Hydrolytic Degradability, Cell Tolerance and On-Demand Antibacterial Effect of Electrospun Photodynamically Active Fibres
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrospinning of Photodynamically Active Fibres (PAFs)
2.3. Scanning Electron Microscopy (SEM)
2.4. Differential Scanning Calorimetry
2.5. Hydrolytic Degradation Tests
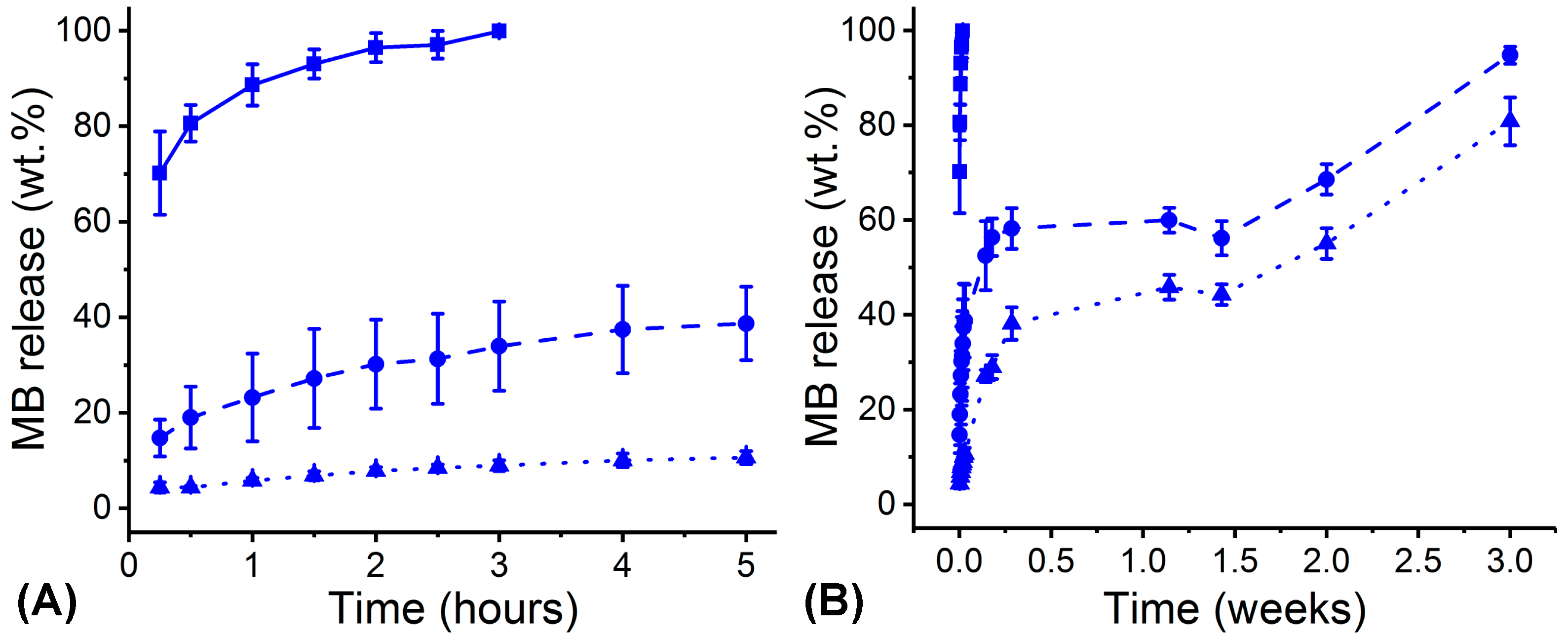
2.6. Methylene Blue (MB) Release Tests
2.7. Photosensitiser Uptake Study
2.7.1. Uptake Study with L929 Cells
2.7.2. Uptake Study with Bacteria
2.8. Cytotoxicity Tests
2.8.1. Extract Cytotoxicity Tests
2.8.2. Contact Cytotoxicity Tests
2.9. Antimicrobial Photodynamic Tests
2.9.1. Extract Photodynamic Tests
2.9.2. Contact Photodynamic Tests
2.10. Statistical Analysis
3. Results and Discussion
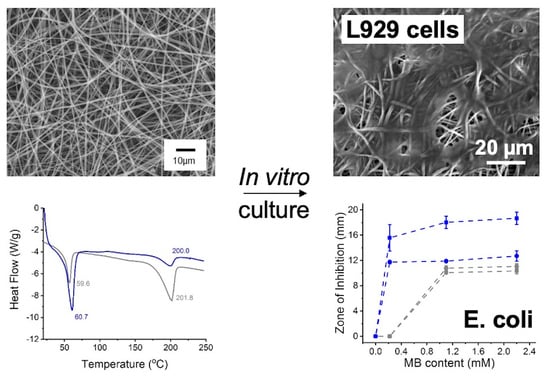
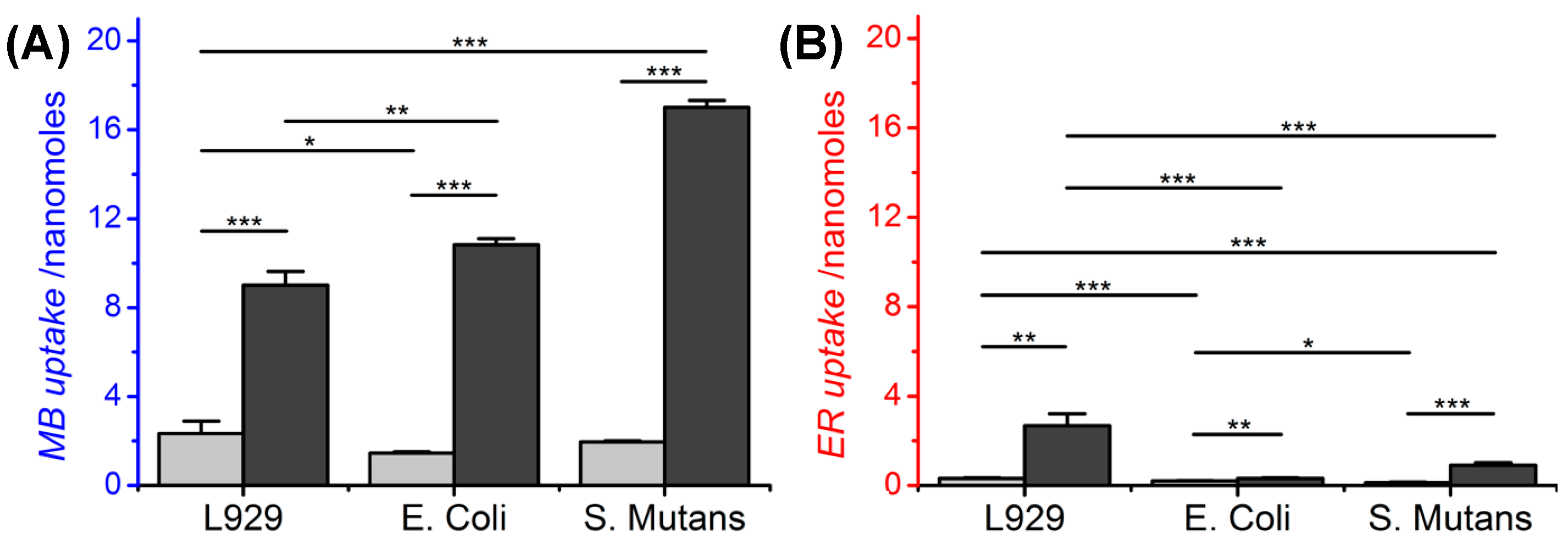
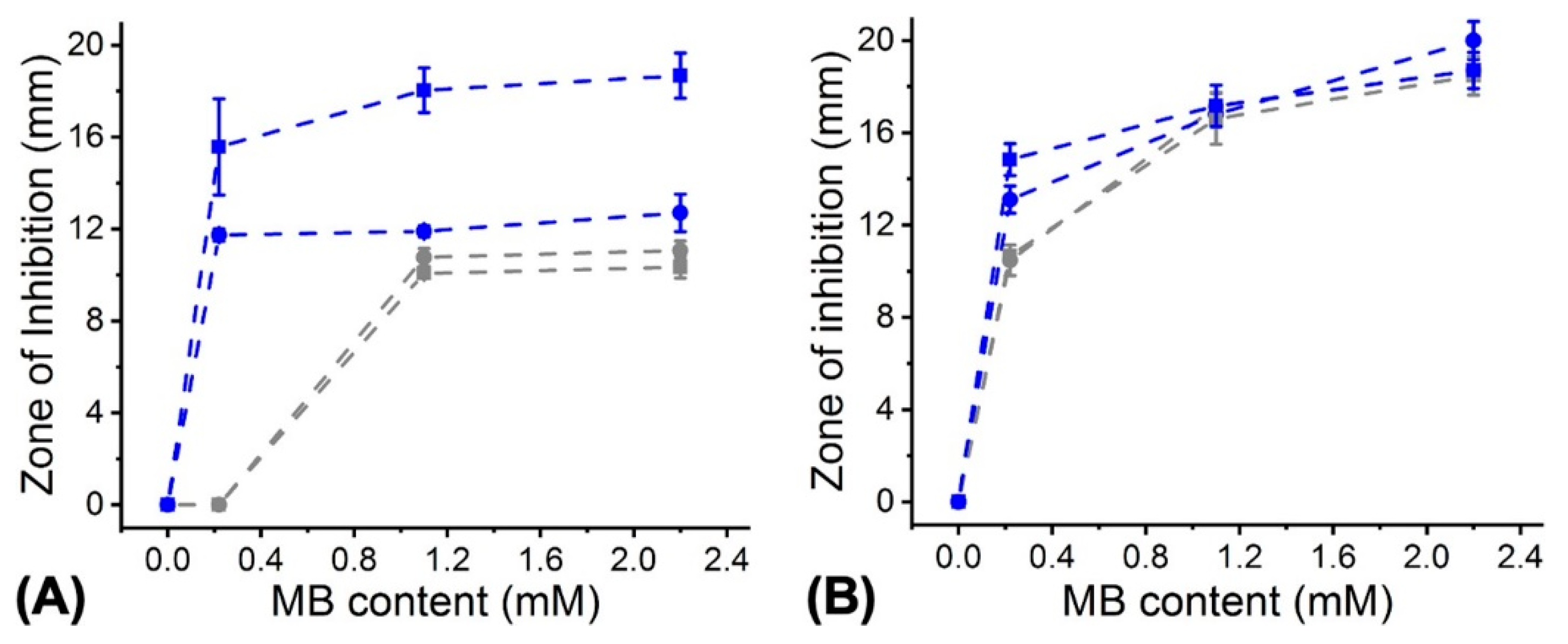
3.1. PS Uptake of Mammalian and Bacterial Cells
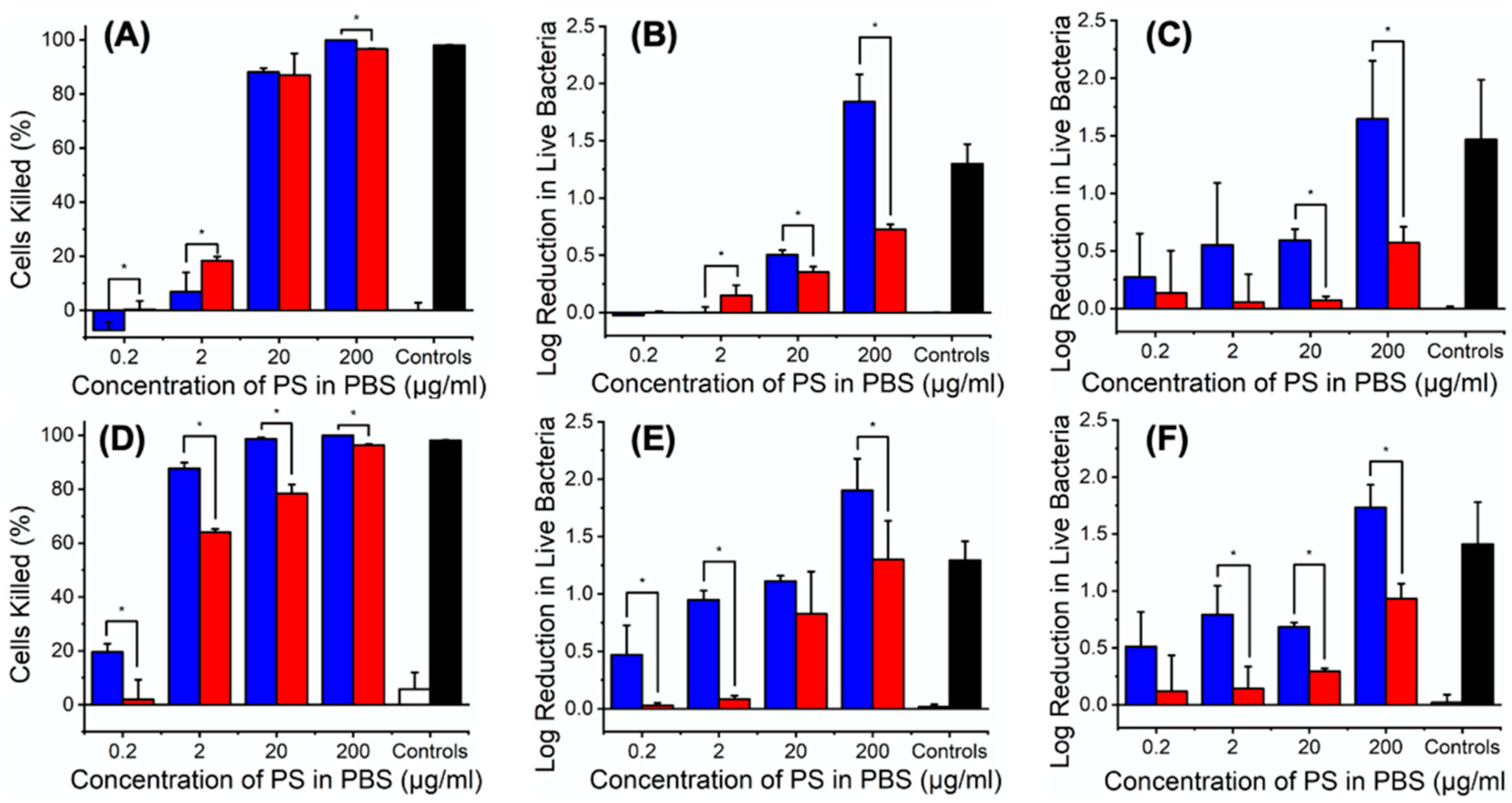
3.2. PS Impact on L929 Cells, E. coli and S. Mutans
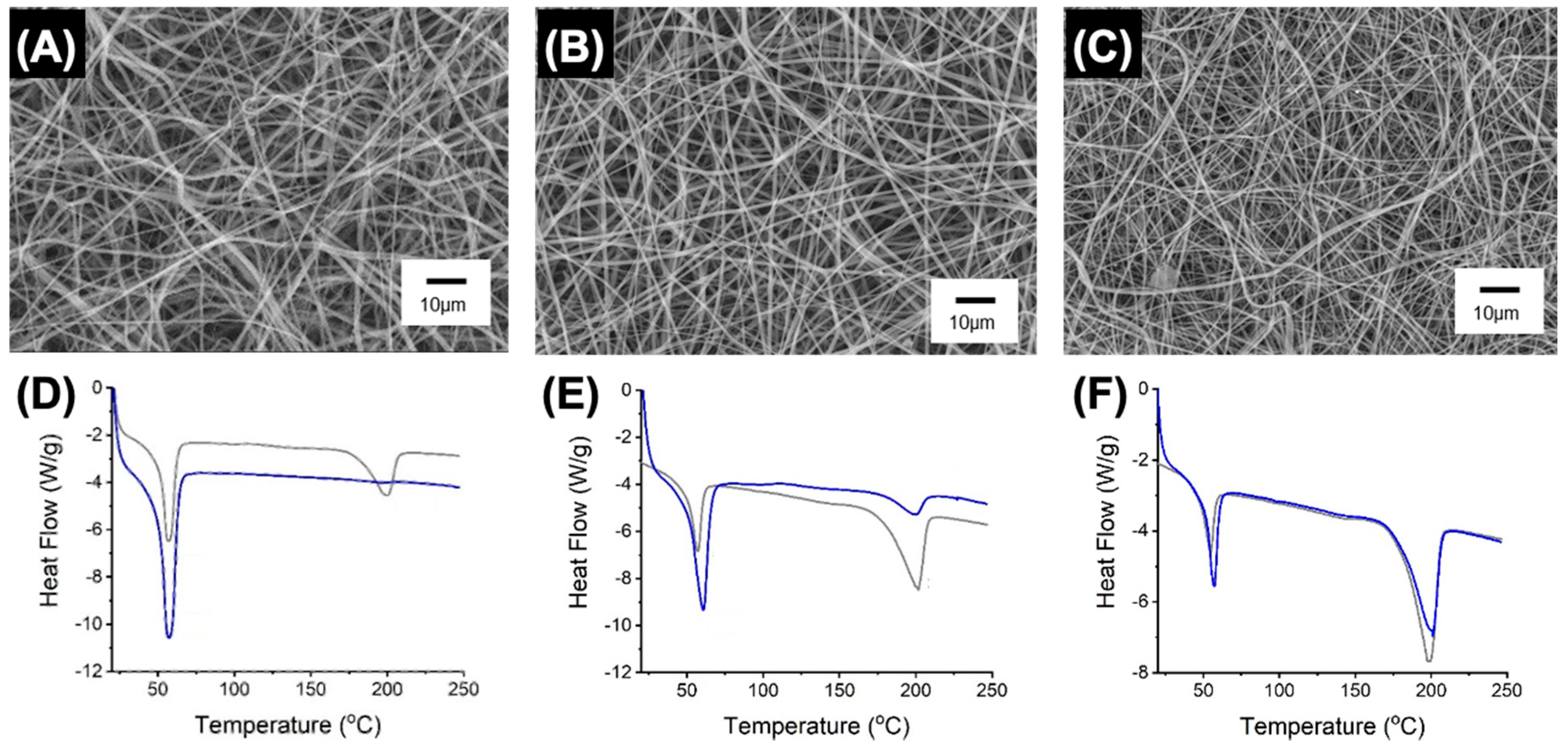
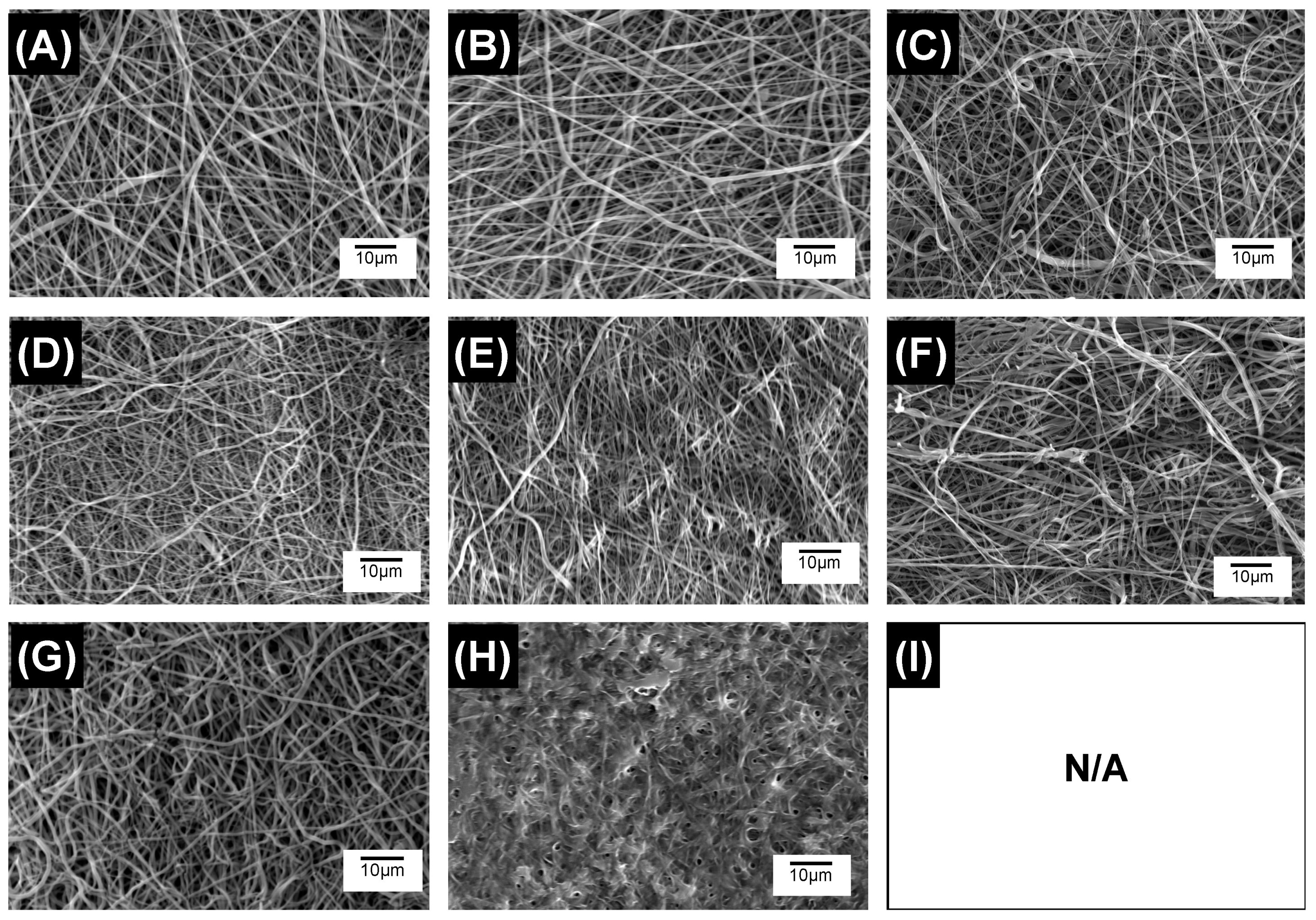
3.3. Microstructure of Electrospun PAFs
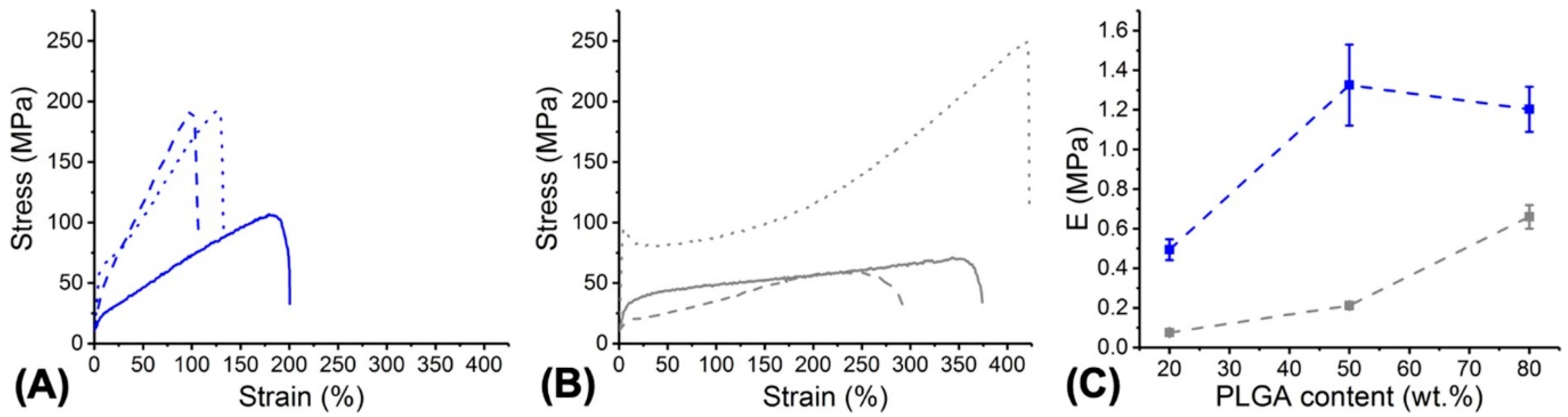
3.4. Fibre Behaviour in Physiological Environment
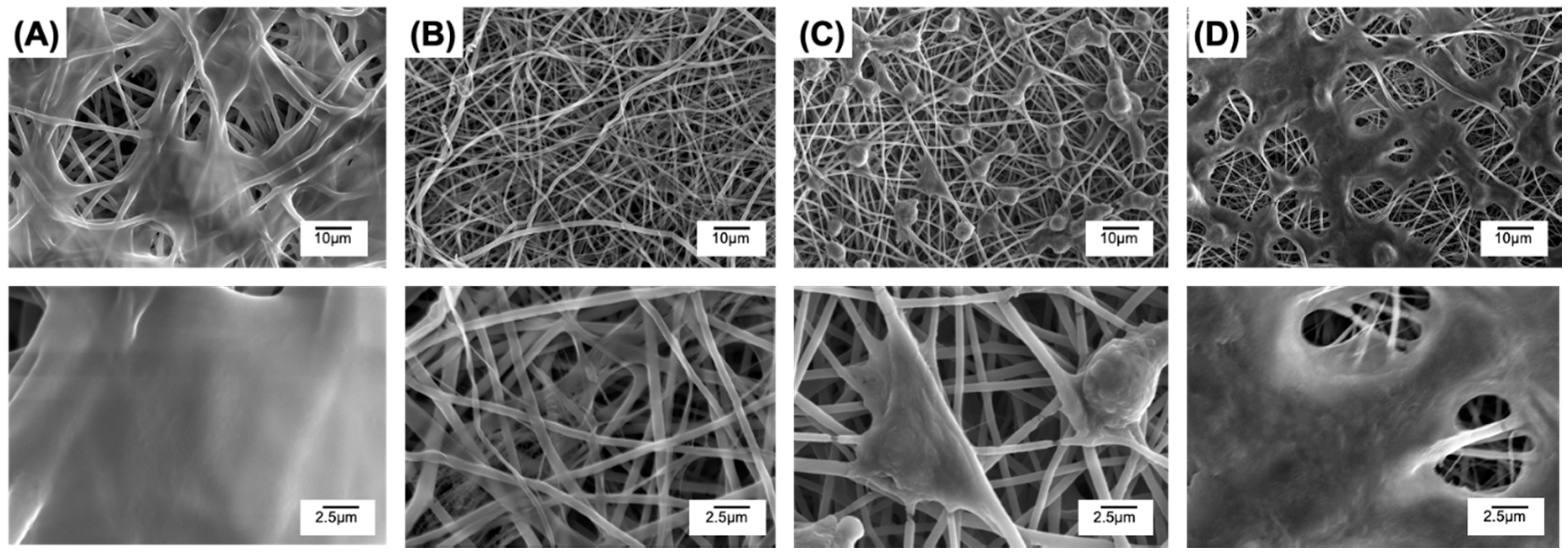
3.5. Contact and Extract Cytotoxicity Tests
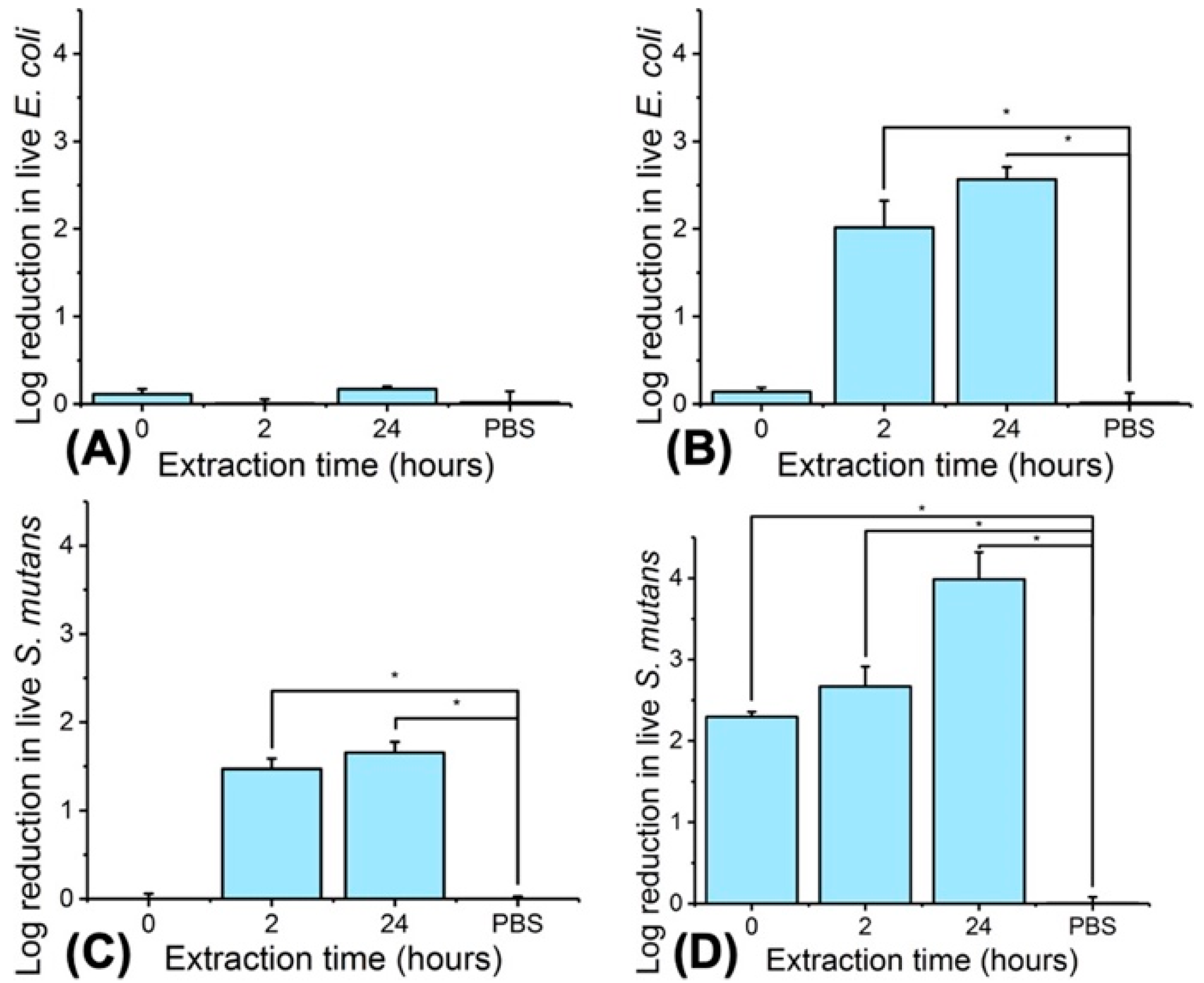
3.6. Antibacterial Photodynamic Capability of PAFs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumari, M.; Kumar, A. Human health risk assessment of antibiotics in binary mixtures for finished drinking water. Chemosphere 2020, 240, 124864. [Google Scholar] [CrossRef]
- Wu, Y.-K.; Cheng, N.-C.; Cheng, C.-M. Biofilms in Chronic Wounds: Pathogenesis and Diagnosis. Trends Biotechnol. 2019, 37, 505–517. [Google Scholar] [CrossRef]
- Bandara, H.M.H.N.; Samaranayake, L.P. Viral, bacterial, and fungal infections of the oral mucosa: Types, incidence, predisposing factors, diagnostic algorithms, and management. Periodontol. 2000 2019, 80, 148–176. [Google Scholar] [CrossRef]
- Shah, S.R.; Tatara, A.M.; Lam, J.; Lu, S.; Scott, D.W.; Bennett, G.N.; van den Beucken, J.J.J.P.; Jansen, J.A.; Wong, M.E.; Mikos, A.G. Polymer-Based Local Antibiotic Delivery for Prevention of Polymicrobial Infection in Contaminated Mandibular Implants. ACS Biomater. Sci. Eng. 2016, 2, 558–566. [Google Scholar] [CrossRef]
- Costley, D.; Nesbitt, H.; Ternan, N.; Dooley, J.; Huang, Y.-Y.; Hamblin, M.R. Sonodynamic inactivation of Gram-positive and Gram-negative bacteria using a Rose Bengal-antimicrobial peptide conjugate. Int. J. Antimicrob. Agents 2017, 49, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Karaman, D.S.; Ercan, U.K.; Bakay, E.; Topaloglu, N.; Rosenholm, J.M. Evolving Technologies and Strategies for Combating Antibacterial Resistance in the Advent of the Postantibiotic Era. Adv. Funct. Mater. 2020, 30, 1908783. [Google Scholar] [CrossRef]
- Wiehe, A.; O’Brien, J.M.; Senge, M.O. Trends and targets in antiviral phototherapy. Photochem. Photobiol. Sci. 2019, 18, 2565–2612. [Google Scholar] [CrossRef] [PubMed]
- Sabino, C.P.; Wainwright, M.; Ribeiro, M.S.; Sellera, F.P.; dos Anjos, C.; da Silva Baptista, M.; Lincopan, N. Global priority multidrug-resistant pathogens do not resist photodynamic therapy. J. Photochem. Photobiol. B 2020, 208, 111893. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Carmona, N.; Ouk, T.-S.; Calvete, M.J.F.; Pereira, M.M.; Villandiera, N.; Leroy-Lhez, S. Conjugating biomaterials with photosensitizers: Advances and perspectives for photodynamic antimicrobial chemotherapy. Photochem. Photobiol. Sci. 2020, 19, 445–461. [Google Scholar] [CrossRef]
- Hohlfeld, B.F.; Gitter, B.; Flanagan, K.J.; Kingsbury, C.J.; Kulak, N.; Senge, M.O.; Wiehe, A. Exploring the relationship between structure and activity in BODIPYs designed for antimicrobial phototherapy. Org. Biomol. Chem. 2020, 18, 2416–2431. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Qian, Y. A water soluble carbazolyl-BODIPY photosensitizer with an orthogonal D–A structure for photodynamic therapy in living cells and zebrafish. Biomater. Sci. 2020, 8, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Moser, D.; Han, F.; Leonhard, M.; Schneider-Stickler, B.; Tan, Y. Preparation and antibiofilm studies of curcumin loaded chitosan nanoparticles against polymicrobial biofilms of Candida albicans and Staphylococcus aureus. Carbohydr. Polym. 2020, 241, 11625. [Google Scholar] [CrossRef]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial Photodynamic Therapy—What We Know and What We Don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Zhao, P.; Li, X.; Fu, H.; Yang, X.; Wang, G.; Yang, Y.; Wei, H.; Zhou, Z.; Liao, W. Evaluating the Photodynamic Biocidal Activity and Investigating the Mechanism of Thiazolium Cyanine Dyes. ACS Appl. Bio Mater. 2020, 3, 1580–1588. [Google Scholar] [CrossRef]
- Aluigi, A.; Sotgiu, G.; Torreggiani, A.; Guerrini, A.; Orlandi, V.T.; Corticelli, F.; Varchi, G. Methylene Blue Doped Films of Wool Keratin with Antimicrobial Photodynamic Activity. ACS Appl. Mater. Interfaces 2015, 7, 17416–17424. [Google Scholar] [CrossRef]
- Contreras, A.; Raxworthy, M.J.; Wood, S.; Schiffman, J.D.; Tronci, G. Photodynamically Active Electrospun Fibers for Antibiotic-Free Infection Control. ACS Appl. Bio Mater. 2019, 2, 4258–4270. [Google Scholar] [CrossRef]
- Castriciano, M.A.; Zagami, R.; Casaletto, M.P.; Martel, B.; Trapani, M.; Romeo, A.; Villari, V.; Sciortino, M.T.; Grasso, L.; Guglielmino, S.; et al. Poly(carboxylic acid)-Cyclodextrin/Anionic Porphyrin Finished Fabrics as Photosensitizer Releasers for Antimicrobial Photodynamic Therapy. Biomacromolecules 2017, 18, 1134–1144. [Google Scholar] [CrossRef]
- Severyukhina, A.N.; Petrova, N.V.; Yashchenok, A.M.; Bratashov, D.N.; Smuda, K.; Mamonova, I.A.; Yurasov, N.A.; Puchinyan, D.M.; Georgieva, R.; Bäumler, H.; et al. Light-induced antibacterial activity of electrospun chitosan-based material containing photosensitizer. Mater. Sci. Eng. C 2017, 70, 311–316. [Google Scholar] [CrossRef]
- Bombin, A.D.J.; Dunn, N.J.; McCarthy, H.O. Electrospinning of natural polymers for the production of nanofibres for wound healing applications. Mater. Sci. Eng. C 2020, 114, 110994. [Google Scholar] [CrossRef]
- Münchow, E.A.; Albuquerque, M.T.P.; Zero, B.; Kamocki, K.; Piva, E.; Gregory, R.L.; Bottino, M.C. Development and Characterization of Novel ZnO-loaded Electrospun Membranes for Periodontal Regeneration. Dent. Mater. 2015, 31, 1038–1051. [Google Scholar] [CrossRef] [Green Version]
- Kersani, D.; Mougin, J.; Lopez, M.; Degoutin, S.; Tabary, N.; Cazaux, F.; Janus, L.; Maton, M.; Chai, F.; Sobocinski, J.; et al. Stent coating by electrospinning with chitosan/poly-cyclodextrin based nanofibers loaded with simvastatin for restenosis prevention. Eur. J. Pharm. Biopharm. 2020, 150, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Li, J.; Zhang, X.; Liu, T.; Ding, J.; Chen, X. Electrospun polymer micro/nanofibers as pharmaceutical repositories for healthcare. J. Control. Release 2019, 302, 19–41. [Google Scholar] [CrossRef]
- Wei, L.; Wu, S.; Shi, W.; Aldrich, A.L.; Kielian, T.; Carlson, M.A.; Sun, R.; Qin, X.; Duan, B. Large-Scale and Rapid Preparation of Nanofibrous Meshes and Their Application for Drug-Loaded Multilayer Mucoadhesive Patch Fabrication for Mouth Ulcer Treatment. ACS Appl. Mater. Interfaces 2019, 11, 28740–28751. [Google Scholar] [CrossRef] [PubMed]
- Shababdoust, A.; Zandi, M.; Ehsani, M.; Shokrollahi, P.; Foudazi, R. Controlled curcumin release from nanofibers based on amphiphilic-block segmented polyurethanes. Int. J. Pharm. 2020, 575, 118947. [Google Scholar] [CrossRef] [PubMed]
- Raxworthy, M.J.; Serino, L.P.; Iddon, P.D. Electrospun bioresorbable tissue repair scaffolds: From laboratory to clinic. In Proceedings of the 3rd Biennial South African Biomedical Engineering Conference (SAIBMEC), Stellenbosch, South Africa, 4–6 April 2018; pp. 1–4. [Google Scholar] [CrossRef] [Green Version]
- Gharaei, R.; Tronci, G.; Davies, R.P.W.; Gough, C.; Alazragi, R.; Goswami, P.; Russell, S.J. A structurally self-assembled peptide nano-architecture by one-step electrospinning. J. Mater. Chem. B 2016, 4, 5475–5485. [Google Scholar] [CrossRef] [Green Version]
- Bazbouz, M.B.; Liang, H.; Tronci, G. A UV-cured nanofibrous membrane of vinylbenzylated gelatin-poly(ɛ-caprolactone) dimethacrylate co-network by scalable free surface electrospinning. Mater. Sci. Eng. C 2018, 91, 541–555. [Google Scholar] [CrossRef]
- Arafat, M.T.; Tronci, G.; Wood, D.J.; Russell, S.J. In-Situ crosslinked wet spun collagen triple helices with nanoscale-regulated ciprofloxacin release capability. Mater. Lett. 2019, 255, 126550. [Google Scholar] [CrossRef]
- Nhi, T.T.; Minh, H.H.; Nam, T.M.P.; Thien, D.B.T.; Hoai, N.T.T.; Phuoc, T.V.; Thai, D.M.; Hai, D.M.; Toi, V.V.; Hiep, N.T. Optimization and characterization of electrospun polycaprolactone coated with gelatin- silver nanoparticles for wound healing application. Mater. Sci. Eng. C 2018, 91, 318–329. [Google Scholar]
- Zhao, R.; Li, X.; Sun, B.; Tong, Y.; Jiang, Z.; Wang, C. Nitrofurazone-Loaded Electrospun PLLA/Sericin-Based Dual-Layer Fiber Mats for Wound Dressing Applications. RSC Adv. 2015, 5, 16940–16949. [Google Scholar] [CrossRef]
- Li, Y.; Kumar, K.N.; Dabkowski, J.M.; Corrigan, M.; Scott, R.W.; Nüsslein, K.; Tew, G.N. New Bactericidal Surgical Suture Coating. Langmuir 2012, 28, 12134–12139. [Google Scholar] [CrossRef]
- Parani, M.; Lokhande, G.; Singh, A.; Gaharwar, A.K. Engineered Nanomaterials for Infection Control and Healing Acute and Chronic Wounds. ACS Appl. Mater. Interfaces 2016, 8, 10049–10069. [Google Scholar] [CrossRef]
- Preis, E.; Anders, T.; Širc, J.; Hobzova, R.; Cocarta, A.-I.; Bakowsky, U.; Jedelská, J. Biocompatible indocyanine green loaded PLA nanofibers for in situ antimicrobial photodynamic therapy. Mater. Sci. Eng. C 2020, 115, 111068. [Google Scholar] [CrossRef] [PubMed]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Song, L.; Zhang, X.; Zhou, R.; Yin, J.; Luan, S. Poly(γ-glutamic acid)-based electrospun nanofibrous mats with photodynamic therapy for effectively combating wound infection. Mater. Sci. Eng. C 2020, 113, 1109362. [Google Scholar] [CrossRef]
- Woodard, L.N.; Grunlan, M.A. Hydrolytic Degradation and Erosion of Polyester Biomaterials. ACS Macro Lett. 2018, 7, 976–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blakney, A.K.; Simonovsky, F.I.; Suydam, I.T.; Ratner, B.D.; Woodrow, K.A. Rapidly Biodegrading PLGA-Polyurethane Fibers for Sustained Release of Physicochemically Diverse Drugs. ACS Biomater. Sci. Eng. 2016, 2, 1595–1607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Homaeigohar, S.; Boccaccini, A.R. Antibacterial biohybrid nanofibers for wound dressings. Acta Biomater. 2020, 107, 25–49. [Google Scholar] [CrossRef]
- Chen, H.; Tang, Y.; Weir, M.D.; Gao, J.; Imazato, S.; Oates, T.W.; Lei, L.; Wang, S.; Hu, T.; Xu, H.H.K. Effects of S. mutans gene-modification and antibacterial monomer dimethylaminohexadecyl methacrylate on biofilm growth and acid production. Dent. Mater. 2020, 36, 296–309. [Google Scholar] [CrossRef]
- Francisco, C.M.L.; Gonçalves, J.M.L.A.; Brum, B.S.; Santos, T.P.C.; Lino-dos-Santos-Franco, A.; Silva, D.F.T.; Pavani, C. The photodynamic efficiency of phenothiazinium dyes is aggregation dependent. New J. Chem. 2017, 41, 14438–14443. [Google Scholar] [CrossRef]
- Parasuraman, P.; Anju, V.T.; Lal, S.B.S.; Sharan, A.; Busi, S.; Kaviyarasu, K.; Arshad, M.; Dawoud, T.M.S.; Syed, A. Synthesis and antimicrobial photodynamic effect of methylene blue conjugated carbon nanotubes on E. coli and S. aureus. Photochem. Photobiol. Sci. 2019, 18, 563–576. [Google Scholar] [CrossRef]
- Tronci, G.; Buiga, P.; Alhilou, A.; Do, T.; Russell, S.J.; Wood, D.J. Hydrolytic and lysozymic degradability of chitosan systems with heparin-mimicking pendant groups. Mater. Lett. 2017, 188, 359–363. [Google Scholar] [CrossRef] [Green Version]
- Garg, A.D.; Bose, M.; Ahmed, M.I.; Bonass, W.A.; Wood, S.R. In Vitro Studies on Erythrosine-Based Photodynamic Therapy of Malignant and Pre-Malignant Oral Epithelial Cells. PLoS ONE 2012, 7, e34475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Midori Tokubo, L.; Luiz Rosalen, P.; de Cássia Orlandi Sardi, J.; Almeida Freires, I.; Fujimaki, M.; Emiko Umeda, J.; Barbosa, P.M.; Tecchio, G.O.; Hioka, N.; de Freitas, C.F.; et al. Antimicrobial effect of photodynamic therapy using erythrosine/methylene blue combination on Streptococcus mutans biofilm. Photodiagn. Photodyn. Ther. 2018, 23, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Holmes, R.; Yang, X.B.; Dunne, A.; Florea, L.; Wood, D.; Tronci, G. Thiol-Ene Photo-Click Collagen-PEG Hydrogels: Impact of Water-Soluble Photoinitiators on Cell Viability, Gelation Kinetics and Rheological Properties. Polymers 2017, 9, 226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, J.; Clegg, J.R.; Heersema, L.A.; Peppas, N.A.; Smyth, H.D.C. Optimization of Cationic Nanogel PEGylation to Achieve Mammalian Cytocompatibility with Limited Loss of Gram-Negative Bactericidal Activity. Biomacromolecules 2020, 21, 1528–1538. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, D.; Robinson, C.; Devine, D.; Wood, S. Enhancement of erythrosine-mediated photodynamic therapy of Streptococcus mutans biofilms by light fractionation. J. Antimicrob.Chemother. 2006, 58, 190–192. [Google Scholar] [CrossRef] [Green Version]
- Boncu, T.E.; Guclu, A.U.; Catma, M.F.; Savaser, A.; Gokce, A.; Ozdemir, N. In Vitro and in Vivo Evaluation of Linezolid Loaded Electrospun PLGA and PLGA/PCL Fiber Mats for Prophylaxis and Treatment of MRSA Induced Prosthetic Infections. Int. J. Pharm. 2020, 573, 118758. [Google Scholar] [CrossRef]
- Chou, S.-F.; Woodrow, K.A. Relationships between mechanical properties and drug release from electrospun fibers of PCL and PLGA blends. J. Mech. Behav. Biomed. Mater. 2017, 65, 724–733. [Google Scholar] [CrossRef] [Green Version]
- Carson, D.; Jiang, Y.; Woodrow, K.A. Tunable Release of Multiclass Anti-HIV Drugs that are Water-Soluble and Loaded at High Drug Content in Polyester Blended Electrospun Fibers. Pharm. Res. 2016, 33, 125–136. [Google Scholar] [CrossRef]
- Bianco, A.; Calderone, M.; Cacciotti, I. Electrospun PHBV/PEO co-solution blends: Microstructure, thermal and mechanical properties. Mater. Sci. Eng. C 2013, 33, 1067–1077. [Google Scholar] [CrossRef]
- Liang, J.-Z.; Duan, D.-R.; Tang, C.-Y.; Tsui, C.-P.; Chen, D.-Z. Tensile properties of PLLA/PCL composites filled with nanometer calcium carbonate. Polym. Test. 2013, 32, 617–621. [Google Scholar] [CrossRef]
- Torricelli, P.; Gioffrè, M.; Fiorani, A.; Panzavolta, S.; Gualandi, C.; Fini, M.; Focarete, M.L.; Bigi, A. Co-electrospun gelatin-poly(l-lactic acid) scaffolds: Modulation of mechanical properties and chondrocyte response as a function of composition. Mater. Sci. Eng. C 2014, 36, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Neffe, A.T.; Tronci, G.; Alteheld, A.; Lendlein, A. Controlled Change of Mechanical Properties during Hydrolytic Degradation of Polyester Urethane Networks. Macromol. Chem. Phys. 2010, 211, 182–194. [Google Scholar] [CrossRef] [Green Version]
- Duffy, P.; McMahon, S.; Wang, X.; Keaveney, S.; O’Cearbhaill, E.D.; Quintana, I.; Rodríguez, F.J.; Wang, W. Synthetic bioresorbable poly-α-hydroxyesters as peripheral nerve guidance conduits; a review of material properties, design strategies and their efficacy to date. Biomater. Sci. 2019, 7, 4912–4943. [Google Scholar] [CrossRef]
- Bandelli, D.; Helbing, C.; Weber, C.; Seifert, M.; Muljajew, I.; Jandt, K.D.; Schubert, U.S. Maintaining the Hydrophilic−Hydrophobic Balance of Polyesters with Adjustable Crystallinity for Tailor-Made Nanoparticles. Macromolecules 2018, 51, 5567–5576. [Google Scholar] [CrossRef]
- Narayanan, N.; Jiang, C.; Wang, C.; Uzunalli, G.; Whittern, N.; Chen, D.; Jones, O.G.; Kuang, S.; Deng, M. Harnessing Fiber Diameter-Dependent Effects of Myoblasts Toward Biomimetic Scaffold-Based Skeletal Muscle Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 203. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.-H.; Chang, S.-Y.; Biswas, R.; Chung, P.-S.; Mo, S.; Lee, M.Y.; Ahn, J.C. Light-emitting diode irradiation using 660 nm promotes human fibroblast HSP90 expression and changes cellular activity and morphology. J. Biophotonics 2019, 12, e201900063. [Google Scholar] [CrossRef]
- Tun Han, W.; Jang, T.; Chen, S.; Chong, L.S.H.; Jung, H.-D.; Song, J. Improved cell viability for large-scale biofabrication with photo-crosslinkable hydrogel systems through a dual-photoinitiator approach. Biomater. Sci. 2020, 8, 450–461. [Google Scholar] [CrossRef]
- Jin, G.; Prabhakaran, M.P.; Kai, D.; Kotaki, M.; Ramakrishna, S. Electrospun photosensitive nanofibers: Potential for photocurrent therapy in skin regeneration. Photochem. Photobiol. Sci. 2013, 12, 124–134. [Google Scholar] [CrossRef]




| Sample ID | PLGA (wt.%) a | PCL (wt.%) a | MB (mM) a |
|---|---|---|---|
| MB20-PLGA20-CL80 | 20 | 80 | 2.2 |
| MB20-PLGA50-CL50 | 50 | 50 | 2.2 |
| MB20-PLGA80-CL20 | 80 | 20 | 2.2 |
| MB2-PLGA50-CL50 | 50 | 50 | 0.2 |
| MB10-PLGA50-CL50 | 50 | 50 | 1.1 |
| PLGA20-CL80 | 20 | 80 | 0 |
| PLGA50-CL50 | 50 | 50 | 0 |
| PLGA80-CL20 | 80 | 20 | 0 |
| Sample ID | Ø (μm) | Tm1 (°C) | ∆Hm1 (J g−1) | Tm2 (°C) | ∆Hm2 (J g−1) |
|---|---|---|---|---|---|
| MB20-PLGA20-CL80 | 0.44 ± 0.17 | 57 | 72 | n.o. | n.o. |
| MB20-PLGA50-CL50 | 0.83 ± 0.22 | 61 | 59 | 200 | 20 |
| MB20-PLGA80-CL20 | 0.54 ± 0.18 | 61 | 16 | 201 | 47 |
| MB2-PLGA50-CL50 | 0.76 ± 0.23 | 58 | 32 | 201 | 30 |
| MB10-PLGA50-CL50 | 0.83 ± 0.29 | n.a. | n.a. | n.a. | n.a. |
| PLGA20-CL80 | 1.68 ± 0.52 | 57 | 33 | 200 | 37 |
| PLGA50-CL50 | 1.48 ± 0.56 | 61 | 22 | 202 | 44 |
| PLGA80-CL20 | 1.15 ± 0.54 | 57 | 10 | 197 | 58 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contreras, A.; Raxworthy, M.J.; Wood, S.; Tronci, G. Hydrolytic Degradability, Cell Tolerance and On-Demand Antibacterial Effect of Electrospun Photodynamically Active Fibres. Pharmaceutics 2020, 12, 711. https://doi.org/10.3390/pharmaceutics12080711
Contreras A, Raxworthy MJ, Wood S, Tronci G. Hydrolytic Degradability, Cell Tolerance and On-Demand Antibacterial Effect of Electrospun Photodynamically Active Fibres. Pharmaceutics. 2020; 12(8):711. https://doi.org/10.3390/pharmaceutics12080711
Chicago/Turabian StyleContreras, Amy, Michael J. Raxworthy, Simon Wood, and Giuseppe Tronci. 2020. "Hydrolytic Degradability, Cell Tolerance and On-Demand Antibacterial Effect of Electrospun Photodynamically Active Fibres" Pharmaceutics 12, no. 8: 711. https://doi.org/10.3390/pharmaceutics12080711
APA StyleContreras, A., Raxworthy, M. J., Wood, S., & Tronci, G. (2020). Hydrolytic Degradability, Cell Tolerance and On-Demand Antibacterial Effect of Electrospun Photodynamically Active Fibres. Pharmaceutics, 12(8), 711. https://doi.org/10.3390/pharmaceutics12080711