Acemannan Used as an Implantable Biomaterial for Vital Pulp Therapy of Immature Permanent Teeth Induced Continued Root Formation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Acemannan Sponge Preparation
2.2. Study Design
2.3. Study Participants
- The patient had a history of systemic disease that interferes with pulp healing.
- The tooth had clinical signs and symptoms of irreversible pulp pulpitis, e.g., spontaneous throbbing pain, tenderness to percussion, abnormal tooth mobility, swelling, or sinus tract.
- Radiographic evidence of internal or external resorption, inter-radicular bone loss, or periapical pathology.
- The tooth was non-restorable.
2.4. Research Ethics
2.5. Research Sample Size
2.6. Study Intervention
2.7. The Follow-Up Examination Procedure
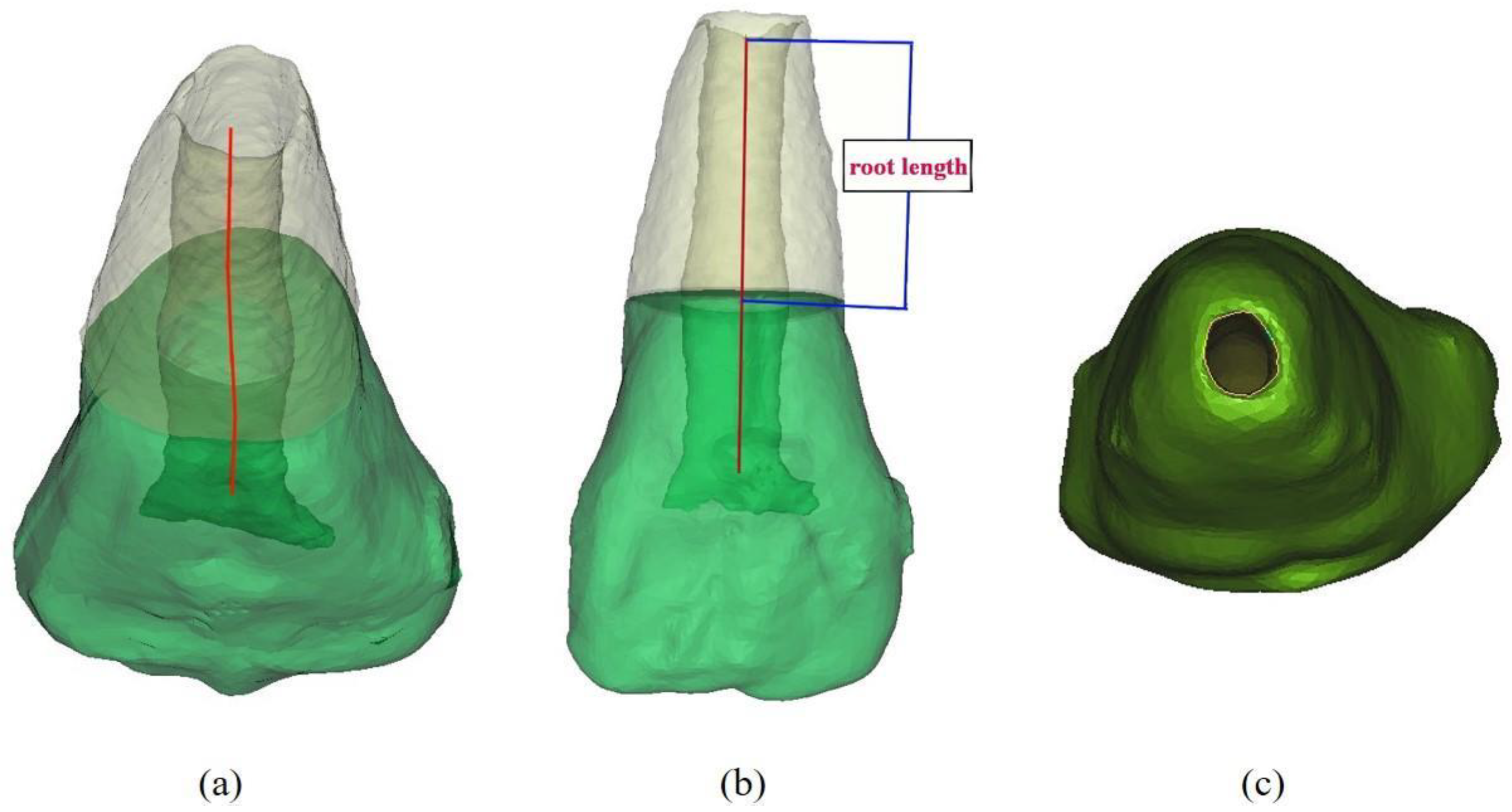
2.8. 3D Tooth Reconstruction and Analysis of the Partial Pulpotomy Treatment
2.9. Criteria for the Evaluation of Overall Research Treatment
2.10. Statistical Analyses
3. Results
3.1. Overall Clinical and Radiographic Success of Acemannan Treatment
3.1.1. Acemannan Demonstrated Similar Overall Clinical and Radiographic Success Rates to Those of MTA
3.1.2. Acemannan Induced Continued Root Formation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
- κ = nA/nB is the ratio between the sample sizes of the two groups (κ = 1 means that each group has an equal sample size)
- Φ is the standard Normal distribution function
- α is a Type I error (α = 0.05)
- β is a Type II error, i.e., 1 − β is power (β = 0.2)
- δ is the testing non-inferiority margin (δ = 0.2)

References
- Guideline on pulp therapy for primary and immature permanent teeth. Pediatr. Dent. 2016, 38, 280–288.
- Witherspoon, D.E. Vital pulp therapy with new materials: New directions and treatment perspectives—Permanent teeth. J. Endod. 2008, 34, S25–S28. [Google Scholar] [CrossRef] [PubMed]
- Kogan, P.; He, J.; Glickman, G.N.; Watanabe, I. The effects of various additives on setting properties of MTA. J. Endod. 2006, 32, 569–572. [Google Scholar] [CrossRef]
- Parinyaprom, N.; Nirunsittirat, A.; Chuveera, P.; Na Lampang, S.; Srisuwan, T.; Sastraruji, T.; Bua-On, P.; Simprasert, S.; Khoipanich, I.; Sutharaphan, T.; et al. Outcomes of direct pulp capping by using either proroot mineral trioxide aggregate or biodentine in permanent teeth with carious pulp exposure in 6- to 18-year-old patients: A randomized controlled trial. J. Endod. 2018, 44, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Stuart, R.W.; Lefkowitz, D.L.; Lincoln, J.A.; Howard, K.; Gelderman, M.P.; Lefkowitz, S.S. Upregulation of phagocytosis and candidicidal activity of macrophages exposed to the immunostimulant acemannan. Int. Immunopharmacol. 1997, 19, 75–82. [Google Scholar] [CrossRef]
- Xing, W.; Guo, W.; Zou, C.H.; Fu, T.T.; Li, X.Y.; Zhu, M.; Qi, J.H.; Song, J.; Dong, C.H.; Li, Z.; et al. Acemannan accelerates cell proliferation and skin wound healing through AKT/mTOR signaling pathway. J. Dermatol. Sci. 2015, 79, 101–109. [Google Scholar] [CrossRef]
- Pachimalla, P.R.; Mishra, S.K.; Chowdhary, R. Evaluation of hydrophilic gel made from Acemannan and Moringa oleifera in enhancing osseointegration of dental implants. A preliminary study in rabbits. J. Oral Biol. Craniofac. Res. 2020, 10, 13–19. [Google Scholar] [CrossRef]
- Liu, C.; Cui, Y.; Pi, F.; Cheng, Y.; Guo, Y.; Qian, H. Extraction, purification, structural characteristics, biological activities and pharmacological applications of acemannan, a polysaccharide from aloe vera: A review. Molecules 2019, 24, 1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Songsiripradubboon, S.; Kladkaew, S.; Trairatvorakul, C.; Sangvanich, P.; Soontornvipart, K.; Banlunara, W.; Thunyakitpisal, P. Stimulation of dentin regeneration by using acemannan in teeth with lipopolysaccharide-induced pulp inflammation. J. Endod. 2017, 43, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Songsiripradubboon, S.; Banlunara, W.; Sangvanich, P.; Trairatvorakul, C.; Thunyakitpisal, P. Clinical, radiographic, and histologic analysis of the effects of acemannan used in direct pulp capping of human primary teeth: Short-term outcomes. Odontology 2016, 104, 329–337. [Google Scholar] [CrossRef]
- Chantarawaratit, P.; Sangvanich, P.; Banlunara, W.; Soontornvipart, K.; Thunyakitpisal, P. Acemannan sponges stimulate alveolar bone, cementum and periodontal ligament regeneration in a canine class II furcation defect model. J. Periodontal Res. 2014, 49, 164–178. [Google Scholar] [CrossRef]
- Boonyagul, S.; Banlunara, W.; Sangvanich, P.; Thunyakitpisal, P. Effect of acemannan, an extracted polysaccharide from Aloe vera, on BMSCs proliferation, differentiation, extracellular matrix synthesis, mineralization, and bone formation in a tooth extraction model. Odontology 2014, 102, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Jittapiromsak, N.; Sahawat, D.; Banlunara, W.; Sangvanich, P.; Thunyakitpisal, P. Acemannan, an extracted product from Aloe vera, stimulates dental pulp cell proliferation, differentiation, mineralization, and dentin formation. Tissue Eng. Part A 2010, 16, 1997–2006. [Google Scholar] [CrossRef] [PubMed]
- Thunyakitpisal, P.; Ruangpornvisuti, V.; Kengkwasing, P.; Chokboribal, J.; Sangvanich, P. Acemannan increases NF-kappaB/DNA binding and IL-6/-8 expression by selectively binding Toll-like receptor-5 in human gingival fibroblasts. Carbohydr. Polym. 2017, 161, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Bouman, A.C.; ten Cate-Hoek, A.J.; Ramaekers, B.L.; Joore, M.A. Sample size estimation for non-inferiority trials: Frequentist approach versus decision theory approach. PLoS ONE 2015, 10, e0130531. [Google Scholar] [CrossRef] [PubMed]
- Ozgur, B.; Uysal, S.; Gungor, H.C. Partial pulpotomy in immature permanent molars after carious exposures using different hemorrhage control and capping materials. Pediatr. Dent. 2017, 39, 364–370. [Google Scholar]
- Fayad, M.I.; Nair, M.; Levin, M.D.; Benavides, E.; Rubinstein, R.A.; Barghan, S.; Hirschberg, C.S.; Ruprecht, A. AAE and AAOMR joint position statement: Use of cone beam computed tomography in endodontics 2015 update. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 120, 508–512. [Google Scholar] [CrossRef]
- Patel, S.; Brown, J.; Semper, M.; Abella, F.; Mannocci, F. European society of endodontology position statement: Cone beam computed tomography. Int. Endod. J. 2019, 52, 1675–1678. [Google Scholar] [CrossRef] [Green Version]
- DiAngelis, A.J.; Andreasen, J.O.; Ebeleseder, K.A.; Kenny, D.J.; Trope, M.; Sigurdsson, A.; Andersson, L.; Bourguignon, C.; Flores, M.T.; Hicks, M.L.; et al. Guidelines for the management of traumatic dental injuries: 1. Fractures and luxations of permanent teeth. Pediatr. Dent. 2016, 38, 358–368. [Google Scholar]
- Li, G. Patient radiation dose and protection from cone-beam computed tomography. Imaging Sci. Dent. 2013, 43, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Chailertvanitkul, P.; Paphangkorakit, J.; Sooksantisakoonchai, N.; Pumas, N.; Pairojamornyoot, W.; Leela-Apiradee, N.; Abbott, P.V. Randomized control trial comparing calcium hydroxide and mineral trioxide aggregate for partial pulpotomies in cariously exposed pulps of permanent molars. Int. Endod. J. 2014, 47, 835–842. [Google Scholar] [CrossRef]
- Fong, C.D.; Davis, M.J. Partial pulpotomy for immature permanent teeth, its present and future. Pediatr. Dent. 2002, 24, 29–32. [Google Scholar]
- Mente, J.; Leo, M.; Panagidis, D.; Ohle, M.; Schneider, S.; Lorenzo Bermejo, J.; Pfefferle, T. Treatment outcome of mineral trioxide aggregate in open apex teeth. J. Endod. 2013, 39, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.W.; Toth, J.M.; Berzins, D.W.; Charlton, D.G. Mineral trioxide aggregate material use in endodontic treatment: A review of the literature. Dent. Mater. 2008, 24, 149–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okiji, T.; Yoshiba, K. Reparative dentinogenesis induced by mineral trioxide aggregate: A review from the biological and physicochemical points of view. Int. J. Dent. 2009, 2009, 464280. [Google Scholar] [CrossRef] [PubMed]
- Tziafas, D.; Pantelidou, O.; Alvanou, A.; Belibasakis, G.; Papadimitriou, S. The dentinogenic effect of mineral trioxide aggregate (MTA) in short-term capping experiments. Int. Endod. J. 2002, 35, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.M.; Sun, Y.; Song, J.S.; Pang, N.S.; Roh, B.D.; Lee, C.Y.; Shin, Y.A. randomized controlled trial of various MTA materials for partial pulpotomy in permanent teeth. J. Dent. 2017, 60, 8–13. [Google Scholar] [CrossRef]
- El-Meligy, O.A.; Avery, D.R. Comparison of mineral trioxide aggregate and calcium hydroxide as pulpotomy agents in young permanent teeth (apexogenesis). Pediatr. Dent. 2006, 28, 399–404. [Google Scholar]
- Bogen, G.; Kim, J.S.; Bakland, L.K. Direct pulp capping with mineral trioxide aggregate: An observational study. J. Am. Dent. Assoc. 2008, 139, 305–315. [Google Scholar] [CrossRef]
- Tai-Nin Chow, J.; Williamson, D.A.; Yates, K.M.; Goux, W.J. Chemical characterization of the immunomodulating polysaccharide of Aloe vera L. Carbohydr. Res. 2005, 340, 1131–1142. [Google Scholar] [CrossRef]
- Chokboribal, J.; Tachaboonyakiat, W.; Sangvanich, P.; Ruangpornvisuti, V.; Jettanacheawchankit, S.; Thunyakitpisal, P. Deacetylation affects the physical properties and bioactivity of acemannan, an extracted polysaccharide from Aloe vera. Carbohydr. Polym. 2015, 133, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Jittapiromsak, N.; Jettanacheawchankit, S.; Lardungdee, P.; Sangvanich, P.; Thunyakitpisal, P. Effect of acemannan on BMP-2 expression in primary pulpal fibroblasts and periodontal fibroblasts, in vitro study. J. Oral Tissue Eng. 2007, 4, 149–154. [Google Scholar]
- Gopikrishna, V.; Pradeep, G.; Venkateshbabu, N. Assessment of pulp vitality: A review. Int. J. Paediatr. Dent. 2009, 19, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Abbott, P.V. Dental pulp testing: A review. Int. J. Dent. 2009, 2009, 365785. [Google Scholar] [CrossRef]
- Gopakumar, R.; Gopakumar, M. Diagnostic aids in pediatric dentistry. Int. J. Clin. Pediatr. Dent. 2011, 4, 1–7. [Google Scholar] [CrossRef]
- Low, K.M.; Dula, K.; Burgin, W.; von Arx, T. Comparison of periapical radiography and limited cone-beam tomography in posterior maxillary teeth referred for apical surgery. J. Endod. 2008, 34, 557–562. [Google Scholar] [CrossRef]
- Mehrvarzfar, P.; Abbott, P.V.; Mashhadiabbas, F.; Vatanpour, M.; Tour Savadkouhi, S. Clinical and histological responses of human dental pulp to MTA and combined MTA/treated dentin matrix in partial pulpotomy. Aust. Endod. J. 2018, 44, 46–53. [Google Scholar] [CrossRef]
- Patel, S.; Durack, C.; Abella, F.; Shemesh, H.; Roig, M.; Lemberg, K. Cone beam computed tomography in Endodontics—A review. Int. Endod. J. 2015, 48, 3–15. [Google Scholar] [CrossRef]
- Meschi, N.; EzEldeen, M.; Torres Garcia, A.E.; Jacobs, R.; Lambrechts, P. A retrospective case series in regenerative endodontics: Trend analysis based on clinical evaluation and 2- and 3-dimensional radiology. J. Endod. 2018, 44, 1517–1525. [Google Scholar] [CrossRef]
- Barrieshi-Nusair, K.M.; Qudeimat, M.A. A prospective clinical study of mineral trioxide aggregate for partial pulpotomy in cariously exposed permanent teeth. J. Endod. 2006, 32, 731–735. [Google Scholar] [CrossRef]
- Waterhouse, P.J.; Whitworth, J.M. Pediatric endodontics: Endodontic treatment for the primary and yong permanent dentition. In Cohen’s Pathways of the Pulp Expert Consult—E-Book, 11th ed.; Berman, L.H., Hargeaves, K.M., Eds.; Elsevier: St. Louis, MO, USA, 2015; pp. e1–e44. [Google Scholar]
- Nowicka, A.; Wilk, G.; Lipski, M.; Kotecki, J.; Buczkowska-Radlinska, J. Tomographic evaluation of reparative dentin formation after direct pulp capping with Ca(OH)2, MTA, Biodentine, and Dentin bonding system in human teeth. J. Endod. 2015, 41, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Valles, M.; Mercade, M.; Duran-Sindreu, F.; Bourdelande, J.L.; Roig, M. Color stability of white mineral trioxide aggregate. Clin. Oral Investig. 2013, 17, 1155–1159. [Google Scholar] [CrossRef] [PubMed]



| Groups | Observation Time | Root Length (mm) | Apical Foramen Area (mm2) | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | SE | Mean | SD | SE | ||
| Acemannan (n = 20) | Immediate | 10.163 | 1.917 | 0.336 | 2.632 | 2.979 | 0.535 |
| 6 months | 10.753 | 1.772 | 0.325 * | 1.912 | 2.299 | 0.387 * | |
| 12 months | 11.263 | 1.525 | 0.292 * | 1.137 | 1.367 | 0.235 * | |
| MTA (n = 22) | Immediate | 10.735 | 1.331 | 0.349 | 2.289 | 1.701 | 0.511 |
| 6 months | 11.565 | 1.092 | 0.310 * | 1.288 | 0.954 | 0.369 * | |
| 12 months | 12.021 | 1.065 | 0.278 * | 0.629 | 0.646 | 0.224 * | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vu, T.T.; Nguyen, M.T.; Sangvanich, P.; Nguyen, Q.N.; Thunyakitpisal, P. Acemannan Used as an Implantable Biomaterial for Vital Pulp Therapy of Immature Permanent Teeth Induced Continued Root Formation. Pharmaceutics 2020, 12, 644. https://doi.org/10.3390/pharmaceutics12070644
Vu TT, Nguyen MT, Sangvanich P, Nguyen QN, Thunyakitpisal P. Acemannan Used as an Implantable Biomaterial for Vital Pulp Therapy of Immature Permanent Teeth Induced Continued Root Formation. Pharmaceutics. 2020; 12(7):644. https://doi.org/10.3390/pharmaceutics12070644
Chicago/Turabian StyleVu, Tien Thuy, Minh Truong Nguyen, Polkit Sangvanich, Quang Ngoc Nguyen, and Pasutha Thunyakitpisal. 2020. "Acemannan Used as an Implantable Biomaterial for Vital Pulp Therapy of Immature Permanent Teeth Induced Continued Root Formation" Pharmaceutics 12, no. 7: 644. https://doi.org/10.3390/pharmaceutics12070644






