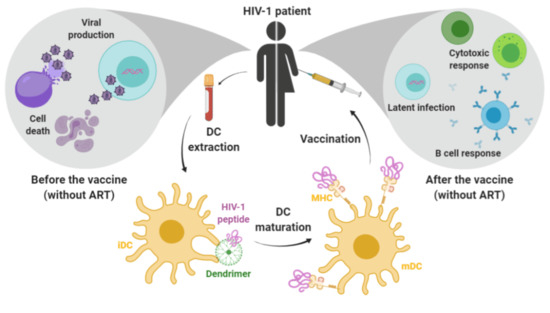
Nanoparticle-Delivered HIV Peptides to Dendritic Cells a Promising Approach to Generate a Therapeutic Vaccine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanocompounds
2.2. Primary Cell Cultures, Purification and Differentiation
2.2.1. Human Peripheral Blood Mononuclear Cells
2.2.2. Monocytes and Monocyte-Derived Dendritic Cells
2.3. HIV-1 Derived Peptide
2.4. Cell Viability Assay
2.5. Flow Cytometry
2.6. Complex Formation and Electrophoretic Measurements
2.7. Dendrimer Visualization by Confocal Microscopy
2.8. Autologous Mixed Lymphocyte Reaction
2.9. Bright field Microscopy
2.10. Cytokine Quantification
2.11. Autologous Mixed Lymphocyte Response
2.12. Reagents
2.13. Statistical Analysis
3. Results
3.1. In Vitro Toxicity of AMC6 Nanoparticle and G4-70/30 Dendrimer
3.2. AMC6 Nanoparticle and G4-70/30 Dendrimer Complex HIV-1 Derived Peptides
3.3. Dendrimers are Effective Carriers for Peptides into the Dendritic Cells
3.4. Transfection with Complexes Has No Effect on DCs Maturation
3.5. Treated DCs Induce Some Changes on Autologous PBMCs
3.6. Transfected DCs Do Not Modify the Expression of Activation Markers on T or B Cells
3.7. Transfection of DCs with G4-70/30 Dendrimer Increases the Release of TNF-α, IL-2 and IL-12 after Autologous Mixed Lymphocyte Reaction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hammer, S.M.; Squires, K.E.; Hughes, M.D.; Grimes, J.M.; Demeter, L.M.; Currier, J.S.; Eron, J.J.; Feinberg, J.E.; Balfour, H.H.; Deyton, L.R. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N. Engl. J. Med. 1997, 337, 725–733. [Google Scholar] [CrossRef] [Green Version]
- Egger, M.; May, M.; Chene, G.; Phillips, A.N.; Ledergerber, B.; Dabis, F.; Costagliola, D.; D’Arminio Monforte, A.; de Wolf, F.; Reiss, P.; et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: A collaborative analysis of prospective studies. Lancet 2002, 360, 119–129. [Google Scholar] [CrossRef]
- Wood, E.; Hogg, R.S.; Yip, B.; Harrigan, P.R.; O’Shaughnessy, M.V.; Montaner, J.S. Effect of medication adherence on survival of HIV-infected adults who start highly active antiretroviral therapy when the CD4+ cell count is 0.200 to 0.350 x 10(9) cells/L. Ann. Intern. Med. 2003, 139, 810–816. [Google Scholar]
- Uniaids. Available online: https://www.unaids.org/en (accessed on 13 February 2020).
- Schackman, B.R.; Fleishman, J.A.; Su, A.E.; Berkowitz, B.K.; Moore, R.D.; Walensky, R.P.; Becker, J.E.; Voss, C.; Paltiel, A.D.; Weinstein, M.C.; et al. The lifetime medical cost savings from preventing HIV in the United States. Med. Care 2015, 53, 293–301. [Google Scholar] [CrossRef] [Green Version]
- Kippax, S. HIV and technology: The issue of prophylactic vaccines. Dev. Bull. 2000, 52, 24–25. [Google Scholar]
- Xu, W.; Li, H.; Wang, Q.; Hua, C.; Zhang, H.; Li, W.; Jiang, S.; Lu, L. Advancements in Developing Strategies for Sterilizing and Functional HIV Cures. Biomed. Res. Int. 2017, 2017, 6096134. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ma, X.; Liu, B.; Chen, C.; Zhang, H.; Li, W.; Jiang, S.; and Lu, L. HIV-1 functional cure: Will the dream come true? BMC Med. 2015, 13, 284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davenport, M.P.; Khoury, D.S.; Cromer, D.; Lewin, S.R.; Kelleher, A.D.; Kent, S.J. Functional cure of HIV: The scale of the challenge. Nat. Rev. Immunol. 2019, 19, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Ensoli, B.; Cafaro, A.; Monini, P.; Marcotullio, S.; Ensoli, F. Challenges in HIV vaccine research for treatment and prevention. Front. Immunol. 2014, 5, 417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tongo, M.; Burgers, W.A. Challenges in the design of a T cell vaccine in the context of HIV-1 diversity. Viruses 2014, 6, 3968–3990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Churchyard, G.J.; Morgan, C.; Adams, E.; Hural, J.; Graham, B.S.; Moodie, Z.; Grove, D.; Gray, G.; Bekker, L.G.; McElrath, M.J.; et al. A phase IIA randomized clinical trial of a multiclade HIV-1 DNA prime followed by a multiclade rAd5 HIV-1 vaccine boost in healthy adults (HVTN204). PLoS ONE 2011, 6, e21225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vacas-Córdoba, E.; Bastida, H.; Pion, M.; Hameau, A.; Ionov, M.; Bryszewska, M.; Caminade, A.M.; Majoral, J.P.; Muñoz-Fernández, M.A. HIV-antigens charged on phosphorus dendrimers as tools for tolerogenic dendritic cells-based immunotherapy. Curr. Med. Chem. 2014, 21, 1898–1909. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.E.; Poteet, E.C.; Liu, D.; Chen, C.; LaBranche, C.E.; Stanfield-Oakley, S.A.; Montefiori, C.; Ferrari, G.; Yao, Q. CTLA-4 blockade, during HIV virus-like particles immunization, alters HIV-specific B-cell responses. Vaccines 2020, 6, 284. [Google Scholar] [CrossRef]
- Palucka, K.; Banchereau, J. Human dendritic cell subsets in vaccination. Curr. Opin. Immunol. 2013, 25, 396–402. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.S.; Bae, Y.S. Dendritic cell-based therapeutic cancer vaccines: Past, present and future. Clin. Exp. Vaccine Res. 2014, 3, 113–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinageshwar, B.; Peruzzaro, S.; Andrews, M.; Johnson, K.; Hietpas, A.; Clark, B.; McGuire, C.; Petersen, E.; Kippe, J.; Stewart, A.; et al. PAMAM Dendrimers Cross the Blood-Brain Barrier When Administered through the Carotid Artery in C57BL/6J Mice. Int. J. Mol. Sci. 2017, 18, 628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinageshwar, B.; Dils, A.; Sturgis, J.; Wedster, A.; Kathirvelu, B.; Baiyasi, S.; Swanson, D.; Dunbar, G.L.; Sharma, A.; Rossignol, J. Surface-Modified G4 PAMAM Dendrimers Cross the Blood-Brain Barrier Following Multiple Tail-Vein Injections in C57BL/6J Mice. ACS Chem. Neurosci. 2019, 10, 4145–4150. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.; Bienvenu, C.; Jiménez Blanco, J.L.; Vierling, P.; Ortiz Mellet, C.; García-Fernandez, J.M.; Di Giorgio, C. Amphiphilic Oligoethyleneimine-ß-Cyclodextrin “Click” Clusters for Enhanced DNA Delivery. J. Org. Chem. 2013, 78, 8143–8148. [Google Scholar] [CrossRef]
- Manzanares, D.; Araya-Duran, I.; Gallego-Yerga, L.; Játiva, P.; Márquez-Miranda, V.; Canan, J.; Manzanares, D.; Araya-Durán, I.; Gallego-Yerga, L.; Játiva, P.; et al. Molecular determinants for cyclo-oligosaccharide-based nanoparticle-mediated effective siRNA transfection. Nanomedicine (Lond) 2017, 12, 1607–1621. [Google Scholar] [CrossRef]
- García-Merino, I.; de las Cuevas, N.; Jiménez, J.L.; Gallego, J.; Gómez, C.; Prieto, C.; Serramía, M.J.; Lorente, R.; Muñoz-Fernández, M.A. The Spanish HIV BioBank: A model of cooperative HIV research. Retrovirology 2009, 6, 27. [Google Scholar]
- García-Merino, I.; de las Cuevas, N.; Jiménez, J.L.; García, A.; Gallego, J.; Gómez, C.; García, D.; Muñoz-Fernández, M.A. Pediatric HIV BioBank: A new role of the Spanish HIV BioBank in pediatric HIV research. AIDS Res. Hum. Retrovir. 2010, 26, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Consuegra, I.; Rodríguez-Aierbe, C.; Santiuste, I.; Bosch, A.; Martínez-Marín, R.; Fortuto, M.A.; Díaz, T.; Martí, S.; Muñoz-Fernández, M.A. Isolation Methods of Peripheral Blood Mononuclear Cells in Spanish Biobanks: An Overview. Biopreserv. Biobank. 2017, 15, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Klenerman, P.; Luzzi, G.; McIntyre, K.; Phillips, R.; McMichael, A. Identification of a Novel HLA-A25-restricted Epitope in a Conserved Region of p24 Gag (Positions 71-80). AIDS 1996, 10, 348–350. [Google Scholar] [CrossRef] [PubMed]
- Ionov, M.; Ciepluch, K.; Moreno, B.R.; Appelhans, D.; Sánchez-Nieves, J.; Gómez, R.; de la Mata, F.J.; Muñoz-Fernández, M.A.; Bryszewska, M. Biophysical characterization of glycodendrimers as nano-carriers for HIV peptides. Curr. Med. Chem. 2013, 20, 3935–3943. [Google Scholar] [CrossRef]
- González-Serna, A.; Ferrando-Martínez, S.; Tarancón-Diez, L.; De Pablo-Bernal, S.R.; Domínguez-Molina, B.; Jiménez, J.L.; Muñz-Fernández, M.A.; Leal, M.; Ruiz-Mateos, E. Increased CD127+ and decreased CD57+ T cell expression levels in HIV-infected patients on NRTI-sparing regimens. J. Transl. Med. 2017, 15, 259. [Google Scholar]
- Díaz, L.; Méndez-Lagares, G.; Correa-Rocha, R.; Pacheco, Y.M.; Ferrando-Martínez, S.; Ruiz-Mateos, E.; del Pozo-Balado, M.; León, J.A.; Gurbindo, M.D.; Isabel de José, M.; et al. Detectable viral load aggravates immunosenescence features of CD8 T-cell subsets in vertically HIV-infected children. J. Acquir. Immune Defic Syndr. 2012, 60, 447–454. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Thudium, K.B.; Han, M.; Wang, X.T.; Huang, H.; Feingersh, D.; Garcia, C.; Wu, Y.; Kuhne, M.; Srinivasan, M.; et al. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol. Res. 2014, 2, 846–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balasubramaniam, M.; Pandhare, J.; Dash, C. Immune Control of HIV. J. Life Sci. (Westlake Village) 2019, 1, 4–37. [Google Scholar] [CrossRef]
- Moretti, S.; Cafaro, A.; Tripiciano, A.; Picconi, O.; Butto, S.; Ensoli, F.; Sgadari, C.; Monini, P.; Ensoli, B. HIV therapeutic vaccines aimed at intensifying combination antiretroviral therapy. Expert Rev. Vaccines 2020, 19, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Surenaud, M.; Montes, M.; Lindestam Arlehamn, C.S.; Sette, A.; Banchereau, J.; Palucka, K.; Lelièvre, J.D.; Lacabaratz, C.; Lévy, Y. Anti-HIV potency of T-cell responses elicited by dendritic cell therapeutic vaccination. PLoS Pathog. 2019, 9, e1008011. [Google Scholar] [CrossRef]
- Jin, Z.; Gao, S.; Cui, X.; Sun, D.; Zhao, K. Adjuvants and delivery systems based on polymeric nanoparticles for mucosal vaccines. Int. J. Pharm. 2019, 572, 118731. [Google Scholar] [CrossRef] [PubMed]
- Jativa, P.; Ceña, V. Use of nanoparticles for glioblastoma treatment: A new approach. Nanomedicine (Lond) 2017, 12, 2533–2554. [Google Scholar] [CrossRef] [PubMed]
- Pion, M.; Serramía, M.J.; Diaz, L.; Bryszewska, M.; Gallart, T.; García, F.; Gómez, R.; de la Mata, F.J.; Muñoz-Fernandez, M.A. Phenotype and functional analysis of human monocytes-derived dendritic cells loaded with a carbosilane dendrimer. Biomaterials 2010, 31, 8749–8758. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, D.; Ceña, V. Endocytosis: The Nanoparticle and Submicron Nanocompounds Gateway into the Cell. Pharmaceutics 2020, 12, 371. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Moscoso, A.; Vercauteren, D.; Rejman, J.; Benito, J.M.; Ortiz Mellet, C.; De Smedt, S.C.; García Fernández, J.M. Insights in cellular uptake mechanisms of pDNA-polycationic amphiphilic cyclodextrin nanoparticles (CDplexes). J. Control Release 2010, 143, 318–325. [Google Scholar] [CrossRef] [Green Version]
- Sundar, R.; Cho, B.C.; Brahmer, J.R.; Soo, R.A. Nivolumab in NSCLC: Latest evidence and clinical potential. Ther. Adv. Med. Oncol. 2015, 7, 85–96. [Google Scholar] [CrossRef] [Green Version]
- Akiyama, Y.; Nonomura, C.; Kondou, R.; Miyata, H.; Ashizawa, T.; Maeda, C.; Mitsuya, K.; Hayashi, N.; Nakasu, Y.; Yamaguchi, K. Immunological effects of the anti-programmed death-1 antibody on human peripheral blood mononuclear cells. Int. J. Oncol. 2016, 49, 1099–1107. [Google Scholar] [CrossRef] [Green Version]
- Hsu, M.L.; Naidoo, J. Principles of Immunotherapy in Non-Small Cell Lung Cancer. Thorac Surg. Clin. 2020, 30, 187–198. [Google Scholar] [CrossRef] [PubMed]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Moreno, A.; Jiménez Blanco, J.L.; Mosher, J.; Swanson, D.R.; García Fernández, J.M.; Sharma, A.; Ceña, V.; Muñoz-Fernández, M.A. Nanoparticle-Delivered HIV Peptides to Dendritic Cells a Promising Approach to Generate a Therapeutic Vaccine. Pharmaceutics 2020, 12, 656. https://doi.org/10.3390/pharmaceutics12070656
Martín-Moreno A, Jiménez Blanco JL, Mosher J, Swanson DR, García Fernández JM, Sharma A, Ceña V, Muñoz-Fernández MA. Nanoparticle-Delivered HIV Peptides to Dendritic Cells a Promising Approach to Generate a Therapeutic Vaccine. Pharmaceutics. 2020; 12(7):656. https://doi.org/10.3390/pharmaceutics12070656
Chicago/Turabian StyleMartín-Moreno, Alba, José L. Jiménez Blanco, Jamie Mosher, Douglas R. Swanson, José M. García Fernández, Ajit Sharma, Valentín Ceña, and María Angeles Muñoz-Fernández. 2020. "Nanoparticle-Delivered HIV Peptides to Dendritic Cells a Promising Approach to Generate a Therapeutic Vaccine" Pharmaceutics 12, no. 7: 656. https://doi.org/10.3390/pharmaceutics12070656
APA StyleMartín-Moreno, A., Jiménez Blanco, J. L., Mosher, J., Swanson, D. R., García Fernández, J. M., Sharma, A., Ceña, V., & Muñoz-Fernández, M. A. (2020). Nanoparticle-Delivered HIV Peptides to Dendritic Cells a Promising Approach to Generate a Therapeutic Vaccine. Pharmaceutics, 12(7), 656. https://doi.org/10.3390/pharmaceutics12070656