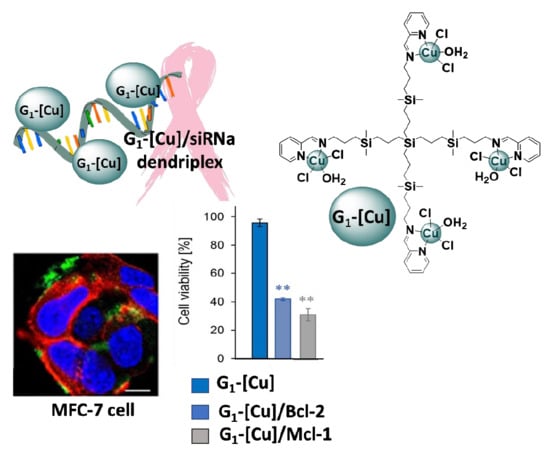
Copper (II) Metallodendrimers Combined with Pro-Apoptotic siRNAs as a Promising Strategy Against Breast Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Considerations
2.2. Biophysical Evaluation of the Dendriplexes
2.2.1. Gel Electrophoresis
2.2.2. Transmission Electron Microscopy (TEM)
2.2.3. Zeta Potential Measurements
2.2.4. Hydrodynamic Diameter of the Dendriplexes
2.2.5. Fluorescence Polarisation Measurements
2.2.6. Circular Dichroism
2.3. Evaluation of Anticancer In Vitro
2.3.1. Cell Cultures
2.3.2. Cytotoxicity
2.3.3. Statistical Analysis
3. Results and Discussion
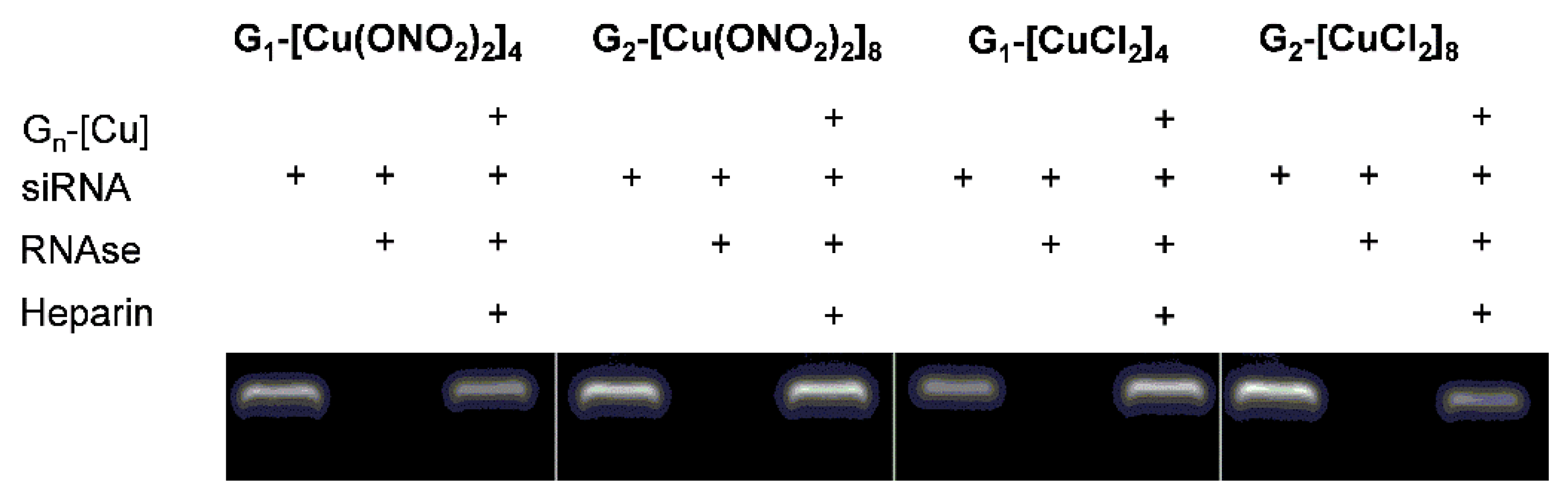
3.1. Evaluation of the Interaction Between Ru(II) Metallodendrimers and siRNA
Electrophoresis Assays
3.2. Biophysical Characterisation of Dendriplexes
3.2.1. Zeta Potential, the Hydrodynamic Diameter of Dendriplexes, and TEM Assays
3.2.2. Fluorescence Polarisation and Circular Dichroism
3.3. Biological Evaluation of CCD-siRNA Complexes: Cellular Uptake and Anticancer Activity
3.3.1. Cellular Uptake
3.3.2. Anticancer Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chalbatani, G.M.; Dana, H.; Gharagouzloo, E.; Grijalvo, S.; Eritja, R.; Logsdon, C.D.; Memari, F.; Miri, S.R.; Rad, M.R.; Marmari, V. Small interfering RNAs (siRNAs) in cancer therapy: A nano-based approach. Int. J. Nanomed. 2019, 14, 3111–3128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahir-Jouzdani, F.; Mottaghitalab, F.; Dinarvand, M.; Atyabi, F.; Zahir, F. siRNA delivery for treatment of degenerative diseases, new hopes and challenges. J. Drug Deliv. Sci. Technol. 2018, 45, 428–441. [Google Scholar] [CrossRef]
- Levanova, A.; Poranen, M.M. RNA Interference as a Prospective Tool for the Control of Human Viral Infections. Front. Microbiol. 2018, 9, 2151. [Google Scholar] [CrossRef] [PubMed]
- Ionov, M.; Lazniewska, J.; Dzmitruk, V.; Halets, I.; Loznikova, S.; Novopashina, D.; Apartsin, E.; Krasheninina, O.; Venyaminova, A.; Milowska, K.; et al. Anticancer siRNA cocktails as a novel tool to treat cancer cells. Part (A). Mechanisms of interaction. Int. J. Pharm. 2015, 485, 261–269. [Google Scholar] [CrossRef]
- Dzmitruk, V.; Szulc, A.; Shcharbin, D.; Janaszewska, A.; Shcharbina, N.; Lazniewska, J.; Novopashina, D.; Buyanova, M.; Ionov, M.; Klajnert-Maculewicz, B.; et al. Anticancer siRNA cocktails as a novel tool to treat cancer cells. Part (B). Efficiency of pharmacological action. Int. J. Pharm. 2015, 485, 288–294. [Google Scholar] [CrossRef]
- Truong, N.P.; Gu, W.; Prasadam, I.; Jia, Z.; Crawford, R.; Xiao, Y.; Monteiro, M.J. An influenza virus-inspired polymer system for the timed release of siRNA. Nat. Commun. 2013, 4, 1902. [Google Scholar] [CrossRef] [Green Version]
- Subhan, A.; Torchilin, V. Efficient nanocarriers of siRNA therapeutics for cancer treatment. Transl. Res. 2019, 214, 62–91. [Google Scholar] [CrossRef]
- Xiao, B.; Ma, L.; Merlin, D. Nanoparticle-mediated co-delivery of chemotherapeutic agent and siRNA for combination cancer therapy. Expert Opin. Drug Deliv. 2016, 14, 65–73. [Google Scholar] [CrossRef]
- Nam, J.-P.; Nam, K.; Jung, S.; Nah, J.-W.; Kim, S.W. Evaluation of dendrimer type bio-reducible polymer as a siRNA delivery carrier for cancer therapy. J. Control. Release 2015, 209, 179–185. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Zhou, B.; Du, X.; Wang, Y.; Zhang, J.; Ai, Y.; Xia, Z.; Zhao, G. Folic acid (FA)-conjugated mesoporous silica nanoparticles combined with MRP-1 siRNA improves the suppressive effects of myricetin on non-small cell lung cancer (NSCLC). Biomed. Pharmacother. 2020, 125, 109561. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yu, T.; Ding, L.; Laurini, E.; Huang, Y.; Zhang, M.; Weng, Y.; Lin, S.; Chen, P.; Marson, D.; et al. A Dual Targeting Dendrimer-Mediated siRNA Delivery System for Effective Gene Silencing in Cancer Therapy. J. Am. Chem. Soc. 2018, 140, 16264–16274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, V.; Rajani, C.; Paul, D.; Borisa, P.; Rajpoot, K.; Youngren-Ortiz, S.R.; Tekade, R.K. Chapter 8-Dendrimers as novel drug-delivery system and its applications. In Drug Delivery Systems; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 333–392. [Google Scholar]
- Michlewska, S.; Ionov, M.; Maroto-Diaz, M.; Szwed, A.; Ihnatsyeu-Kachan, A.; Loznikova, S.; Shcharbin, D.; Malý, M.; Ramírez, R.G.; De La Mata, F.J.; et al. Ruthenium dendrimers as carriers for anticancer siRNA. J. Inorg. Biochem. 2018, 181, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Mignani, S.; Caminade, A.; Majoral, J.-P. Metal-based phosphorus dendrimers as novel nanotherapeutic strategies to tackle cancers: A concise overview. Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol. 2019, 11, e1577. [Google Scholar] [CrossRef] [PubMed]
- Hołota, M.; Magiera, J.; Michlewska, S.; Kubczak, M.; Del Olmo, N.S.; Gallego, S.G.; Ortega, P.; De La Mata, F.; Ionov, M.; Bryszewska, M.; et al. In Vitro Anticancer Properties of Copper Metallodendrimers. Biomolecules 2019, 9, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maroto-Díaz, M.; Elie, B.T.; Gómez-Sal, P.; Pérez-Serrano, J.; Gómez, R.; Contel, M.; De La Mata, F. Synthesis and anticancer activity of carbosilane metallodendrimers based on arene ruthenium(ii) complexes. Dalton Trans. 2016, 45, 7049–7066. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Yu, Q.; Liu, Y.; Bhavsar, D.; Yang, L.; Ren, X.; Sun, N.; Zheng, W.; Liu, J.; Chen, L. Multifunctional selenium nanoparticles: Chiral selectivity of delivering MDR-siRNA for reversal of multidrug resistance and real-time biofluorescence imaging. Nanomed. Nanotechnol. Boil. Med. 2015, 11, 1773–1784. [Google Scholar] [CrossRef]
- Hussain, A.; Alajmi, M.F.; Rehman, T.; Amir, S.; Husain, F.M.; Alsalme, A.; Siddiqui, M.A.; Alkhedhairy, A.A.; Khan, R.A. Copper(II) complexes as potential anticancer and Nonsteroidal anti-inflammatory agents: In vitro and in vivo studies. Sci. Rep. 2019, 9, 5237. [Google Scholar] [CrossRef] [Green Version]
- Jopp, M.; Becker, J.; Becker, S.; Miska, A.; Gandin, V.; Marzano, C.; Schindler, S. Anticancer activity of a series of copper(II) complexes with tripodal ligands. Eur. J. Med. Chem. 2017, 132, 274–281. [Google Scholar] [CrossRef]
- Del Olmo, N.S.; Maroto-Díaz, M.; Gómez, R.; Ortega, P.; Cangiotti, M.; Ottaviani, M.F.; De La Mata, F.J. Carbosilane metallodendrimers based on copper (II) complexes: Synthesis, EPR characterization and anticancer activity. J. Inorg. Biochem. 2017, 177, 211–218. [Google Scholar] [CrossRef]
- Del Olmo, N.S.; Carloni, R.; Bajo, A.M.; Ortega, P.; Fattori, A.; Gómez, R.; Ottaviani, M.F.; Gallego, S.G.; Cangiotti, M.; De La Mata, F.; et al. Insight into the antitumor activity of carbosilane Cu(ii)–metallodendrimers through their interaction with biological membrane models. Nanoscale 2019, 11, 13330–13342. [Google Scholar] [CrossRef]
- Campbell, K.J.; Dhayade, S.; Ferrari, N.; Sims, A.H.; Johnson, E.; Mason, S.; Dickson, A.; Ryan, K.M.; Kalna, G.; Edwards, J.; et al. MCL-1 is a prognostic indicator and drug target in breast cancer. Cell Death Dis. 2018, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Kirkin, V.; Joos, S.; Zörnig, M. The role of Bcl-2 family members in tumorigenesis. Biochim. Biophys. Acta 2004, 1644, 229–249. [Google Scholar] [CrossRef] [PubMed]
- Akar, U.; Chaves-Reyez, A.; Barria, M.; Tari, A.; Sanguino, A.; Kondo, Y.; Kondo, S.; Arun, B.; Lopez-Berestein, G.; Ozpolat, B. Silencing of Bcl-2 expression by small interfering RNA induces autophagic cell death in MCF-7 breast cancer cells. Autophagy 2008, 4, 669–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ionov, M.; Garaiova, Z.; Waczulikova, I.; Wrobel, D.; Pedziwiatr-Werbicka, E.; Gomez-Ramirez, R.; De La Mata, F.; Klajnert, B.; Hianik, T.; Bryszewska, M.; et al. siRNA carriers based on carbosilane dendrimers affect zeta potential and size of phospholipid vesicles. Biochim. Biophys. Acta (BBA)-Biomembr. 2012, 1818, 2209–2216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shcharbin, D.; Pedziwiatr-Werbicka, E.; Nowacka, O.; Kumar, M.; Zaborski, M.; Ortega, P.; De La Mata, F.; Gómez, R.; Muñoz-Fernández, M. Ángeles; Bryszewska, M. Carbosilane dendrimers NN8 and NN16 form a stable complex with siGAG1. Colloids Surf. B Biointerfaces 2011, 83, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Weber, N.; Ortega, P.; Clemente, M.I.; Shcharbin, D.; Bryszewska, M.; De La Mata, F.J.; Gómez, R.; Muñoz, M. Ángeles Characterization of carbosilane dendrimers as effective carriers of siRNA to HIV-infected lymphocytes. J. Control. Release 2008, 132, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Perez, A.; Romero, E.L.; Morilla, M.J. Ethylendiamine core PAMAM dendrimers/siRNA complexes as in vitro silencing agents. Int. J. Pharm. 2009, 380, 189–200. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, J.; Hafdi, N.; Behr, J.-P.; Erbacher, P.; Peng, L. PAMAM dendrimers for efficient siRNA delivery and potent gene silencing. Chem. Commun. 2006, 2362. [Google Scholar] [CrossRef]
- Ferenc, M.; Pedziwiatr-Werbicka, E.; Nowak, K.E.; Klajnert-Maculewicz, B.; Majoral, J.-P.; Bryszewska, M. Phosphorus Dendrimers as Carriers of siRNA—Characterisation of Dendriplexes. Molecules 2013, 18, 4451–4466. [Google Scholar] [CrossRef]
- Vu, M.N.; Kelly, H.G.; Wheatley, A.K.; Peng, S.; Pilkington, E.H.; Veldhuis, N.A.; Davis, T.P.; Kent, S.J.; Truong, N.P. Cellular Interactions of Liposomes and PISA Nanoparticles during Human Blood Flow in a Microvascular Network. Small 2020. [Google Scholar] [CrossRef]
- Khor, S.Y.; Vu, M.N.; Pilkington, E.H.; Johnston, A.P.R.; Whittaker, M.R.; Quinn, J.F.; Truong, N.P.; Davis, T.P. Elucidating the Influences of Size, Surface Chemistry, and Dynamic Flow on Cellular Association of Nanoparticles Made by Polymerization-Induced Self-Assembly. Small 2018, 14, 1801702. [Google Scholar] [CrossRef] [PubMed]
- Pędziwiatr-Werbicka, E.; Shcharbin, D.; Malý, J.; Malý, M.; Zaborski, M.; Gabara, B.; Ortega, P.; De La Mata, F.J.; Gómez, R.; Muñoz-Fernández, M.; et al. Carbosilane Dendrimers are a Non-Viral Delivery System for Antisense Oligonucleotides: Characterization of Dendriplexes. J. Biomed. Nanotechnol. 2012, 8, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Ambesajir, A.; Kaushik, A.; Kaushik, J.J.; Petros, S.T. RNA interference: A futuristic tool and its therapeutic applications. Saudi J. Boil. Sci. 2012, 19, 395–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tekedereli, I.; Alpay, S.N.; Akar, U.; Yuca, E.; Ayugo-Rodriguez, C.; Han, H.-D.; Sood, A.K.; Lopez-Berestein, G.; Ozpolat, B. Therapeutic Silencing of Bcl-2 by Systemically Administered siRNA Nanotherapeutics Inhibits Tumor Growth by Autophagy and Apoptosis and Enhances the Efficacy of Chemotherapy in Orthotopic Xenograft Models of ER (−) and ER (+) Breast Cancer. Mol. Ther.-Nucleic Acids 2013, 2, e121. [Google Scholar] [CrossRef]








| Strand | Mcl-1 | Bcl-2 |
|---|---|---|
| Sense | 5′-GGACUUUUAUACCUGUUAUtt 3′ | 5′-G CUG CAC CUG ACG CCC UUCtt 3′ |
| Antisense | 5′-AUAACAGGUAUAAAAGUCCtg 3′ | 5′-GAA GGG CGU CAG GUG CAG Ctt 3′ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanz del Olmo, N.; Holota, M.; Michlewska, S.; Gómez, R.; Ortega, P.; Ionov, M.; de la Mata, F.J.; Bryszewska, M. Copper (II) Metallodendrimers Combined with Pro-Apoptotic siRNAs as a Promising Strategy Against Breast Cancer Cells. Pharmaceutics 2020, 12, 727. https://doi.org/10.3390/pharmaceutics12080727
Sanz del Olmo N, Holota M, Michlewska S, Gómez R, Ortega P, Ionov M, de la Mata FJ, Bryszewska M. Copper (II) Metallodendrimers Combined with Pro-Apoptotic siRNAs as a Promising Strategy Against Breast Cancer Cells. Pharmaceutics. 2020; 12(8):727. https://doi.org/10.3390/pharmaceutics12080727
Chicago/Turabian StyleSanz del Olmo, Natalia, Marcin Holota, Sylwia Michlewska, Rafael Gómez, Paula Ortega, Maksim Ionov, Francisco Javier de la Mata, and Maria Bryszewska. 2020. "Copper (II) Metallodendrimers Combined with Pro-Apoptotic siRNAs as a Promising Strategy Against Breast Cancer Cells" Pharmaceutics 12, no. 8: 727. https://doi.org/10.3390/pharmaceutics12080727
APA StyleSanz del Olmo, N., Holota, M., Michlewska, S., Gómez, R., Ortega, P., Ionov, M., de la Mata, F. J., & Bryszewska, M. (2020). Copper (II) Metallodendrimers Combined with Pro-Apoptotic siRNAs as a Promising Strategy Against Breast Cancer Cells. Pharmaceutics, 12(8), 727. https://doi.org/10.3390/pharmaceutics12080727