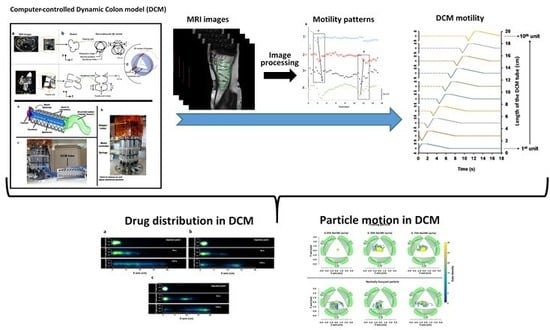
Dynamic Colon Model (DCM): A Cine-MRI Informed Biorelevant In Vitro Model of the Human Proximal Large Intestine Characterized by Positron Imaging Techniques
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. The Dynamic Colon Model (DCM)
2.3. In Vivo cine-MRI of Cecum-Ascending Colon in Healthy Human Subjects
2.4. Reproducing the Wall Motion Patterns in DCM Observed in Cine-MRI Study
2.5. Fluids Used in PEPT and PET Experiments
2.6. Positron Emission Particle Tracking (PEPT) System
2.7. Positron Emission Tomography (PET) to Visualize Fluid Motion within the DCM Tube
2.8. Statistical Analysis
3. Results and Discussion
3.1. Determining Haustral Activity in the Cecum-Ascending Colon Using Cine-MRI
3.2. Replicating the Haustral Motility in DCM
- (1)
- The membrane of the first segment was inflated, whereas, in the second, it was deflated at the same speed, 1.6 cm s−1.
- (2)
- When the first segment reaches the maximum degree of luminal occlusion, it stays at this position for 1 s before going back to its neutral position at a lower speed of 0.35 cm s−1 (Figure 5a).
3.3. Positron Emission Tomography (PET)
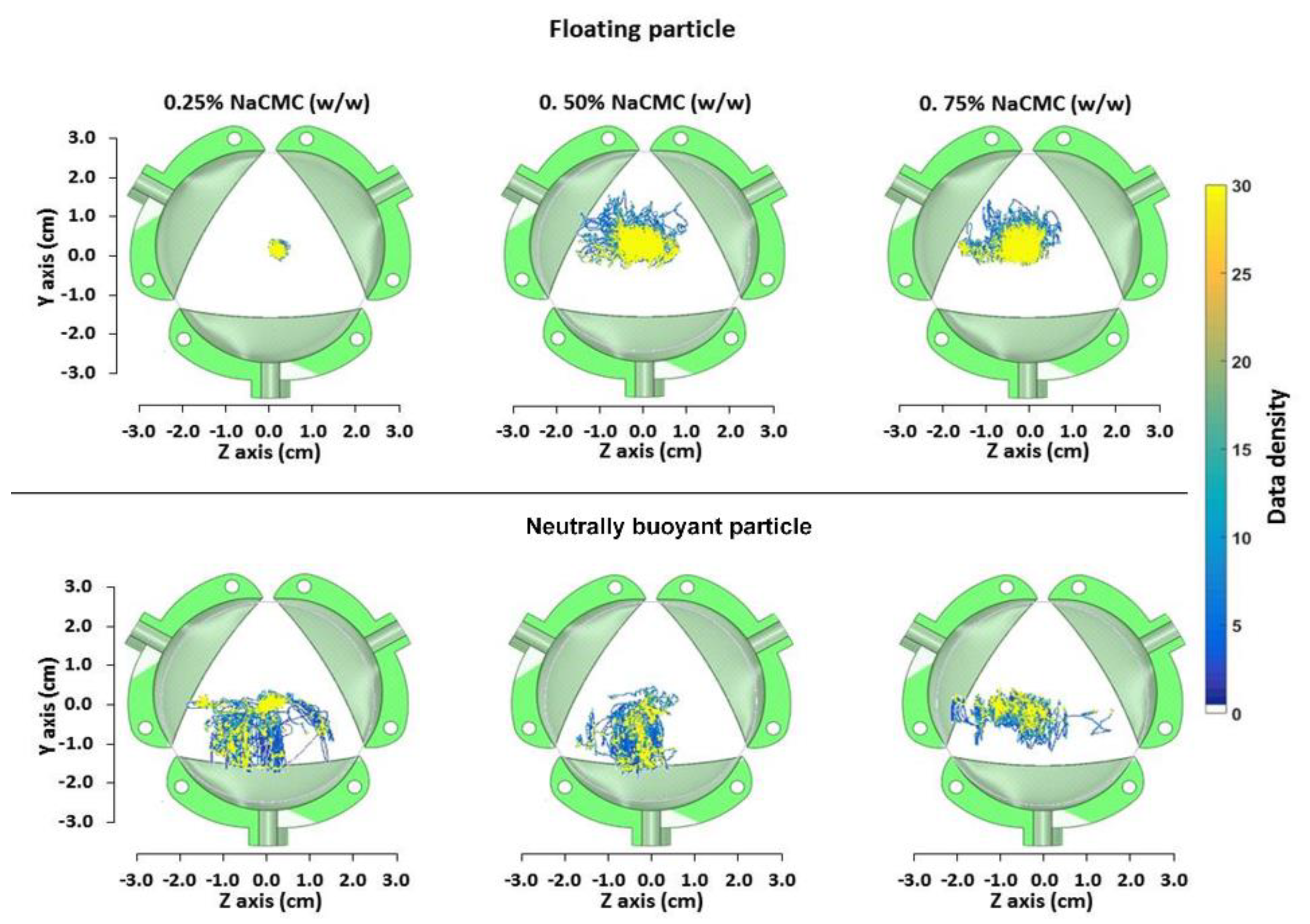
3.4. Assessing Particle Motion in DCM Using PEPT
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burisch, J.; Jess, T.; Martinato, M.; Lakatos, P.L. The burden of inflammatory bowel disease in Europe. J. Crohn’s Colitis 2013, 7, 322–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54.e42. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, G.G. The global burden of ibd: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Amidon, S.; Brown, J.E.; Dave, V.S. Colon-targeted oral drug delivery systems: Design trends and approaches. AAPS PharmSciTech 2015, 16, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.; Hoad, C.L.; Mudie, D.M.; Wright, J.; Heissam, K.; Abrehart, N.; Pritchard, S.E.; Al Atwah, S.; Gowland, P.A.; Garnett, M.C.; et al. Magnetic resonance imaging quantification of fasted state colonic liquid pockets in healthy humans. Mol. Pharm. 2017, 14, 2629–2638. [Google Scholar] [CrossRef] [Green Version]
- Garbacz, G.; Wedemeyer, R.S.; Nagel, S.; Giessmann, T.; Monnikes, H.; Wilson, C.G.; Siegmund, W.; Weitschies, W. Irregular absorption profiles observed from diclofenac extended release tablets can be predicted using a dissolution test apparatus that mimics in vivo physical stresses. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Pharm. Verfahr. 2008, 70, 421–428. [Google Scholar] [CrossRef]
- Garbacz, G.; Klein, S. Dissolution testing of oral modified-release dosage forms. J. Pharm. Pharmacol. 2012, 64, 944–968. [Google Scholar] [CrossRef]
- Fotaki, N.; Aivaliotis, A.; Butler, J.; Dressman, J.; Fischbach, M.; Hempenstall, J.; Klein, S.; Reppas, C. A comparative study of different release apparatus in generating in vitro-in vivo correlations for extended release formulations. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Pharm. Verfahr. 2009, 73, 115–120. [Google Scholar] [CrossRef]
- Zuleger, S.; Fassihi, R.; Lippold, B.C. Polymer particle erosion controlling drug release. Ii. Swelling investigations to clarify the release mechanism. Int. J. Pharm. 2002, 247, 23–37. [Google Scholar] [CrossRef]
- Mitchell, K.; Ford, J.L.; Armstrong, D.J.; Elliott, P.N.C.; Hogan, J.E.; Rostron, C. The influence of drugs on the properties of gels and swelling characteristics of matrices containing methylcellulose or hydroxypropylmethylcellulose. Int. J. Pharm. 1993, 100, 165–173. [Google Scholar] [CrossRef]
- Spratt, P.; Nicolella, C.; Pyle, D.L. An engineering model of the human colon. Food Bioprod. Process. 2005, 83, 147–157. [Google Scholar] [CrossRef]
- Stamatopoulos, K.; Batchelor, H.K.; Simmons, M.J.H. Dissolution profile of theophylline modified release tablets, using a biorelevant dynamic colon model (dcm). Eur. J. Pharm. Biopharm. 2016, 108, 9–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamei, M.; Turner, D.; Yang, J.; Neuhoff, S.; Polak, S.; Rostami-Hodjegan, A.; Tucker, G. Population-based mechanistic prediction of oral drug absorption. AAPS J. 2009, 11, 225–237. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.-H.; Yu, Y.; Yang, Z.; Yu, W.-Z.; Chen, W.L.; Yu, H.; Kim, M.J.-M.; Huang, M.; Tan, S.; Luo, H.; et al. Intraluminal pressure patterns in the human colon assessed by high-resolution manometry. Sci. Rep. 2017, 7, 41436. [Google Scholar] [CrossRef] [Green Version]
- Bharucha, A.E. Editorial: Identifying colonic motor dysfunction in chronic constipation with high-resolution manometry: Pan-colonic pressurizations. Am. J. Gastroenterol. 2017, 112, 490–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinning, P.G.; Wiklendt, L.; Maslen, L.; Gibbins, I.; Patton, V.; Arkwright, J.W.; Lubowski, D.Z.; O’Grady, G.; Bampton, P.A.; Brookes, S.J.; et al. Quantification of in vivo colonic motor patterns in healthy humans before and after a meal revealed by high-resolution fiber-optic manometry. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2014, 26, 1443–1457. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.-H.; Parsons, S.P.; Shokrollahi, M.; Wan, A.; Vincent, A.D.; Yuan, Y.; Pervez, M.; Chen, W.L.; Xue, M.; Zhang, K.K.; et al. Characterization of simultaneous pressure waves as biomarkers for colonic motility assessed by high-resolution colonic manometry. Front. Physiol. 2018, 9, 1248. [Google Scholar] [CrossRef]
- Milkova, N.; Parsons, S.P.; Ratcliffe, E.; Huizinga, J.D.; Chen, J.H. On the nature of high-amplitude propagating pressure waves in the human colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G646–G660. [Google Scholar] [CrossRef] [Green Version]
- Arkwright, J.W.; Dickson, A.; Maunder, S.A.; Blenman, N.G.; Lim, J.; O’Grady, G.; Archer, R.; Costa, M.; Spencer, N.J.; Brookes, S.; et al. The effect of luminal content and rate of occlusion on the interpretation of colonic manometry. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2013, 25, e52–e59. [Google Scholar] [CrossRef] [Green Version]
- Hiroz, P.; Schlageter, V.; Givel, J.C.; Kucera, P. Colonic movements in healthy subjects as monitored by a magnet tracking system. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2009, 21, 838-e57. [Google Scholar] [CrossRef] [Green Version]
- Mark, E.B.; Poulsen, J.L.; Haase, A.M.; Espersen, M.; Gregersen, T.; Schlageter, V.; Scott, S.M.; Krogh, K.; Drewes, A.M. Ambulatory assessment of colonic motility using the electromagnetic capsule tracking system. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2019, 31, e13451. [Google Scholar] [CrossRef]
- Sulaiman, S.; Marciani, L. Mri of the colon in the pharmaceutical field: The future before us. Pharmaceutics 2019, 11, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoad, C.L.; Menys, A.; Garsed, K.; Marciani, L.; Hamy, V.; Murray, K.; Costigan, C.; Atkinson, D.; Major, G.; Spiller, R.C.; et al. Colon wall motility: Comparison of novel quantitative semi-automatic measurements using cine mri. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2016, 28, 327–335. [Google Scholar] [CrossRef] [Green Version]
- Effinger, A.; O’Driscoll, C.M.; McAllister, M.; Fotaki, N. Impact of gastrointestinal disease states on oral drug absorption—Implications for formulation design—A pearrl review. J. Pharm. Pharmacol. 2019, 71, 674–698. [Google Scholar] [CrossRef] [Green Version]
- Fallingborg, J.; Christensen, L.A.; Jacobsen, B.A.; Rasmussen, S.N. Very low intraluminal colonic ph in patients with active ulcerative colitis. Dig. Dis. Sci. 1993, 38, 1989–1993. [Google Scholar] [CrossRef]
- Marciani, L.; Garsed, K.C.; Hoad, C.L.; Fields, A.; Fordham, I.; Pritchard, S.E.; Placidi, E.; Murray, K.; Chaddock, G.; Costigan, C.; et al. Stimulation of colonic motility by oral peg electrolyte bowel preparation assessed by mri: Comparison of split vs single dose. Neurogastroenterol. Motil. 2014, 26, 1426–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broadbent, C.J.; Bridgwater, J.; Parker, D.J.; Keningley, S.T.; Knight, P. A phenomenological study of a batch mixer using a positron camera. Powder Technol. 1993, 76, 317–329. [Google Scholar] [CrossRef]
- Simmons, M.J.H.; Alberini, F.; Tsoligkas, A.N.; Gargiuli, J.; Parker, D.J.; Fryer, P.J.; Robinson, S. Development of a hydrodynamic model for the uv-c treatment of turbid food fluids in a novel ‘surepure turbulator™’ swirl-tube reactor. Innov. Food Sci. Emerg. Technol. 2012, 14, 122–134. [Google Scholar] [CrossRef]
- McConnell, E.L.; Fadda, H.M.; Basit, A.W. Gut instincts: Explorations in intestinal physiology and drug delivery. Int. J. Pharm. 2008, 364, 213–226. [Google Scholar] [CrossRef]
- Leadbeater, T.W.; Parker, D.J. A high speed pc-based data acquisition and control system for positron imaging. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2009, 604, 355–358. [Google Scholar] [CrossRef]
- Savitzky, A.; Golay, M.J.E. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Dinning, P.G.; Szczesniak, M.M.; Cook, I.J. Proximal colonic propagating pressure waves sequences and their relationship with movements of content in the proximal human colon. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2008, 20, 512–520. [Google Scholar] [CrossRef]
- Spencer, N.J.; Dinning, P.G.; Brookes, S.J.; Costa, M. Insights into the mechanisms underlying colonic motor patterns. J. Physiol. 2016, 594, 4099–4116. [Google Scholar] [CrossRef] [Green Version]
- Sinnott, M.D.; Cleary, P.W.; Arkwright, J.W.; Dinning, P.G. Investigating the relationships between peristaltic contraction and fluid transport in the human colon using smoothed particle hydrodynamics. Comput. Biol. Med. 2012, 42, 492–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, I.J.; Furukawa, Y.; Panagopoulos, V.; Collins, P.J.; Dent, J. Relationships between spatial patterns of colonic pressure and individual movements of content. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 278, G329–G341. [Google Scholar] [CrossRef]
- Pritchard, S.E.; Paul, J.; Major, G.; Marciani, L.; Gowland, P.A.; Spiller, R.C.; Hoad, C.L. Assessment of motion of colonic contents in the human colon using mri tagging. Neurogastroenterol. Motil. 2017, 29, e13091. [Google Scholar] [CrossRef] [Green Version]
- Wright, J.; Evans, D.; Gowland, P.; Mansfield, P. Validation of antroduodenal motility measurements made by echo-planar magnetic resonance imaging. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 1999, 11, 19–25. [Google Scholar] [CrossRef]
- Gollifer, R.M.; Menys, A.; Plumb, A.; Mengoudi, K.; Puylaert, C.A.J.; Tielbeek, J.A.W.; Ponsioen, C.Y.; Vos, F.M.; Stoker, J.; Taylor, S.A.; et al. Automated versus subjective assessment of spatial and temporal mri small bowel motility in crohn’s disease. Clin. Radiol. 2019, 74, 814.e9–814.e19. [Google Scholar] [CrossRef]
- Gollifer, R.M.; Menys, A.; Makanyanga, J.; Puylaert, C.A.; Vos, F.M.; Stoker, J.; Atkinson, D.; Taylor, S.A. Relationship between mri quantified small bowel motility and abdominal symptoms in crohn’s disease patients-a validation study. Br. J. Radiol. 2018, 91, 20170914. [Google Scholar] [CrossRef] [PubMed]
- Menys, A.; Hoad, C.; Spiller, R.; Scott, S.M.; Atkinson, D.; Marciani, L.; Taylor, S.A. Spatio-temporal motility mri analysis of the stomach and colon. Neurogastroenterol. Motil. 2019, 31, e13557. [Google Scholar] [CrossRef] [PubMed]
- Boulby, P.; Moore, R.; Gowland, P.; Spiller, R.C. Fat delays emptying but increases forward and backward antral flow as assessed by flow-sensitive magnetic resonance imaging. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 1999, 11, 27–36. [Google Scholar] [CrossRef]
- Gutzeit, A.; Patak, M.A.; von Weymarn, C.; Graf, N.; Doert, A.; Willemse, E.; Binkert, C.A.; Froehlich, J.M. Feasibility of small bowel flow rate measurement with mri. J. Magn. Reson. Imaging JMRI 2010, 32, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Marciani, L.; Gowland, P.A.; Spiller, R.C.; Manoj, P.; Moore, R.J.; Young, P.; Al-Sahab, S.; Bush, D.; Wright, J.; Fillery-Travis, A.J. Gastric response to increased meal viscosity assessed by echo-planar magnetic resonance imaging in humans. J. Nutr. 2000, 130, 122–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reppas, C.; Eleftheriou, G.; Macheras, P.; Symillides, M.; Dressman, J.B. Effect of elevated viscosity in the upper gastrointestinal tract on drug absorption in dogs. Eur. J. Pharm. Sci. 1998, 6, 131–139. [Google Scholar] [CrossRef]
- Reppas, C.; Friedel, H.-D.; Barker, A.R.; Buhse, L.F.; Cecil, T.L.; Keitel, S.; Kraemer, J.; Morris, J.M.; Shah, V.P.; Stickelmeyer, M.P.; et al. Biorelevant in vitro performance testing of orally administered dosage forms—workshop report. Pharm. Res. 2014, 31, 1867–1876. [Google Scholar] [CrossRef]









| Condition | N 1 | Motility Index (Segment x s) | No of Waves 2 | Travel Distance (cm) | Travel Velocity (cm s−1) | Occlusion Degree (%) | Occlusion Velocity (cm s−1) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| A | R | A | R | A | R | |||||
| Baseline | 240 | 120 ± 50 * | 1 * | - | 3.9 * | - | 0.98 * | - | 18 ± 10 * | 0.14 ± 11 * |
| Stimulated | 720 | 320 ± 138 * | 9 ± 1 * | 2 | 5.3 ± 1.4 * | 6.6 ± 2.2 | 2.2 ± 3.3 * | 2.2 ±1.8 | 59 ± 18 * | 3.6 ± 0.17 * |
| DCM | - | 240 | 4 | - | 20 | - | 2 | - | 40 | 1.6 |
| Fluid | %NaCMC (w/w) | μA (m Pa s) | K (Pa s-n) | n | Re | to (s) | Residence Times (s) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Floating Particle | Neutrally Buoyant Particle | |||||||||
| L * | 0.25 | 8 | 0.04 | 0.9 | 80 | 0.059 + | 0.065 ++ | 0.378 +++ 0.178 ++++ | 25 a–125 b | 50 a–300 b |
| M * | 0.50 | 106 | 0.20 | 0.7 | 5.6 | 0.004 + | 0.005 ++ | 0.028 +++ 0.013 ++++ | 50 a–125 b | 150 a–300 b |
| H * | 0.75 | 200 | 0.83 | 0.6 | 0.9 | 0.002 + | 0.003 ++ | 0.015 +++ 0.007 ++++ | 100 a–125 b | 175 a–300 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stamatopoulos, K.; Karandikar, S.; Goldstein, M.; O’Farrell, C.; Marciani, L.; Sulaiman, S.; Hoad, C.L.; Simmons, M.J.H.; Batchelor, H.K. Dynamic Colon Model (DCM): A Cine-MRI Informed Biorelevant In Vitro Model of the Human Proximal Large Intestine Characterized by Positron Imaging Techniques. Pharmaceutics 2020, 12, 659. https://doi.org/10.3390/pharmaceutics12070659
Stamatopoulos K, Karandikar S, Goldstein M, O’Farrell C, Marciani L, Sulaiman S, Hoad CL, Simmons MJH, Batchelor HK. Dynamic Colon Model (DCM): A Cine-MRI Informed Biorelevant In Vitro Model of the Human Proximal Large Intestine Characterized by Positron Imaging Techniques. Pharmaceutics. 2020; 12(7):659. https://doi.org/10.3390/pharmaceutics12070659
Chicago/Turabian StyleStamatopoulos, Konstantinos, Sharad Karandikar, Mark Goldstein, Connor O’Farrell, Luca Marciani, Sarah Sulaiman, Caroline L. Hoad, Mark J. H. Simmons, and Hannah K. Batchelor. 2020. "Dynamic Colon Model (DCM): A Cine-MRI Informed Biorelevant In Vitro Model of the Human Proximal Large Intestine Characterized by Positron Imaging Techniques" Pharmaceutics 12, no. 7: 659. https://doi.org/10.3390/pharmaceutics12070659
APA StyleStamatopoulos, K., Karandikar, S., Goldstein, M., O’Farrell, C., Marciani, L., Sulaiman, S., Hoad, C. L., Simmons, M. J. H., & Batchelor, H. K. (2020). Dynamic Colon Model (DCM): A Cine-MRI Informed Biorelevant In Vitro Model of the Human Proximal Large Intestine Characterized by Positron Imaging Techniques. Pharmaceutics, 12(7), 659. https://doi.org/10.3390/pharmaceutics12070659