Silica Nanoparticles in Transmucosal Drug Delivery
Abstract
:1. Introduction
2. Common Methods of Preparation of Silica Nanoparticles
3. Applications of Silica Nanoparticles in Drug Delivery: Loading Capacity and Release
4. Transmucosal Drug Delivery
5. Factors Influencing Mucosal Drug Delivery
6. Applications of Silica Nanoparticles in Transmucosal Drug Delivery
7. Safety Considerations and Biodistribution of Silica Nanoparticles
8. Potential for Future Research
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FDA. Synthetic Amorphous Silica. Available online: https://www.accessdata.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&id=321 (accessed on 5 March 2020).
- Chen, L.; Liu, J.; Zhang, Y.; Zhang, G.; Kang, Y.; Chen, A.; Feng, X.; Shao, L. The toxicity of silica nanoparticles to the immune system. Nanomedicine 2018, 13, 1939–1962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croissant, J.G.; Fatieiev, Y.; Khashab, N.M. Degradability and Clearance of Silicon, Organosilica, Silsesquioxane, Silica Mixed Oxide, and Mesoporous Silica Nanoparticles. Adv. Mater. 2017, 29, 1604634. [Google Scholar] [CrossRef]
- Tang, L.; Cheng, J. Nonporous silica nanoparticles for nanomedicine application. Nano Today 2013, 8, 290–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jiang, H.; Zhao, Q.; Wang, S.; Zou, M.; Cheng, G. Enhanced mucosal and systemic immune responses obtained by porous silica nanoparticles used as an oral vaccine adjuvant: Effect of silica architecture on immunological properties. Int. J. Pharm. 2012, 436, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Hamilton, P.D.; Reilly, M.A.; d’Avignon, A.; Biswas, P.; Ravi, N. A facile synthesis of highly water-soluble, core–shell organo-silica nanoparticles with controllable size via sol–gel process. J. Colloid Interface Sci. 2009, 340, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Malugin, A.; Ghandehari, H. Impact of Silica Nanoparticle Design on Cellular Toxicity and Hemolytic Activity. ACS Nano 2011, 5, 5717–5728. [Google Scholar] [CrossRef]
- Martínez-Edo, G.; Llinàs, M.C.; Borrós, S.; Sánchez-García, D. Isothiocyanate-Functionalized Mesoporous Silica Nanoparticles as Building Blocks for the Design of Nanovehicles with Optimized Drug Release Profile. Nanomaterials 2019, 9, 1219. [Google Scholar] [CrossRef] [Green Version]
- Slowing, I.; Viveroescoto, J.; Wu, C.; Lin, V. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers☆. Adv. Drug Deliv. Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef]
- Sprenger, S. Epoxy resins modified with elastomers and surface-modified silica nanoparticles. Polymer 2013, 54, 4790–4797. [Google Scholar] [CrossRef] [Green Version]
- Liberman, A.; Mendez, N.; Trogler, W.C.; Kummel, A.C. Synthesis and surface functionalization of silica nanoparticles for nanomedicine. Surf. Sci. Rep. 2014, 69, 132–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khung, Y.L.; Narducci, D. Surface modification strategies on mesoporous silica nanoparticles for anti-biofouling zwitterionic film grafting. Adv. Colloid Interface Sci. 2015, 226, 166–186. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Barnes, J.C.; Bosoy, A.; Stoddart, J.F.; Zink, J.I. Mesoporous silica nanoparticles in biomedical applications. Chem. Soc. Rev. 2012, 41, 2590–2605. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 313–327. [Google Scholar] [CrossRef]
- Jafari, S.; Derakhshankhah, H.; Alaei, L.; Fattahi, A.; Varnamkhasti, B.S.; Saboury, A.A. Mesoporous silica nanoparticles for therapeutic/diagnostic applications. Biomed. Pharmacother. 2019, 109, 1100–1111. [Google Scholar] [CrossRef]
- Li, T.; Shi, S.; Goel, S.; Shen, X.; Xie, X.; Chen, Z.; Zhang, H.; Li, S.; Qin, X.; Yang, H.; et al. Recent advancements in mesoporous silica nanoparticles towards therapeutic applications for cancer. Acta Biomater. 2019, 89, 1–13. [Google Scholar] [CrossRef]
- Sábio, R.M.; Meneguin, A.B.; Ribeiro, T.C.; Silva, R.R.; Chorilli, M. New insights towards mesoporous silica nanoparticles as a technological platform for chemotherapeutic drugs delivery. Int. J. Pharm. 2019, 564, 379–409. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Bogush, G.H.; Zukoski, C.F. Studies of the kinetics of the precipitation of uniform silica particles through the hydrolysis and condensation of silicon alkoxides. J. Colloid Interface Sci. 1991, 142, 1–18. [Google Scholar] [CrossRef]
- Carcouët, C.C.; Van De Put, M.W.; Mezari, B.; Magusin, P.C.; Laven, J.; Bomans, P.H.; Friedrich, H.; Esteves, A.C.; Sommerdijk, N.A.; Van Benthem, R.A.; et al. Nucleation and Growth of Monodisperse Silica Nanoparticles. Nano Lett. 2014, 14, 1433–1438. [Google Scholar] [CrossRef]
- Green, D.L.; Lin, J.S.; Lam, Y.-F.; Hu, M.Z.C.; Schaefer, D.W.; Harris, M.T. Size, volume fraction, and nucleation of Stober silica nanoparticles. J. Colloid Interface Sci. 2003, 266, 346–358. [Google Scholar] [CrossRef]
- Matsoukas, T.; Gulari, E. Dynamics of growth of silica particles from ammonia-catalyzed hydrolysis of tetra-ethyl-orthosilicate. J. Colloid Interface Sci. 1988, 124, 252–261. [Google Scholar] [CrossRef] [Green Version]
- Matsoukas, T.; Gulari, E. Monomer-addition growth with a slow initiation step: A growth model for silica particles from alkoxides. J. Colloid Interface Sci. 1989, 132, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Bogush, G.H.; Zukoski, C.F. Uniform silica particle precipitation: An aggregative growth model. J. Colloid Interface Sci. 1991, 142, 19–34. [Google Scholar] [CrossRef]
- Narayan, R.; Nayak, U.; Raichur, A.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Q.; Yang, G.; Huang, D.; Cao, W.; Ge, L.; Li, L. Synthesis and characterization of spherical silica nanoparticles by modified Stöber process assisted by slow-hydrolysis catalyst. Colloid. Polym. Sci. 2018, 296, 379–384. [Google Scholar] [CrossRef]
- Rao, K.S.; El-Hami, K.; Kodaki, T.; Matsushige, K.; Makino, K. A novel method for synthesis of silica nanoparticles. J. Colloid Interface Sci. 2005, 289, 125–131. [Google Scholar] [CrossRef]
- Gao, W.; Rigout, M.; Owens, H. Facile control of silica nanoparticles using a novel solvent varying method for the fabrication of artificial opal photonic crystals. J. Nanopart. Res. 2016, 18, 387. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Haynes, C.L. Impacts of mesoporous silica nanoparticle size, pore ordering, and pore integrity on hemolytic activity. J. Am. Chem. Soc. 2010, 132, 4834–4842. [Google Scholar] [CrossRef]
- Qiao, Z.-A.; Zhang, L.; Guo, M.; Liu, Y.; Huo, Q. Synthesis of mesoporous silica nanoparticles via controlled hydrolysis and condensation of silicon alkoxide. Chem. Mater. 2009, 21, 3823–3829. [Google Scholar] [CrossRef]
- Kao, K.-C.; Mou, C.-Y. Pore-expanded mesoporous silica nanoparticles with alkanes/ethanol as pore expanding agent. Microporous Mesoporous Mater. 2013, 169, 7–15. [Google Scholar] [CrossRef]
- Nakamura, M.; Ishimura, K. Synthesis and characterization of organosilica nanoparticles prepared from 3-mercaptopropyltrimethoxysilane as the single silica source. J. Phys. Chem. C 2007, 111, 18892–18898. [Google Scholar] [CrossRef]
- Nakamura, M.; Ishimura, K. One-pot synthesis and characterization of three kinds of thiol−organosilica nanoparticles. Langmuir 2008, 24, 5099–5108. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Ishimura, K. Size-Controlled, One-Pot Synthesis, Characterization, and Biological Applications of Epoxy-Organosilica Particles Possessing Positive Zeta Potential. Langmuir 2008, 24, 12228–12234. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Ozaki, S.; Abe, M.; Doi, H.; Matsumoto, T.; Ishimura, K. Size-controlled synthesis, surface functionalization, and biological applications of thiol-organosilica particles. Colloids Surf. B Biointerfaces 2010, 79, 19–26. [Google Scholar] [CrossRef]
- Doura, T.; Nishio, T.; Tamanoi, F.; Nakamura, M. Relationship between the glutathione-responsive degradability of thiol-organosilica nanoparticles and the chemical structures. J. Mater. Res. 2019, 34, 1266–1278. [Google Scholar] [CrossRef]
- Chiu, S.-J.; Wang, S.-Y.; Chou, H.-C.; Liu, Y.-L.; Hu, T.-M. Versatile Synthesis of Thiol- and Amine-Bifunctionalized Silica Nanoparticles Based on the Ouzo Effect. Langmuir 2014, 30, 7676–7686. [Google Scholar] [CrossRef]
- Osseo-Asare, K.; Arriagada, F.J. Preparation of SiO2 nanoparticles in a non-ionic reverse micellar system. ColSu 1990, 50, 321–339. [Google Scholar] [CrossRef]
- Finnie, K.S.; Bartlett, J.R.; Barbé, C.J.A.; Kong, L. Formation of silica nanoparticles in microemulsions. Langmuir 2007, 23, 3017–3024. [Google Scholar] [CrossRef]
- Jin, Y.; Lohstreter, S.; Pierce, D.T.; Parisien, J.; Wu, M.; Hall, C.; Zhao, J.X. Silica nanoparticles with continuously tunable sizes: Synthesis and size effects on cellular contrast imaging. Chem. Mater. 2008, 20, 4411–4419. [Google Scholar] [CrossRef]
- Irmukhametova, G.S.; Mun, G.A.; Khutoryanskiy, V.V. Thiolated Mucoadhesive and PEGylated Nonmucoadhesive Organosilica Nanoparticles from 3-Mercaptopropyltrimethoxysilane. Langmuir 2011, 27, 9551–9556. [Google Scholar] [CrossRef] [PubMed]
- Al Mahrooqi, J.H.; Mun, E.A.; Williams, A.C.; Khutoryanskiy, V.V. Controlling the size of thiolated organosilica nanoparticles. Langmuir 2018, 34, 8347–8354. [Google Scholar] [CrossRef] [PubMed]
- Bremmell, K.E.; Prestidge, C.A. Enhancing oral bioavailability of poorly soluble drugs with mesoporous silica based systems: Opportunities and challenges. Drug Dev. Ind. Pharm. 2019, 45, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Feng, N. Mesoporous silica nanoparticles: Synthesis, classification, drug loading, pharmacokinetics, biocompatibility, and application in drug delivery. Expert Opin. Drug Deliv. 2019, 16, 219–237. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.R.; Lozano, D.; González, B.; Manzano, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Advances in mesoporous silica nanoparticles for targeted stimuli-responsive drug delivery: An update. Expert Opin. Drug Deliv. 2019, 16, 415–439. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regi, M.; Rámila, A.; del Real, R.P.; Pérez-Pariente, J. A New Property of MCM-41: Drug Delivery System. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Muñoz, B.; Rámila, A.; Pérez-Pariente, J.; Díaz, I.; Vallet-Regí, M. MCM-41 Organic Modification as Drug Delivery Rate Regulator. Chem. Mater. 2003, 15, 500–503. [Google Scholar] [CrossRef]
- Slowing, I.I.; Trewyn, B.G.; Giri, S.; Lin, V.S.-Y. Mesoporous Silica Nanoparticles for Drug Delivery and Biosensing Applications. Adv. Funct. Mater. 2007, 17, 1225–1236. [Google Scholar] [CrossRef]
- Abdo, G.G.; Zagho, M.M.; Khalil, A. Recent advances in stimuli-responsive drug release and targeting concepts using mesoporous silica nanoparticles. Emerg. Mater. 2020, 3, 407–425. [Google Scholar] [CrossRef]
- Wani, A.; Muthuswamy, E.; Savithra, G.H.L.; Mao, G.; Brock, S.; Oupický, D. Surface functionalization of mesoporous silica nanoparticles controls loading and release behavior of mitoxantrone. Pharm. Res. 2012, 29, 2407–2418. [Google Scholar] [CrossRef]
- Datt, A.; El-Maazawi, I.; Larsen, S.C. Aspirin Loading and Release from MCM-41 Functionalized with Aminopropyl Groups via Co-condensation or Postsynthesis Modification Methods. J. Phys. Chem. C 2012, 116, 18358–18366. [Google Scholar] [CrossRef]
- Varache, M.; Bezverkhyy, I.; Weber, G.; Saviot, L.; Chassagnon, R.; Baras, F.; Bouyer, F. Loading of Cisplatin into Mesoporous Silica Nanoparticles: Effect of Surface Functionalization. Langmuir 2019, 35, 8984–8995. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Gao, L.; Wang, L.; Shi, L.; Wei, E.; Zhou, B.; Zhou, L.; Ge, B. Polydopamine and peptide decorated doxorubicin-loaded mesoporous silica nanoparticles as a targeted drug delivery system for bladder cancer therapy. Drug Deliv. 2017, 24, 681–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadi, E.; Dehghannejad, N.; Hashemikia, S.; Ghasemnejad, M.; Tabebordbar, H. Synthesis and surface modification of mesoporous silica nanoparticles and its application as carriers for sustained drug delivery. Drug Deliv. 2014, 21, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.F.; Liu, F.; Brown, M.B. Advances in oral transmucosal drug delivery. J. Control. Release 2011, 153, 106–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drumond, N.; Stegemann, S. Polymer adhesion predictions for oral dosage forms to enhance drug administration safety. Part 3: Review of in vitro and in vivo methods used to predict esophageal adhesion and transit time. Colloids Surf. B Biointerfaces 2018, 165, 303–314. [Google Scholar] [CrossRef]
- Ensign, L.M.; Cone, R.; Hanes, J. Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barriers. Adv. Drug Deliv. Rev. 2012, 64, 557–570. [Google Scholar] [CrossRef] [Green Version]
- Ugwoke, M.; Agu, R.; Verbeke, N.; Kinget, R. Nasal mucoadhesive drug delivery: Background, applications, trends and future perspectives. Adv. Drug Deliv. Rev. 2005, 57, 1640–1665. [Google Scholar] [CrossRef]
- Morrison, P.W.J.; Khutoryanskiy, V.V. Advances in ophthalmic drug delivery. Ther. Deliv. 2014, 5, 1297–1315. [Google Scholar] [CrossRef] [Green Version]
- Purohit, T.J.; Hanning, S.M.; Wu, Z. Advances in rectal drug delivery systems. Pharm. Dev. Technol. 2018, 23, 942–952. [Google Scholar] [CrossRef]
- das Neves, J.; Nunes, R.; Machado, A.; Sarmento, B. Polymer-based nanocarriers for vaginal drug delivery. Adv. Drug Deliv. Rev. 2015, 92, 53–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolawole, O.M.; Lau, W.M.; Mostafid, H.; Khutoryanskiy, V.V. Advances in intravesical drug delivery systems to treat bladder cancer. Int. J. Pharm. 2017, 532, 105–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Date, A.A.; Hanes, J.; Ensign, L.M. Nanoparticles for oral delivery: Design, evaluation and state-of-the-art. J. Control. Release 2016, 240, 504–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Florek, J.; Caillard, R.; Kleitz, F. Evaluation of mesoporous silica nanoparticles for oral drug delivery – current status and perspective of MSNs drug carriers. Nanoscale 2017, 9, 15252–15277. [Google Scholar] [CrossRef] [PubMed]
- Laffleur, F.; Bernkop-Schnürch, A. Strategies for improving mucosal drug delivery. Nanomedicine 2013, 8, 2061–2075. [Google Scholar] [CrossRef]
- Vllasaliu, D.; Fowler, R.; Garnett, M.; Eaton, M.; Stolnik, S. Barrier characteristics of epithelial cultures modelling the airway and intestinal mucosa. Comparison 2011, 415, 579–585. [Google Scholar] [CrossRef]
- Khutoryanskiy, V.V. Advances in mucoadhesion and mucoadhesive polymers. Macromol. Biosci. 2011, 11, 748–764. [Google Scholar] [CrossRef]
- Peppas, N.A.; Thomas, J.B.; McGinty, J. Molecular Aspects of Mucoadhesive Carrier Development for Drug Delivery and Improved Absorption. J. Biomater. Sci. Polym. Ed. 2009, 20, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Serra, L.; Doménech, J.; Peppas, N.A. Engineering design and molecular dynamics of mucoadhesive drug delivery systems as targeting agents. Eur. J. Pharm. Biopharm. 2009, 71, 519–528. [Google Scholar] [CrossRef] [Green Version]
- Nagai, T. Adhesive topical drug delivery system. J. Control. Release 1985, 2, 121–134. [Google Scholar] [CrossRef]
- Ishida, M.; Nambu, N.; Nagai, T. Highly viscous gel ointment containing carbopol for application to the oral mucosa. Chem. Pharm. Bull. 1983, 31, 4561–4564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernkop-Schnürch, A.; Schwarz, V.; Steininger, S. Polymers with Thiol Groups: A New Generation of Mucoadhesive Polymers? Pharm. Res. 1999, 16, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnürch, A.; Hornof, M.; Guggi, D. Thiolated chitosans. Eur. J. Pharm. Biopharm. 2004, 57, 9–17. [Google Scholar] [CrossRef]
- Cheewatanakornkool, K.; Niratisai, S.; Manchun, S.; Dass, C.R.; Sriamornsak, P. Thiolated pectin–doxorubicin conjugates: Synthesis, characterization and anticancer activity studies. Carbohydr. Polym. 2017, 174, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Ahuja, M. Thiolated pectin: Synthesis, characterization and evaluation as a mucoadhesive polymer. Carbohydr. Polym. 2011, 85, 658–663. [Google Scholar] [CrossRef]
- Pereira de Sousa, I.; Buttenhauser, K.; Suchaoin, W.; Partenhauser, A.; Perrone, M.; Matuszczak, B.; Bernkop-Schnürch, A. Thiolated graphene oxide as promising mucoadhesive carrier for hydrophobic drugs. Int. J. Pharm. 2016, 509, 360–367. [Google Scholar] [CrossRef]
- Jalil, A.; Matuszczak, B.; Nguyen Le, N.-M.; Mahmood, A.; Laffleur, F.; Bernkop-Schnürch, A. Synthesis and Characterization of Thiolated PVP–Iodine Complexes: Key to Highly Mucoadhesive Antimicrobial Gels. Mol. Pharm. 2018, 15, 3527–3534. [Google Scholar] [CrossRef]
- Laffleur, F.; Netsomboon, K.; Erman, L.; Partenhauser, A. Evaluation of modified hyaluronic acid in terms of rheology, enzymatic degradation and mucoadhesion. Int. J. Biol. Macromol. 2019, 123, 1204–1210. [Google Scholar] [CrossRef]
- Müller, C.; Bernkop-Schnürch, A. Thiomers. In Mucoadhesive Materials and Drug Delivery Systems; Khutoryanskiy, V.V., Ed.; John Wiley & Sons, Ltd: Chichester, UK, 2014; pp. 255–278. [Google Scholar]
- Brannigan, R.P.; Khutoryanskiy, V.V. Progress and Current Trends in the Synthesis of Novel Polymers with Enhanced Mucoadhesive Properties. Macromol. Biosci. 2019, 19, 1900194. [Google Scholar] [CrossRef]
- Khutoryanskiy, V.V. Beyond PEGylation: Alternative surface-modification of nanoparticles with mucus-inert biomaterials. Adv. Drug Deliv. Rev. 2018, 124, 140–149. [Google Scholar] [CrossRef]
- Akkus, Z.B.; Nazir, I.; Jalil, A.; Tribus, M.; Bernkop-Schnürch, A. Zeta Potential Changing Polyphosphate Nanoparticles: A Promising Approach To Overcome the Mucus and Epithelial Barrier. Mol. Pharm. 2019, 16, 2817–2825. [Google Scholar] [CrossRef]
- Müller, C.; Perera, G.; König, V.; Bernkop-Schnürch, A. Development and in vivo evaluation of papain-functionalized nanoparticles. Eur. J. Pharm. Biopharm. 2014, 87, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Huckaby, J.T.; Lai, S.K. PEGylation for enhancing nanoparticle diffusion in mucus. Adv. Drug Deliv. Rev. 2018, 124, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Kaminskas, L.M.; McLeod, V.M.; Porter, C.J.H.; Boyd, B.J. Differences in colloidal structure of PEGylated nanomaterials dictate the likelihood of accelerated blood clearance. J. Pharm. Sci. 2011, 100, 5069–5077. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Du, S.; Liu, Y.; Lv, C.; Song, Y.; Chen, X.; Zhang, B.; Li, D.; Gao, S.; Cui, W.; et al. Mucoadhesive-to-penetrating controllable peptosomes-in-microspheres co-loaded with anti-miR-31 oligonucleotide and Curcumin for targeted colorectal cancer therapy. Theranostics 2020, 10, 3594–3611. [Google Scholar] [CrossRef] [PubMed]
- Köllner, S.; Dünnhaupt, S.; Waldner, C.; Hauptstein, S.; Pereira de Sousa, I.; Bernkop-Schnürch, A. Mucus permeating thiomer nanoparticles. Eur. J. Pharm. Biopharm. 2015, 97, 265–272. [Google Scholar] [CrossRef]
- Netsomboon, K.; Bernkop-Schnürch, A. Mucoadhesive vs. mucopenetrating particulate drug delivery. Eur. J. Pharm. Biopharm. 2016, 98, 76–89. [Google Scholar] [CrossRef]
- Ways, T.M.; Lau, W.M.; Ng, K.W.; Khutoryanskiy, V.V. Synthesis of thiolated, PEGylated and POZylated silica nanoparticles and evaluation of their retention on rat intestinal mucosa in vitro. Eur. J. Pharm. Sci. 2018, 122, 230–238. [Google Scholar] [CrossRef]
- Maleki, A.; Kettiger, H.; Schoubben, A.; Rosenholm, J.M.; Ambrogi, V.; Hamidi, M. Mesoporous silica materials: From physico-chemical properties to enhanced dissolution of poorly water-soluble drugs. J. Control. Release 2017, 262, 329–347. [Google Scholar] [CrossRef]
- Watermann, A.; Brieger, J. Mesoporous silica nanoparticles as drug delivery vehicles in cancer. Nanomaterials 2017, 7, 189. [Google Scholar] [CrossRef] [Green Version]
- Cha, B.G.; Kim, J. Functional mesoporous silica nanoparticles for bio-imaging applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenholm, J.M.; Zhang, J.; Linden, M.; Sahlgren, C. Mesoporous silica nanoparticles in tissue engineering—A perspective. Nanomedicine 2016, 11, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.; Liu, X.; Huang, Q.; Zhang, R.; Feng, Q. Incorporation of silica nanoparticles to PLGA electrospun fibers for osteogenic differentiation of human osteoblast-like cells. Regen. Biomater. 2018, 5, 229–238. [Google Scholar] [CrossRef]
- Mansfield, E.D.; Sillence, K.; Hole, P.; Williams, A.C.; Khutoryanskiy, V.V. POZylation: A new approach to enhance nanoparticle diffusion through mucosal barriers. Nanoscale 2015, 7, 13671–13679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansfield, E.D.; de la Rosa, V.R.; Kowalczyk, R.M.; Grillo, I.; Hoogenboom, R.; Sillence, K.; Hole, P.; Williams, A.C.; Khutoryanskiy, V.V. Side chain variations radically alter the diffusion of poly(2-alkyl-2-oxazoline) functionalised nanoparticles through a mucosal barrier. Biomater. Sci. 2016, 4, 1318–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansfield, E.D.H.; Pandya, Y.; Mun, E.A.; Rogers, S.E.; Abutbul-Ionita, I.; Danino, D.; Williams, A.C.; Khutoryanskiy, V.V. Structure and characterisation of hydroxyethylcellulose–silica nanoparticles. RSC Adv. 2018, 8, 6471–6478. [Google Scholar] [CrossRef] [Green Version]
- Mun, E.A.; Williams, A.C.; Khutoryanskiy, V.V. Adhesion of thiolated silica nanoparticles to urinary bladder mucosa: Effects of PEGylation, thiol content and particle size. Int. J. Pharm. 2016, 512, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Mun, E.A.; Morrison, P.W.; Williams, A.C.; Khutoryanskiy, V.V. On the barrier properties of the cornea: A microscopy study of the penetration of fluorescently labeled nanoparticles, polymers, and sodium fluorescein. Mol. Pharm. 2014, 11, 3556–3564. [Google Scholar] [CrossRef]
- Zhang, Q.; Neoh, K.G.; Xu, L.; Lu, S.; Kang, E.T.; Mahendran, R.; Chiong, E. Functionalized mesoporous silica nanoparticles with mucoadhesive and sustained drug release properties for potential bladder cancer therapy. Langmuir 2014, 30, 6151–6161. [Google Scholar] [CrossRef]
- Araújo, F.; Shrestha, N.; Shahbazi, M.-A.; Fonte, P.; Mäkilä, E.M.; Salonen, J.J.; Hirvonen, J.T.; Granja, P.L.; Santos, H.A.; Sarmento, B. The impact of nanoparticles on the mucosal translocation and transport of GLP-1 across the intestinal epithelium. Biomaterials 2014, 35, 9199–9207. [Google Scholar] [CrossRef]
- Shrestha, N.; Shahbazi, M.-A.; Araújo, F.; Mäkilä, E.; Raula, J.; Kauppinen, E.I.; Salonen, J.; Sarmento, B.; Hirvonen, J.; Santos, H.A. Multistage pH-responsive mucoadhesive nanocarriers prepared by aerosol flow reactor technology: A controlled dual protein-drug delivery system. Biomaterials 2015, 68, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, N.; Araújo, F.; Shahbazi, M.-A.; Mäkilä, E.; Gomes, M.J.; Herranz-Blanco, B.; Lindgren, R.; Granroth, S.; Kukk, E.; Salonen, J.; et al. Thiolation and cell-penetrating peptide surface functionalization of porous silicon nanoparticles for oral delivery of insulin. Adv. Funct. Mater. 2016, 26, 3405–3416. [Google Scholar] [CrossRef]
- Sun, J.; Liu, Y.; Ge, M.; Zhou, G.; Sun, W.; Liu, D.; Liang, X.-J.; Zhang, J. A Distinct Endocytic Mechanism of Functionalized-Silica Nanoparticles in Breast Cancer Stem Cells. Sci. Rep. 2017, 7, 16236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slowing, I.; Trewyn, B.G.; Lin, V.S.Y. Effect of Surface Functionalization of MCM-41-Type Mesoporous Silica Nanoparticles on the Endocytosis by Human Cancer Cells. J. Am. Chem. Soc. 2006, 128, 14792–14793. [Google Scholar] [CrossRef] [Green Version]
- Gessner, I.; Klimpel, A.; Klußmann, M.; Neundorf, I.; Mathur, S. Interdependence of charge and secondary structure on cellular uptake of cell penetrating peptide functionalized silica nanoparticles. Nanoscale Adv. 2020, 2, 453–462. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, L.F.; Bouchmella, K.; Gonçalves, K.d.A.; Bettini, J.; Kobarg, J.; Cardoso, M.B. Functionalized Silica Nanoparticles As an Alternative Platform for Targeted Drug-Delivery of Water Insoluble Drugs. Langmuir 2016, 32, 3217–3225. [Google Scholar] [CrossRef]
- Sarparanta, M.P.; Bimbo, L.M.; Mäkilä, E.M.; Salonen, J.J.; Laaksonen, P.H.; Helariutta, A.M.K.; Linder, M.B.; Hirvonen, J.T.; Laaksonen, T.J.; Santos, H.A.; et al. The mucoadhesive and gastroretentive properties of hydrophobin-coated porous silicon nanoparticle oral drug delivery systems. Biomaterials 2012, 33, 3353–3362. [Google Scholar] [CrossRef]
- Desai, D.; Prabhakar, N.; Mamaeva, V.; Sen Karaman, D.; Lähdeniemi, I.; Sahlgren, C.; Rosenholm, J.; Toivola, D. Targeted modulation of cell differentiation in distinct regions of the gastrointestinal tract via oral administration of differently PEG-PEI functionalized mesoporous silica nanoparticles. Int. J. Nanomed. 2016, 11, 299. [Google Scholar] [CrossRef] [Green Version]
- Andreani, T.; Miziara, L.; Lorenzón, E.N.; de Souza, A.L.R.; Kiill, C.P.; Fangueiro, J.F.; Garcia, M.L.; Gremião, P.D.; Silva, A.M.; Souto, E.B. Effect of mucoadhesive polymers on the in vitro performance of insulin-loaded silica nanoparticles: Interactions with mucin and biomembrane models. Eur. J. Pharm. Biopharm. 2015, 93, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Bimbo, L.M.; Mäkilä, E.; Villanova, F.; Kaasalainen, M.; Herranz-Blanco, B.; Caramella, C.M.; Lehto, V.-P.; Salonen, J.; Herzig, K.-H.; et al. Co-delivery of a hydrophobic small molecule and a hydrophilic peptide by porous silicon nanoparticles. J. Control. Release 2013, 170, 268–278. [Google Scholar] [CrossRef]
- Moulari, B.; Pertuit, D.; Pellequer, Y.; Lamprecht, A. The targeting of surface modified silica nanoparticles to inflamed tissue in experimental colitis. Biomaterials 2008, 29, 4554–4560. [Google Scholar] [CrossRef] [PubMed]
- Lungare, S.; Hallam, K.; Badhan, R.K.S. Phytochemical-loaded mesoporous silica nanoparticles for nose-to-brain olfactory drug delivery. Int. J. Pharm. 2016, 513, 280–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Zhang, K.; Wang, J.; Zhao, R.; Zhang, Q.; Kong, X. Poly(amidoamine)-modified mesoporous silica nanoparticles as a mucoadhesive drug delivery system for potential bladder cancer therapy. Colloids Surf. B Biointerfaces 2020, 189, 110832. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Y.; Cui, Y.; Zhao, Q.; Zhang, Q.; Musetti, S.; Kinghorn, K.A.; Wang, S. Overcoming multiple gastrointestinal barriers by bilayer modified hollow mesoporous silica nanocarriers. Acta Biomater. 2018, 65, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Feng, S.; Zhang, X.; Zhao, Q.; Fang, Y.; Wang, S. Thiolated polymer and Cell-Penetrating Peptide dual-surface functionalization of mesoporous silicon nanoparticles to overcome intestinal barriers. J. Drug Deliv. Sci. Technol. 2019, 53, 101184. [Google Scholar] [CrossRef]
- Amin, M.K.; Boateng, J.S. Surface Modification of Mobile Composition of Matter (MCM)-41 Type Silica Nanoparticles for Potential Oral Mucosa Vaccine Delivery. J. Pharm. Sci. 2020. [Google Scholar] [CrossRef]
- Yoshida, T.; Yoshioka, Y.; Takahashi, H.; Misato, K.; Mori, T.; Hirai, T.; Nagano, K.; Abe, Y.; Mukai, Y.; Kamada, H.; et al. Intestinal absorption and biological effects of orally administered amorphous silica particles. Nanoscale Res. Lett. 2014, 9, 532. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, T.; Yoshioka, Y.; Tochigi, S.; Hirai, T.; Uji, M.; Ichihashi, K.-I.; Nagano, K.; Abe, Y.; Kamada, H.; Tsunoda, S.-I.; et al. Intranasal exposure to amorphous nanosilica particles could activate intrinsic coagulation cascade and platelets in mice. Part. Fibre Toxicol. 2013, 10, 41. [Google Scholar] [CrossRef] [Green Version]
- Möller, K.; Bein, T. Degradable Drug Carriers: Vanishing Mesoporous Silica Nanoparticles. Chem. Mater. 2019, 31, 4364–4378. [Google Scholar] [CrossRef]
- Croissant, J.G.; Brinker, C.J. Chapter Eight—Biodegradable Silica-Based Nanoparticles: Dissolution Kinetics and Selective Bond Cleavage. In The Enzymes; Tamanoi, F., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 43, pp. 181–214. [Google Scholar]
- Shabir, Q. Biodegradability of Porous Silicon. In Handbook of Porous Silicon; Canham, L., Ed.; Springer International Publishing: Cham, Switzerland, 2014; pp. 395–401. [Google Scholar] [CrossRef]
- Hudson, S.P.; Padera, R.F.; Langer, R.; Kohane, D.S. The biocompatibility of mesoporous silicates. Biomaterials 2008, 29, 4045–4055. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Park, J.-H.; Jeong, H.; Hong, J.; Choi, W.S.; Lee, B.-H.; Park, C.Y. An evaluation of the in vivo safety of nonporous silica nanoparticles: Ocular topical administration versus oral administration. Sci. Rep. 2017, 7, 8238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Liu, T.; Fu, C.; Tan, L.; Meng, X.; Liu, H. Biodistribution, excretion, and toxicity of mesoporous silica nanoparticles after oral administration depend on their shape. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1915–1924. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, L.; Liu, T.; Hao, N.; Liu, H.; Chen, D.; Tang, F. The Shape Effect of Mesoporous Silica Nanoparticles on Biodistribution, Clearance, and Biocompatibility in Vivo. ACS Nano 2011, 5, 5390–5399. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Y.; Ran, F.; Cui, Y.; Liu, C.; Zhao, Q.; Gao, Y.; Wang, D.; Wang, S. A comparison between sphere and rod nanoparticles regarding their in vivo biological behavior and pharmacokinetics. Sci. Rep. 2017, 7, 4131. [Google Scholar] [CrossRef] [PubMed]
- Bukara, K.; Schueller, L.; Rosier, J.; Martens, M.A.; Daems, T.; Verheyden, L.; Eelen, S.; Van Speybroeck, M.; Libanati, C.; Martens, J.A.; et al. Ordered mesoporous silica to enhance the bioavailability of poorly water-soluble drugs: Proof of concept in man. Eur. J. Pharm. Biopharm. 2016, 108, 220–225. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. Hydrogels as mucoadhesive and bioadhesive materials: A review. Biomaterials 1996, 17, 1553–1561. [Google Scholar] [CrossRef]
- Lee, J.W.; Park, J.H.; Robinson, J.R. Bioadhesive-Based Dosage Forms: The Next Generation. J. Pharm. Sci. 2000, 89, 850–866. [Google Scholar] [CrossRef]
- Ouellette, M.; Masse, F.; Lefebvre-Demers, M.; Maestracci, Q.; Grenier, P.; Millar, R.; Bertrand, N.; Prieto, M.; Boisselier, É. Insights into gold nanoparticles as a mucoadhesive system. Sci. Rep. 2018, 8, 14357. [Google Scholar] [CrossRef] [Green Version]
- Lai, S.K.; O’Hanlon, D.E.; Harrold, S.; Man, S.T.; Wang, Y.-Y.; Cone, R.; Hanes, J. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc. Natl. Acad. Sci. USA 2007, 104, 1482–1487. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-Y.; Lai, S.K.; Suk, J.S.; Pace, A.; Cone, R.; Hanes, J. Addressing the PEG Mucoadhesivity Paradox to Engineer Nanoparticles that “Slip” through the Human Mucus Barrier. Angew. Chem. Int. Ed. 2008, 47, 9726–9729. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Lai, S.K.; Wang, Y.-Y.; Zhong, W.; Happe, C.; Zhang, M.; Fu, J.; Hanes, J. Biodegradable Nanoparticles Composed Entirely of Safe Materials that Rapidly Penetrate Human Mucus. Angew. Chem. Int. Ed. 2011, 50, 2597–2600. [Google Scholar] [CrossRef]
- Vllasaliu, D.; Fowler, R.; Stolnik, S. PEGylated nanomedicines. Recent Prog. Remain. Concerns 2014, 11, 139–154. [Google Scholar] [CrossRef]
- Wang, X.; Fu, L.; Lin, W.; Zhang, W.; Pei, Q.; Zheng, X.; Liu, S.; Zhang, T.; Xie, Z. Vaginal delivery of mucus-penetrating organic nanoparticles for photothermal therapy against cervical intraepithelial neoplasia in mice. J. Mater. Chem. B 2019, 7, 4528–4537. [Google Scholar] [CrossRef]
- Li, H.; Guissi, N.E.I.; Su, Z.; Ping, Q.; Sun, M. Effects of surface hydrophilic properties of PEG-based mucus-penetrating nanostructured lipid carriers on oral drug delivery. RSC Adv. 2016, 6, 84164–84176. [Google Scholar] [CrossRef]
- Tan, X.; Zhang, Y.; Wang, Q.; Ren, T.; Gou, J.; Guo, W.; Yin, T.; He, H.; Zhang, Y.; Tang, X. Cell-penetrating peptide together with PEG-modified mesostructured silica nanoparticles promotes mucous permeation and oral delivery of therapeutic proteins and peptides. Biomater. Sci. 2019, 7, 2934–2950. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Park, J.-H.; Kim, M.; Jeong, H.; Hong, J.; Chuck, R.S.; Park, C.Y. Safety of Nonporous Silica Nanoparticles in Human Corneal Endothelial Cells. Sci. Rep. 2017, 7, 14566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadpour, R.; Cheney, D.L.; Grunberger, J.W.; Yazdimamaghani, M.; Jedrzkiewicz, J.; Isaacson, K.J.; Dobrovolskaia, M.A.; Ghandehari, H. One-year chronic toxicity evaluation of single dose intravenously administered silica nanoparticles in mice and their Ex vivo human hemocompatibility. J. Control. Release 2020, 324, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, J.; Dong, S.; Cai, X.; Simaiti, A.; Yang, X.; Zhu, X.; Luo, J.; Jiang, L.-H.; Du, B.; et al. Silica nanoparticles induce lung inflammation in mice via ROS/PARP/TRPM2 signaling-mediated lysosome impairment and autophagy dysfunction. Part. Fibre Toxicol. 2020, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Yazdimamaghani, M.; Moos, P.J.; Dobrovolskaia, M.A.; Ghandehari, H. Genotoxicity of amorphous silica nanoparticles: Status and prospects. Nanomed. Nanotechnol. Biol. Med. 2019, 16, 106–125. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, S.; Hu, X.; Sun, D.; Yang, J.; Yang, C.; Wu, W.; Li, Y.; Gu, X.; Li, M.; et al. Toxicity and mechanism of mesoporous silica nanoparticles in eyes. Nanoscale 2020, 12, 13637–13653. [Google Scholar] [CrossRef]
- Hsiao, I.-L.; Fritsch-Decker, S.; Leidner, A.; Al-Rawi, M.; Hug, V.; Diabaté, S.; Grage, S.L.; Meffert, M.; Stoeger, T.; Gerthsen, D.; et al. Biocompatibility of Amine-Functionalized Silica Nanoparticles: The Role of Surface Coverage. Small 2019, 15, 1805400. [Google Scholar] [CrossRef] [PubMed]
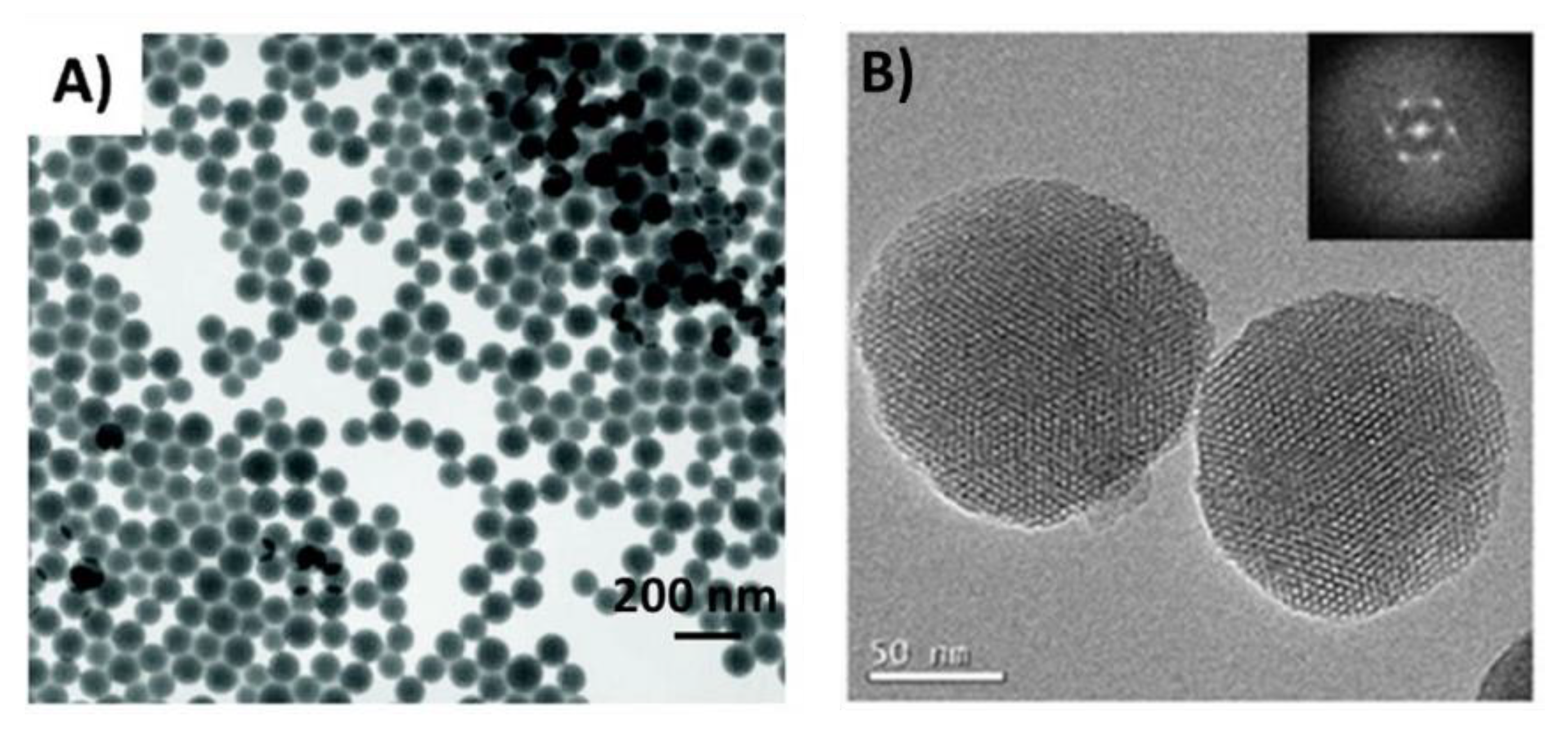
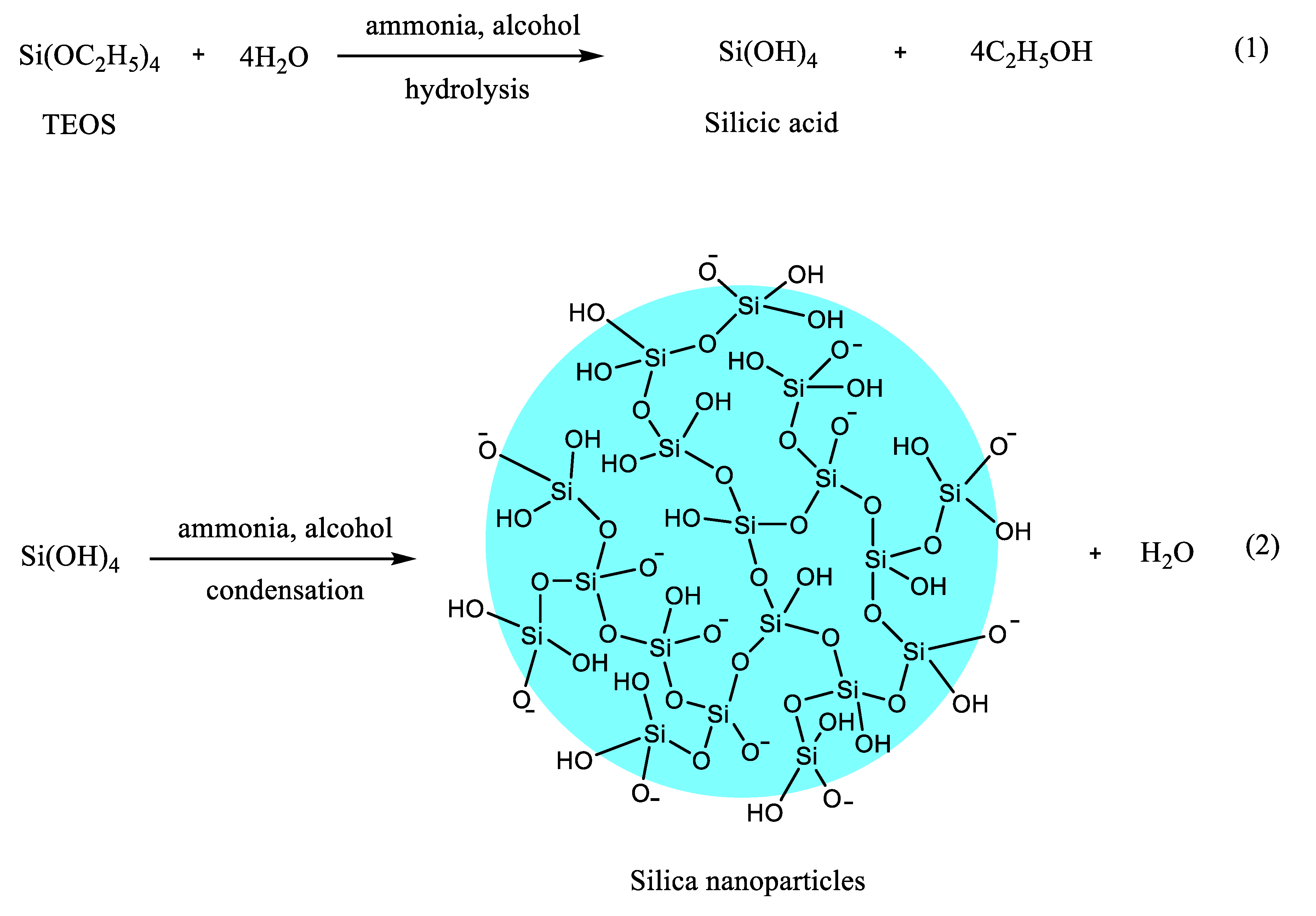
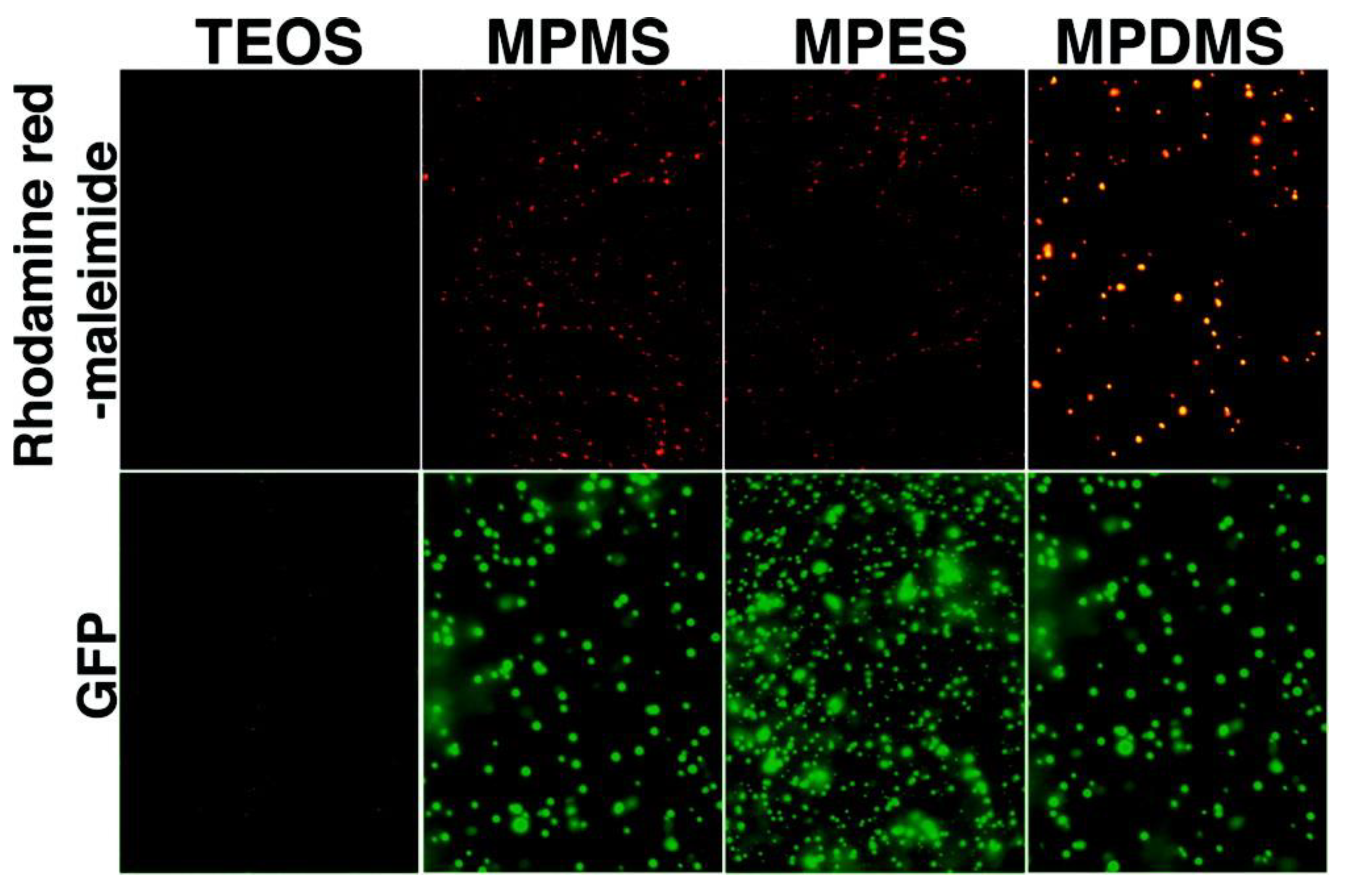
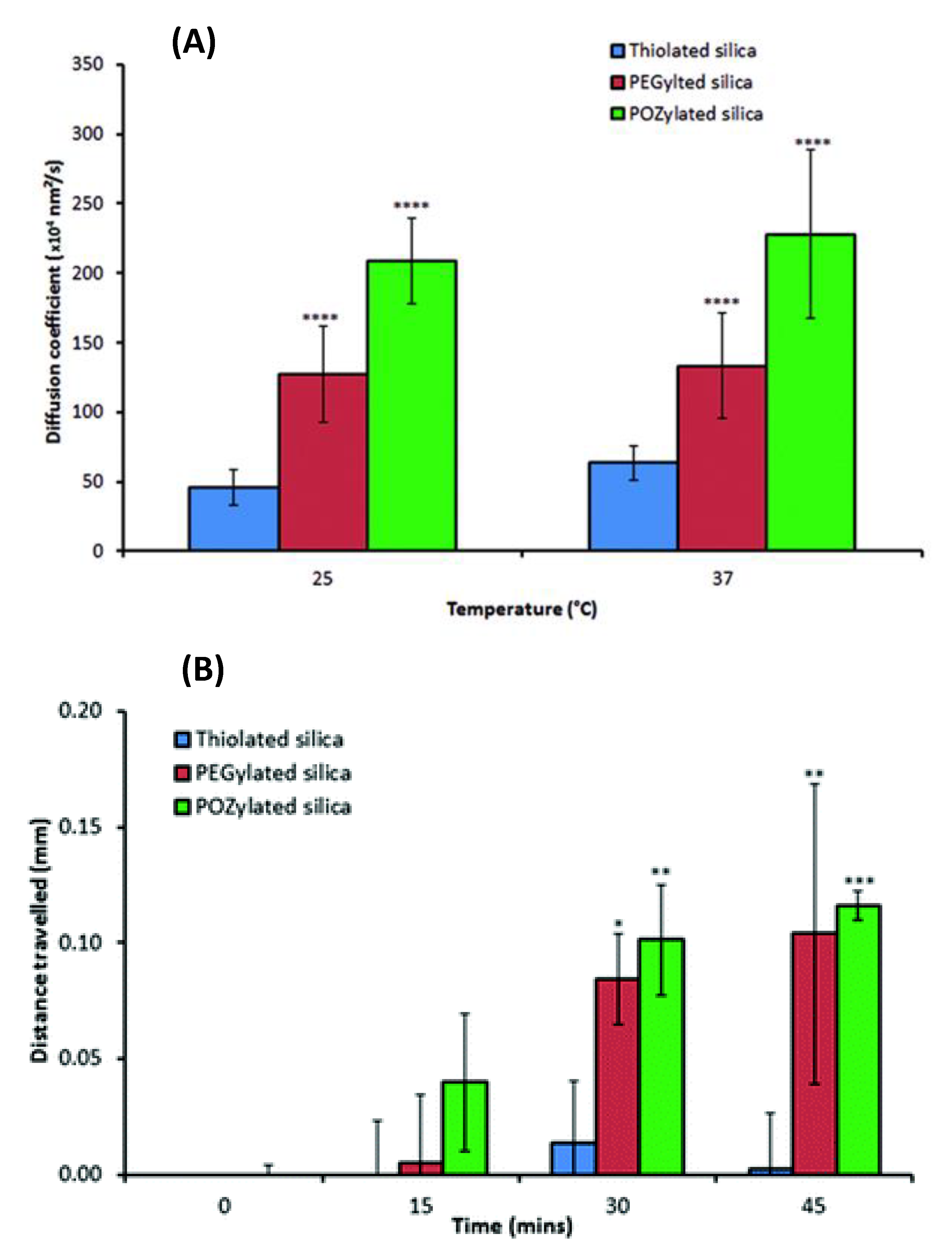

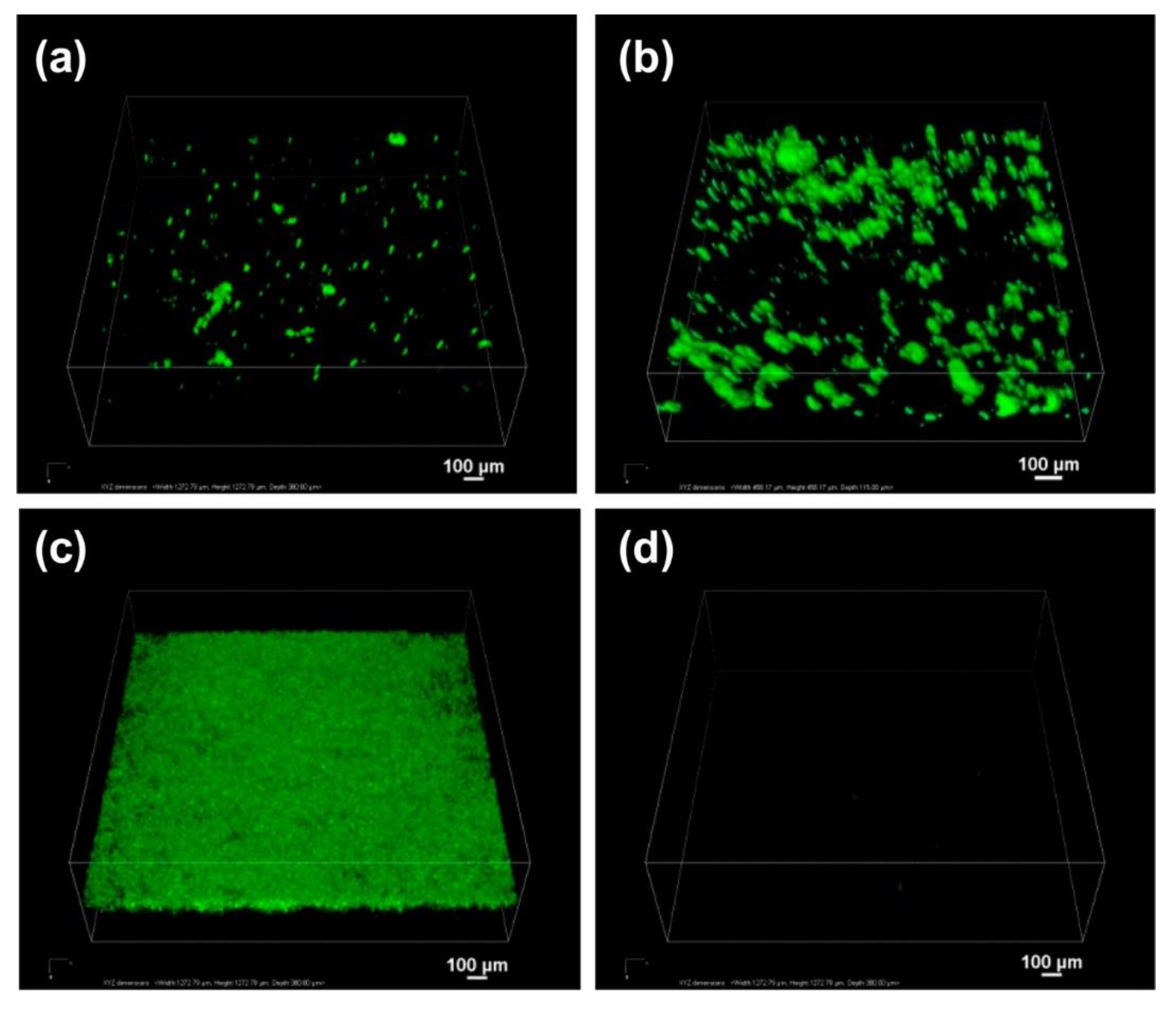

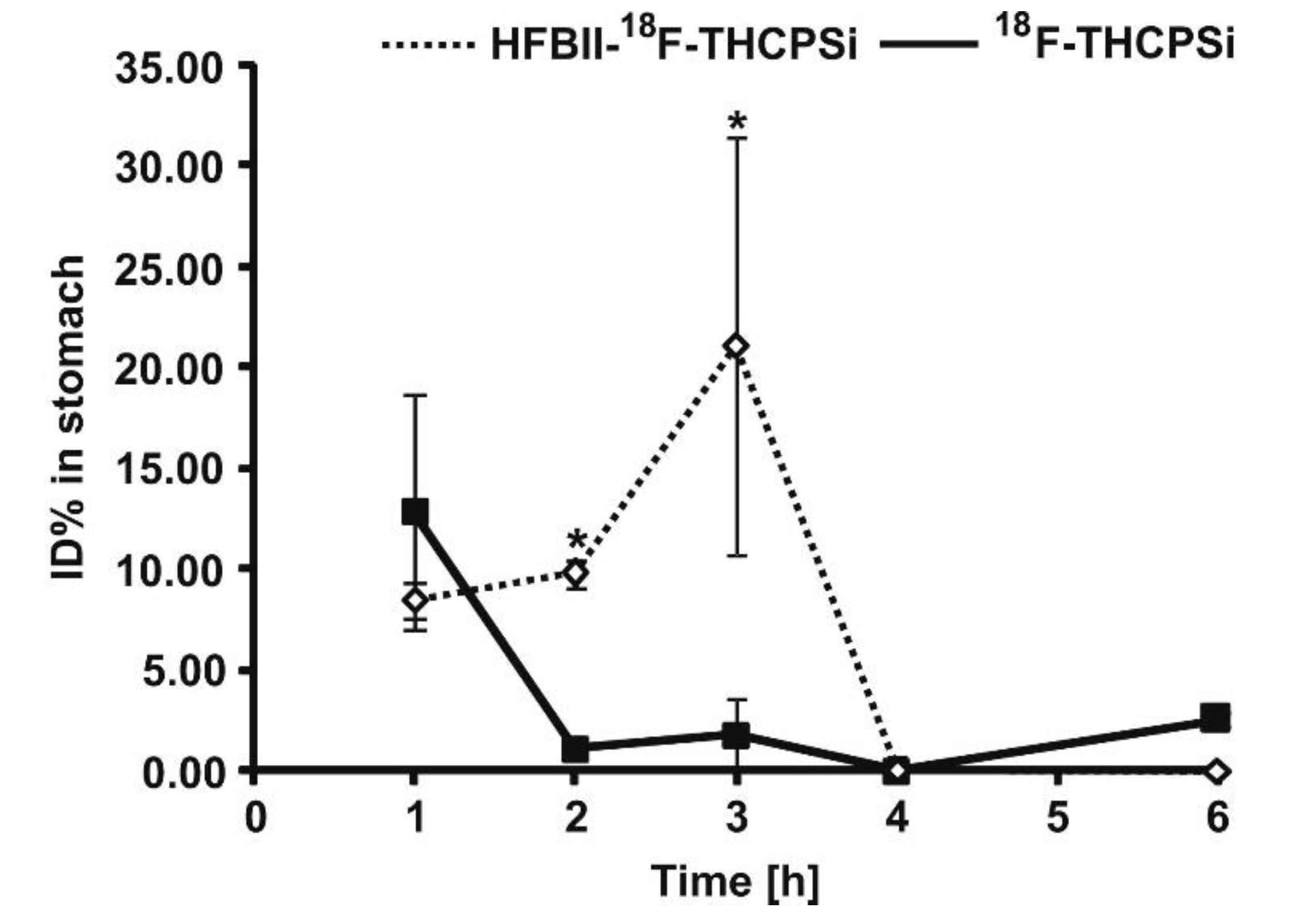
| Drugs | Uses | Routes of Administration | Models for Mucoadhesion and Therapeutic Evaluation | SURFACE Chemistries | Advantages | References |
|---|---|---|---|---|---|---|
| 5-amino salicylic acid | Inflammatory bowel disease | Oral | In vivo using mice | Coated with chitosan | Delayed drug release and targeted delivery to the inflamed tissues | [113] |
| Glucagon like peptide-1 | Type 2 diabetes mellitus | Oral | In vitro using intestinal cells | Coated with chitosan | Chitosan coated silica nanoparticles provided high drug loading capacity, sustained drug release and enhanced drug permeation | [102] |
| Curcumin | Neurodegenerative diseases | Nasal | In vitro using olfactory neuroblastoma cells | No coating | Targeting the brain, better chemical stability of the loaded drug | [114] |
| Doxorubicin | Bladder Cancer | Intravesical | In vitro porcine bladder mucosa | Poly(amidoamine) dendrimers | Controlling the level of surface layer though a layer-by-layer grafting method, Enhanced retention in bladder mucosa, Sustained drug release which was triggered in acidic environment | [115] |
| Paclitaxel (as a model drug) | Cancer | Oral | Incubating particles in mucin suspension, Caco-2 cells, In vivo studies in rats | Quantum dots doped hollow silica nanoparticles were first coated with cationic cell-penetrating peptides and then with a mucus-inert hydrophilic succinylated casein layer | Protects the drug from gastric acid, Degrades and then releases the drug in small intestine, Enhanced mucus-penetration, Strong interaction with epithelial membranes and a 5-fold increase in cellular uptake, Enhanced absolute bioavailability and in vivo antitumor activities | [116] |
| Lopinavir (as a model drug) | AIDS | Oral | Caco-2/HT29 cells, Everted gut sac method, In vivo bio-distribution studies and pharmacokinetic studies in rats | The core silica nanoparticles were coated with a middle layer of a cell-penetrating peptide and an outer layer of a thiolated polymer | Enhanced mucoadhesion and absorption through epithelial cells simultaneously, Enhanced oral bioavailability | [117] |
| Ovalbumin (as a model antigen) | Vaccination | Oral | Mucin binding assay | 3-aminopropyltriethoxysilane, poly(methyl methacrylate) (PMMA), PEG, chitosan | PMMA, PEG and chitosan modified nanoparticles provided sustained drug release, PEG and chitosan modified nanoparticles showed high encapsulation efficiency, Remained intact in simulated gastric and intestinal fluids, Showed enhanced mucoadhesion | [118] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
M. Ways, T.M.; Ng, K.W.; Lau, W.M.; Khutoryanskiy, V.V. Silica Nanoparticles in Transmucosal Drug Delivery. Pharmaceutics 2020, 12, 751. https://doi.org/10.3390/pharmaceutics12080751
M. Ways TM, Ng KW, Lau WM, Khutoryanskiy VV. Silica Nanoparticles in Transmucosal Drug Delivery. Pharmaceutics. 2020; 12(8):751. https://doi.org/10.3390/pharmaceutics12080751
Chicago/Turabian StyleM. Ways, Twana Mohammed, Keng Wooi Ng, Wing Man Lau, and Vitaliy V. Khutoryanskiy. 2020. "Silica Nanoparticles in Transmucosal Drug Delivery" Pharmaceutics 12, no. 8: 751. https://doi.org/10.3390/pharmaceutics12080751








