Ophthalmic In Situ Gelling System Containing Lanosterol Nanoparticles Delays Collapse of Lens Structure in Shumiya Cataract Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Preparation of LA-NPs/ISG
2.4. Measurement of Characteristics of LA-NPs/ISG
2.5. Evaluation of Cell Toxicity of LA-NPs/ISG Using Culture Human Corneal Epithelial Cell (HCE-T Cell)
2.6. Evaluation of Corneal Toxicity of LA-NPs/ISG Using Rabbits
2.7. Evaluation of Corneal Toxicity of LA-NPs/ISG Using Rat Debrided Corneal Epithelium
2.8. Transcorneal Penetration of LA-NPs/ISG Using Isolated Rabbit Cornea
2.9. Treatment of Inhibitor of Energy-Dependent Endocytosis in the Isolated Rabbit Cornea
2.10. Measurement of Lan Content in Rat Lenses
2.11. Evaluation of Lens Structure in the SCR-N Using Hematoxylin and Eosin (H.E.) Staining
2.12. Scheimpflug Slit Images in the SCR-C
2.13. Measurement of Cataract-Related Factors
2.14. Statistical Analysis
3. Results
3.1. Corneal Toxicity in the Instillation of LA-NPs/ISG
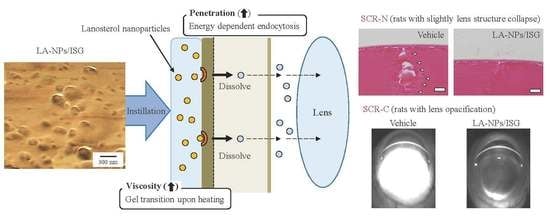
3.2. Mechanism for Drug Delivery into Lens by the Instillation of LA-NPs/ISG
3.3. Therapeutic Potential of LA-NPs/ISG on the Collapse of Lens Structure in SCR-N
3.4. Delay of Lens Opacification in SCR-C by the Instillation of LA-NPs/ISG
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sletten, T.L.; Revell, V.L.; Middleton, B.; Lederle, K.A.; Skenek, D.J. Age-related changes in acute and phase-advancing responses to monochromatic light. J. Biol. Rhythms. 2009, 24, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.A.; Langa, K.M. Untreated poor vision: A contributing factor to late-life dementia. Am. J. Epidemiol. 2010, 171, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Tsukamoto, H.; Mukai, S.; Kato, T.; Minamoto, A.; Ohno, Y.; Yamashita, H.; Mishima, H.K. Improvement in cognitive impairment after cataract surgery in elderly patients. J. Cataract Refract Surg. 2004, 30, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Melillo, P.; Orrico, A.; Attanasio, M.; Rossi, S.; Pecchia, L.; Chirico, F.; Testa, F.; Simonelli, F. A pilot study for development of a novel tool for clinical decision making to identify fallers among ophthalmic patients. BMC. Med. Inform. Decis. Mak. 2015, 15, S6. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Yang, Z.; Zhou, R. Lanosterol disrupts aggregation of human gammaD-crystallin by binding to the hydrophobic dimerization interface. J. Am. Chem. Soc. 2018, 140, 8479–8486. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, X.J.; Zhu, J.; Xi, Y.B.; Yang, X.; Hu, L.D.; Ouyang, H.; Patel, S.H.; Jin, X.; Lin, D.; et al. Lanosterol reverses protein aggregation in cataracts. Nature 2015, 523, 607–611. [Google Scholar] [CrossRef]
- Gan, L.; Wang, J.; Jiang, M.; Bartlett, H.; Ouyang, D.; Eperjesi, F.; Liu, J.; Gan, Y. Recent advances in topical ophthalmic drug delivery with lipid-based nanocarriers. Drug Discov. Today 2013, 18, 290–297. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Zhou, T.; Zhang, J.; Xia, H.; Li, H.; He, J.; He, S.; Wang, L. Positively charged micelles based on a triblock copolymer demonstrate enhanced corneal penetration. Int. J. Nanomed. 2015, 10, 6027–6237. [Google Scholar] [CrossRef]
- Zhu, Q.; Wei, Y.; Li, C.; Mao, S. Inner layer-embedded contact lenses for iontriggered controlled drug delivery. Mater. Sci. Eng. 2018, C93, 36–48. [Google Scholar] [CrossRef]
- Almeida, H.; Amaral, M.H.; Lobao, P.; Sousa Lobo, J.M. In situ gelling systems: A strategy to improve the bioavailability of ophthalmic pharmaceutical formulations. Drug Discov. Today 2014, 19, 400–412. [Google Scholar] [CrossRef]
- Grimaudo, M.A.; Pescina, S.; Padula, C.; Santi, P.; Concheiro, A.; Alvarez-Lorenzo, C.; Nicoli, S. Topical application of polymeric nanomicelles in ophthalmology: A review on research efforts for the non-invasive delivery of ocular therapeutics. Expert Opin. Drug Deliv. 2019, 16, 397–413. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Cheng, H.; Huo, Y.; Mao, S. Sustained ophthalmic delivery of highly soluble drug using pH-triggered inner layer-embedded contact lens. Int. J. Pharm. 2018, 544, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Wang, Y.; Ping, Q.; Xiao, Y.; Huang, X. Mucoadhesive effect of thiolated PEG stearate and its modified NLC for ocular drug delivery. J. Control. Release 2009, 137, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Maeno, H.; Gujrati, M.; Schur, R.; Maeda, A.; Maeda, T.; Palczewski, K.; Lu, Z.R. Self-assembly of a multifunctional lipid with core-shell dendrimer DNA nanoparticles enhanced efficient gene delivery at low charge ratios into RPE cells. Macromol. Biosci. 2015, 15, 1663–1672. [Google Scholar] [CrossRef]
- Gan, L.; Gan, Y.; Zhu, C.; Zhang, X.; Zhu, J. Novel microemulsion in situ electrolyte-triggered gelling system for ophthalmic delivery of lipophilic cyclosporine a: In vitro and in vivo results. Int. J. Pharm. 2009, 365, 143–149. [Google Scholar] [CrossRef]
- Nagai, N.; Ogata, F.; Otake, H.; Nakazawa, Y.; Kawasaki, N. Energy-dependent endocytosis is responsible for drug transcorneal penetration following the instillation of ophthalmic formulations containing indomethacin nanoparticles. Int. J. Nanomed. 2019, 14, 1213–1227. [Google Scholar] [CrossRef]
- Nagai, N.; Minami, M.; Deguchi, S.; Otake, H.; Sasaki, H.; Yamamoto, N. An In Situ Gelling System based on Methylcellulose and Tranilast Solid Nanoparticles Enhances Ocular Residence Time and Drug Absorption into the Cornea and Conjunctiva. Front. Bioeng. Biotechnol. 2020. [Google Scholar] [CrossRef]
- Nagai, N.; Mano, Y.; Ito, Y. An Ophthalmic Formulation of Disulfiram Nanoparticles Prolongs Drug Residence Time in Lens. Biol. Pharm. Bull. 2016, 39, 1881–1887. [Google Scholar] [CrossRef]
- Shumiya, S. Establishment of the hereditary cataract rat strain (SCR) and genetic analysis. Lab. Anim. Sci. 1995, 45, 671–673. [Google Scholar]
- Mori, M.; Li, G.; Abe, I.; Nakayama, J.; Guo, Z.; Sawashita, J.; Ugawa, T.; Nishizono, S.; Serikawa, T.; Higuchi, K.; et al. Lanosterol synthase mutations cause cholesterol deficiency-associated cataracts in the Shumiya cataract rat. J. Clin. Investig. 2006, 116, 395–404. [Google Scholar] [CrossRef]
- Inomata, M.; Hayashi, M.; Shumiya, S.; Kawashima, S.; Ito, Y. Aminoguanidine-treatment results in the inhibition of lens opacification and calpain-mediated proteolysis in Shumiya cataract rats (SCR). J. Biochem. 2000, 128, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Inomata, M.; Hayashi, M.; Shumiya, S.; Kawashima, S.; Ito, Y. Involvement of inducible nitric oxide synthase in cataract formation in Shumiya cataract rats (SCR). Curr. Eye Res. 2001, 23, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ito, Y.; Okamoto, N.; Shimomura, Y. A nanoparticle formulation reduces the corneal toxicity of indomethacin eye drops and enhances its corneal permeability. Toxicology 2014, 319, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Fukuoka, Y.; Sato, K.; Otake, H.; Taga, A.; Oka, M.; Hiramatsu, N.; Yamamoto, N. The Intravitreal Injection of Lanosterol Nanoparticles Rescues Lens Structure Collapse at an Early Stage in Shumiya Cataract Rats. Int. J. Mol. Sci. 2020, 21, 1048. [Google Scholar] [CrossRef] [PubMed]
- Mäger, I.; Langel, K.; Lehto, T.; Eiríksdóttir, E.; Langel, U. The role of endocytosis on the uptake kinetics of luciferin-conjugated cell-penetrating peptides. Biochim. Biophys. Acta 2012, 1818, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Malomouzh, A.I.; Mukhitov, A.R.; Proskurina, S.E.; Vyskocil, F.; Nikolsky, E.E. The effect of dynasore, a blocker of dynamin-dependent endocytosis, on spontaneous quantal and non-quantal release of acetylcholine in murine neuromuscular junctions. Dokl. Biol. Sci. 2014, 459, 330–333. [Google Scholar] [CrossRef]
- Hufnagel, H.; Hakim, P.; Lima, A.; Hollfelder, F. Fluid phase endocytosis contributes to transfection of DNA by PEI-25. Mol. Ther. 2009, 17, 1411–1417. [Google Scholar] [CrossRef]
- Nagai, N.; Ito, Y.; Inomata, M.; Shumiya, S.; Tai, H.; Hataguchi, Y.; Nakagawa, K. Delay of cataract development in the shumiya cataract rat by the administration of drinking water containing high concentration of magnesium ion. Biol. Pharm. Bull. 2006, 29, 1234–1238. [Google Scholar] [CrossRef][Green Version]
- Nagai, N.; Ito, Y. Adverse effects of excessive nitric oxide on cytochrome c oxidase in lenses of hereditary cataract UPL rats. Toxicology 2007, 242, 7–15. [Google Scholar] [CrossRef]
- Nagai, N.; Ito, Y.; Takeuchi, N. Inhibitive effects of enhanced lipid peroxidation on Ca2+-ATPase in lenses of hereditary cataract ICR/f rats. Toxicology 2008, 247, 139–144. [Google Scholar] [CrossRef]
- Nagai, N.; Ito, Y.; Takeuchi, N.; Usui, S.; Hirano, K. Comparison of the Mechanisms of Cataract Development Involving Differences in Ca(2+) Regulation in Lenses among Three Hereditary Cataract Model Rats. Biol. Pharm. Bull. 2008, 31, 1990–1995. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, Y.; Liu, Y.; Li, X.; Kebebe, D.; Zhang, B.; Ren, J.; Lu, J.; Li, J.; Du, S.; Liu, Z. Research progress of in-situ gelling ophthalmic drug delivery system. Asian J. Pharm. Sci. 2019, 14, 1–15. [Google Scholar] [CrossRef]
- Yadav, K.S.; Rajpurohit, R.; Sharma, S. Glaucoma: Current treatment and impact of advanced drug delivery systems. Life Sci. 2019, 221, 362–376. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, N.; Hua, H.; Liu, T.; Tang, Y.; Fu, L.; Yang, Y.; Ma, X.; Zhao, Y. Preparation, pharmacokinetics and pharmacodynamics of ophthalmic thermosensitive in situ hydrogel of betaxolol hydrochloride. Biomed. Pharmacother. 2016, 83, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; Lee, S.K.; Cristol, S.M.; Lee, D.W.; Seo, K.Y.; Kim, E.K. Impact ofshort-term exposure of commercial eyedrops preserved with benzalkoniumchloride on precorneal mucin. Mol. Vis. 2006, 12, 415–421. [Google Scholar]
- Debbasch, C.; Brignole, F.; Pisella, P.J.; Warnet, J.M.; Rat, P.; Baudouin, C. Qua-ternary ammoniums and other preservatives’ contribution in oxidative stressand apoptosis on Chang conjunctival cells. Investig. Ophthalmol. Vis. Sci. 2001, 42, 642–652. [Google Scholar]
- Guenoun, J.M.; Baudouin, C.; Rat, P.; Pauly, A.; Briqnole-Baudouin, F. In vitro comparison of cytoprotective and antioxidative effects of latanoprost, travoprost, and bimatoprost on conjunctiva-derived epithelial cells. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4594–4599. [Google Scholar] [CrossRef]
- Nagai, N.; Yoshioka, C.; Tanino, T.; Ito, Y.; Okamoto, N.; Shimomura, Y. Decrease in Corneal Damage due to Benzalkonium Chloride by the Addition of Mannitol into Timolol Maleate Eye Drops. J. Oleo Sci. 2015, 64, 743–750. [Google Scholar] [CrossRef]
- Nagai, N.; Ishii, M.; Seiriki, R.; Ogata, F.; Otake, H.; Nakazawa, Y.; Okamoto, N.; Kanai, K.; Kawasaki, N. Novel Sustained-Release Drug Delivery System for Dry Eye Therapy by Rebamipide Nanoparticles. Pharmaceutics 2020, 12, 155. [Google Scholar] [CrossRef]
- Jansen, T.; Xhonneux, B.; Mesens, J.; Borgers, M. Beta-cyclodextrins as vehi-cles in eye-drop formulations: An evaluation of their effects on rabbit cornealepithelium. Lens Eye Toxic. Res. 1990, 7, 459–468. [Google Scholar]
- Goto, H.; Yamada, M.; Yoshikawa, K.; Iino, M. Ganka-Kaigyoui Notameno Gimon·nanmon Kaiketusaku; Shindan to Chiryosha Co.: Tokyo, Japan, 2006; pp. 216–217. (In Japanese) [Google Scholar]
- Daszynski, D.M.; Santhoshkumar, P.; Phadte, A.S.; Sharma, K.K.; Zhong, H.A.; Lou, M.F.; Kador, P.F. Failure of Oxysterols Such as Lanosterol to Restore Lens Clarity from Cataracts. Sci. Rep. 2019, 9, 8459. [Google Scholar] [CrossRef] [PubMed]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagai, N.; Umachi, K.; Otake, H.; Oka, M.; Hiramatsu, N.; Sasaki, H.; Yamamoto, N. Ophthalmic In Situ Gelling System Containing Lanosterol Nanoparticles Delays Collapse of Lens Structure in Shumiya Cataract Rats. Pharmaceutics 2020, 12, 629. https://doi.org/10.3390/pharmaceutics12070629
Nagai N, Umachi K, Otake H, Oka M, Hiramatsu N, Sasaki H, Yamamoto N. Ophthalmic In Situ Gelling System Containing Lanosterol Nanoparticles Delays Collapse of Lens Structure in Shumiya Cataract Rats. Pharmaceutics. 2020; 12(7):629. https://doi.org/10.3390/pharmaceutics12070629
Chicago/Turabian StyleNagai, Noriaki, Kazuki Umachi, Hiroko Otake, Mikako Oka, Noriko Hiramatsu, Hiroshi Sasaki, and Naoki Yamamoto. 2020. "Ophthalmic In Situ Gelling System Containing Lanosterol Nanoparticles Delays Collapse of Lens Structure in Shumiya Cataract Rats" Pharmaceutics 12, no. 7: 629. https://doi.org/10.3390/pharmaceutics12070629
APA StyleNagai, N., Umachi, K., Otake, H., Oka, M., Hiramatsu, N., Sasaki, H., & Yamamoto, N. (2020). Ophthalmic In Situ Gelling System Containing Lanosterol Nanoparticles Delays Collapse of Lens Structure in Shumiya Cataract Rats. Pharmaceutics, 12(7), 629. https://doi.org/10.3390/pharmaceutics12070629