. Nanotechnol. 2017, 17, 7775–7782. [Google Scholar] [CrossRef]
- Paddock, E.; Hohenadel, M.G.; Piaggi, P.; Vijayakumar, P.; Hanson, R.L.; Knowler, W.C.; Krakoff, J.; Chang, D.C. One-hour and two-hour postload plasma glucose concentrations are comparable predictors of type 2 diabetes mellitus in Southwestern Native Americans. Diabetologia 2017, 60, 1704–1711. [Google Scholar] [CrossRef]
- Ullah, A.; Kim, C.M.; Kim, G.M. Porous polymer coatings on metal microneedles for enhanced drug delivery. R. Soc. Open Sci. 2018, 5, 171609. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Khan, H.; Choi, H.J.; Kim, G.M. Smart Microneedles with Porous Polymer Coatings for pH-Responsive Drug Delivery. Polymers 2019, 11, 1834. [Google Scholar] [CrossRef] [PubMed]
- Summerfield, A.; Meurens, F.; Ricklin, M.E. The immunology of the porcine skin and its value as a model for human skin. Mol. Immunol. 2015, 66, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Zarnitsyn, V.G.; Ye, L.; Wen, Z.; Gao, Y.; Pan, L.; Skountzou, I.; Gill, H.S.; Prausnitz, M.R.; Yang, C. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proc. Natl. Acad. Sci. USA 2009, 106, 7968–7973. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yan, L.-J. Streptozotocin-induced type 1 diabetes in rodents as a model for studying mitochondrial mechanisms of diabetic β cell glucotoxicity. Diabetes Metab. Syndr. Obes. Targets Ther. 2015, 8, 181. [Google Scholar]
- Zhu, W.; Chen, M.; Shou, Q.; Li, Y.; Hu, F. Biological activities of Chinese propolis and Brazilian propolis on streptozotocin-induced type 1 diabetes mellitus in rats. Evid. Based Complement. Altern. Med. 2011, 2011, 468529. [Google Scholar] [CrossRef] [PubMed]
- Maritim, A.C.; Sanders, A.; Watkins Iii, J.B. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef]
- Erdal, N.; Gürgül, S.; Demirel, C.; Yildiz, A. The effect of insulin therapy on biomechanical deterioration of bone in streptozotocin (STZ)-induced type 1 diabetes mellitus in rats. Diabetes Res. Clin. Pract. 2012, 97, 461–467. [Google Scholar] [CrossRef]
- Abeeleh, M.A.; Ismail, Z.B.; Alzaben, K.R.; Abu-Halaweh, S.A.; Al-Essa, M.K.; Abuabeeleh, J.; Alsmady, M.M. Induction of diabetes mellitus in rats using intraperitoneal streptozotocin: A comparison between 2 strains of rats. Eur. J. Sci. Res. 2009, 32, 398–402. [Google Scholar]
- Hein, T.W.; Potts, L.B.; Xu, W.; Yuen, J.Z.; Kuo, L. Temporal development of retinal arteriolar endothelial dysfunction in porcine type 1 diabetes. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7943–7949. [Google Scholar] [CrossRef]
- Wu, K.K.; Huan, Y. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. Pharmacol. 2008, 40, 5.47.1–5.47.20. [Google Scholar] [CrossRef] [PubMed]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, A.; Choi, H.J.; Jang, M.; An, S.; Kim, G.M. Smart Microneedles with Porous Polymer Layer for Glucose-Responsive Insulin Delivery. Pharmaceutics 2020, 12, 606. https://doi.org/10.3390/pharmaceutics12070606
Ullah A, Choi HJ, Jang M, An S, Kim GM. Smart Microneedles with Porous Polymer Layer for Glucose-Responsive Insulin Delivery. Pharmaceutics. 2020; 12(7):606. https://doi.org/10.3390/pharmaceutics12070606
Chicago/Turabian StyleUllah, Asad, Hye Jin Choi, Mijin Jang, Sanghyun An, and Gyu Man Kim. 2020. "Smart Microneedles with Porous Polymer Layer for Glucose-Responsive Insulin Delivery" Pharmaceutics 12, no. 7: 606. https://doi.org/10.3390/pharmaceutics12070606
APA StyleUllah, A., Choi, H. J., Jang, M., An, S., & Kim, G. M. (2020). Smart Microneedles with Porous Polymer Layer for Glucose-Responsive Insulin Delivery. Pharmaceutics, 12(7), 606. https://doi.org/10.3390/pharmaceutics12070606