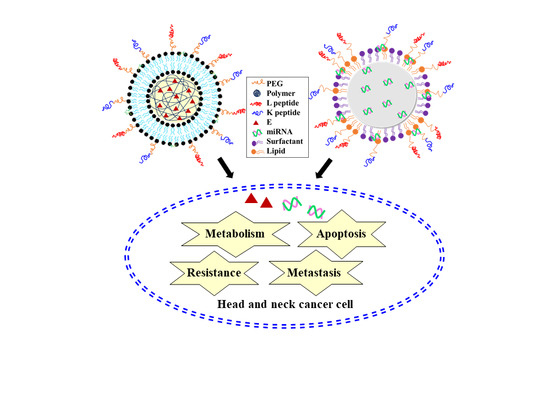
Mitochondrion-Directed Nanoparticles Loaded with a Natural Compound and a microRNA for Promoting Cancer Cell Death via the Modulation of Tumor Metabolism and Mitochondrial Dynamics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Preparation of E/LPN-KL and miR125/SLN-KL
2.3. Size Distribution and Zeta Potential of LPN and SLN
2.4. The Shape and Particle Morphology by Transmission Electron Microscopy (TEM)
2.5. Encapsulation Efficiency (EE%)
2.6. Cell Lines
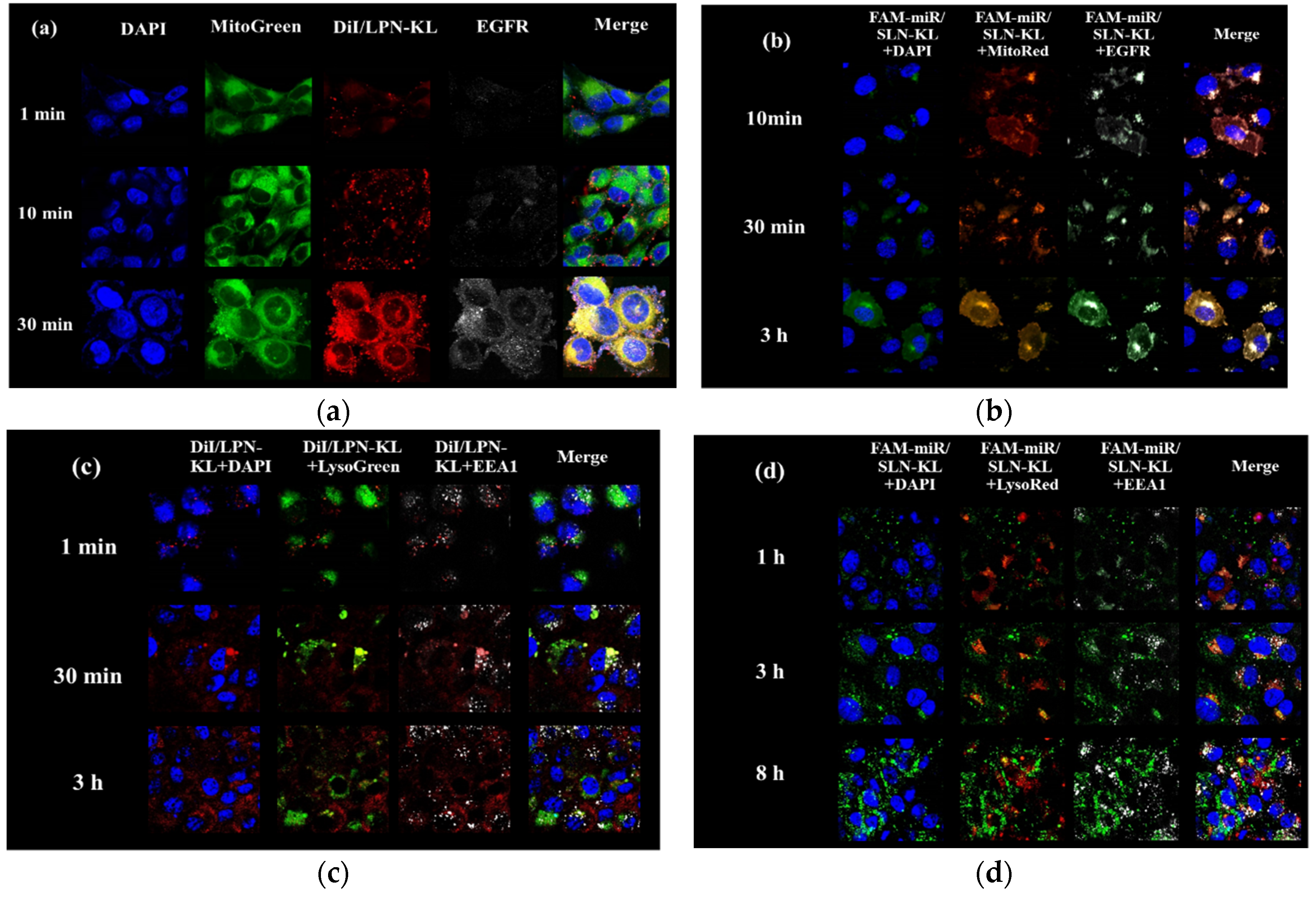
2.7. Identification of Intracellular Localization
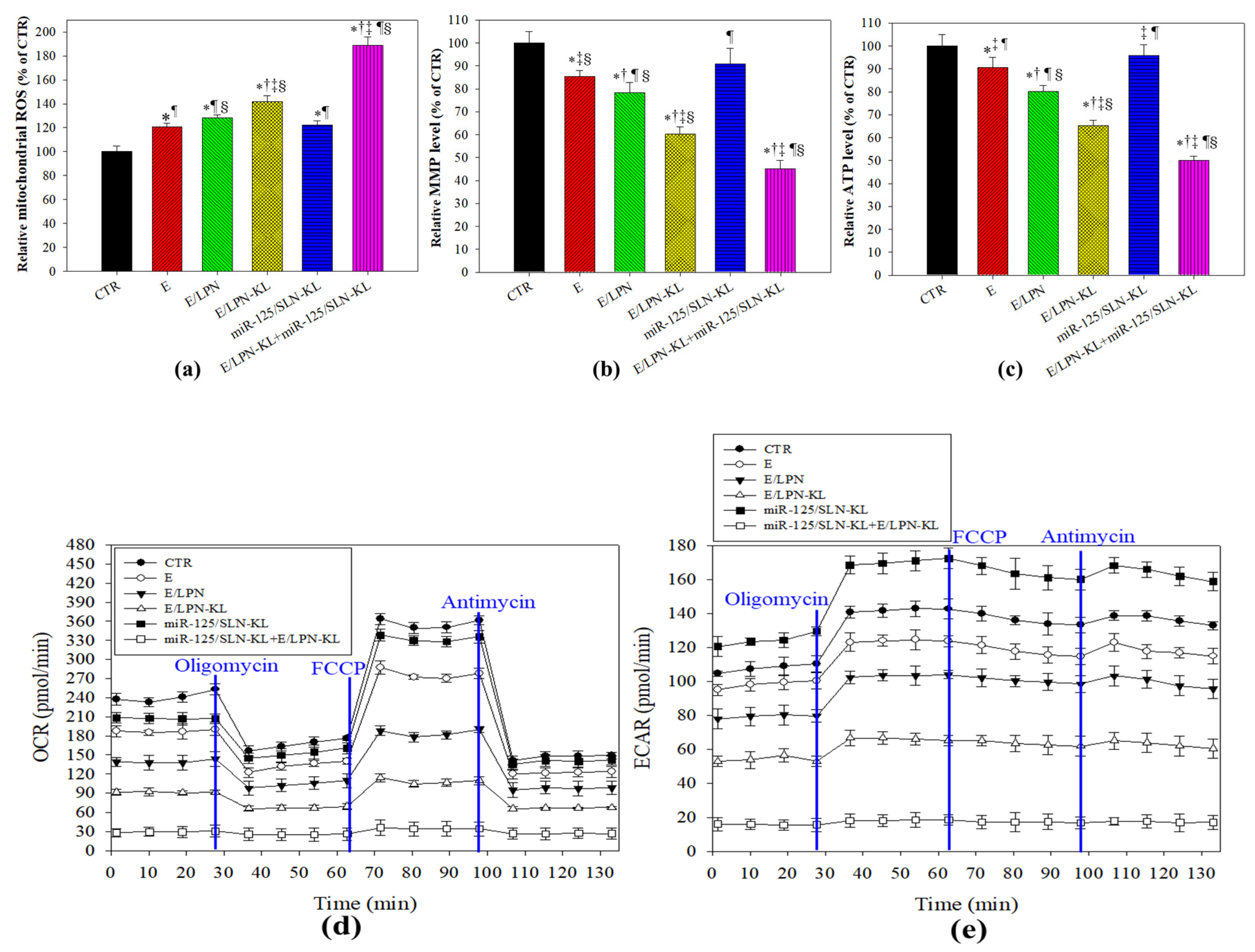
2.8. Detection of Mitochondrial ROS Level Using Mito-SOX
2.9. Measurement of Total Cellular ATP
2.10. Measurements of Mitochondrial Respiration
2.11. Glucose Uptake Assay
2.12. Oil Red O Staining for Intracellular Lipid Accumulation
2.13. Evaluation of Protein Expression Levels via Western Blot
2.14. Migration Assay
2.15. Cytotoxicity by Sulforhodamine B (SRB) Assay
2.16. Apoptosis Detection Assay
2.17. Establishment of In Vivo SAS-Tumor Bearing Mouse Model
2.18. Evaluation of Antitumor Efficacy on SAS Tumor-Bearing Mice
2.19. Biochemical Tests
2.20. Statistical Analysis
3. Results
3.1. Physicochemical Characteristics of E- or miR-125-Loaded Nanoparticles
3.2. Cellular Internalization of DiI/LPN-KL and FAM-miR125/SLN-KL into SAS Cells
3.3. Effect of Different Treatments on Mitochondrial ROS Production and Bioenergenesis in SAS Cells
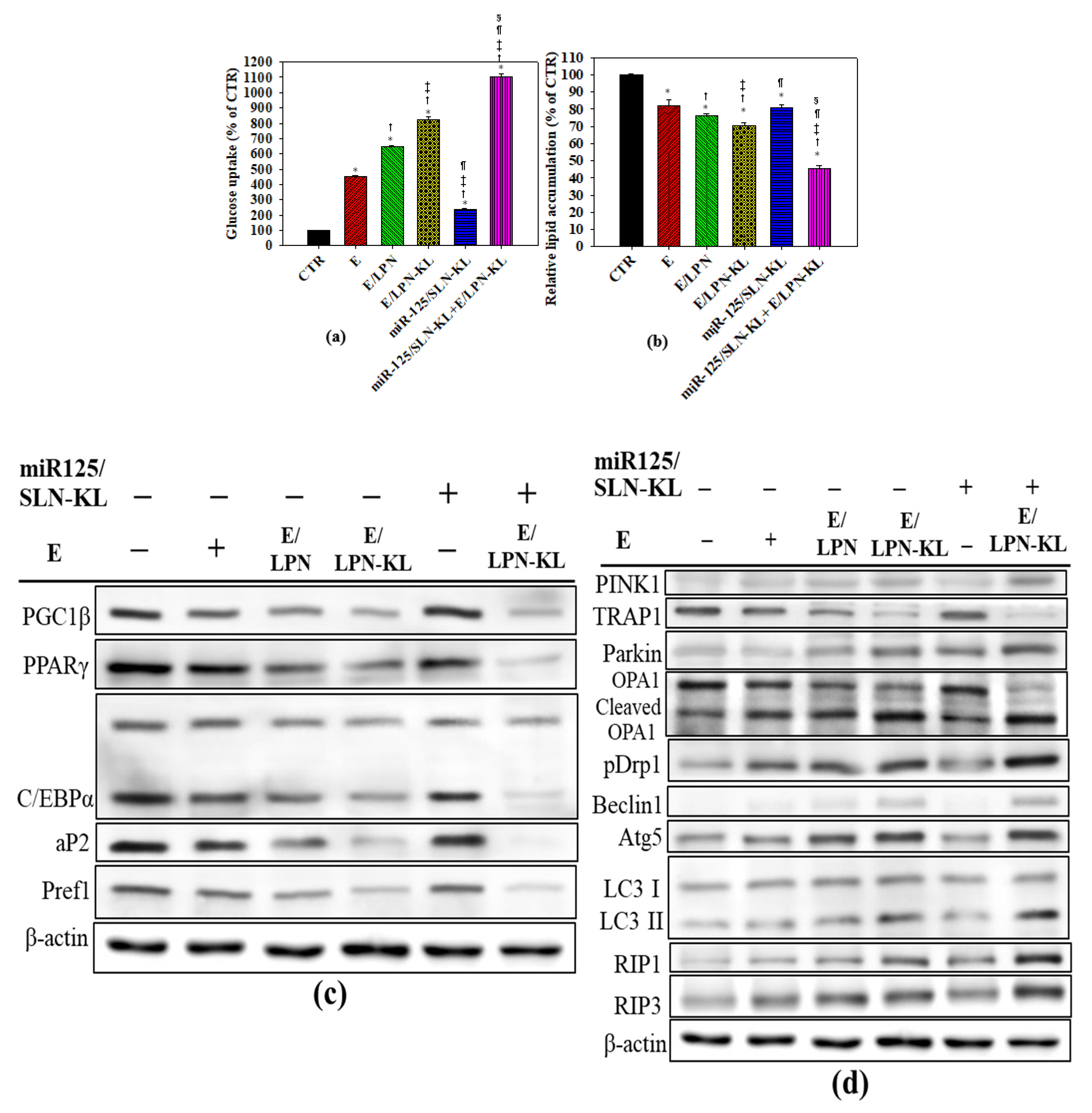
3.4. Increase in Glucose Uptake in SAS Cells Treated with Different Nanoparticle Formulations
3.5. Decreased Accumulation of Oil Droplets in SAS Cells Treated with Different Nanoparticle Formulations
3.6. Evaluation of Proteins Associated with Adipogenesis and Lipid Synthesis by Western Blot
3.7. Assessment of Proteins Related to Mitophagy and Necropotosis by Western Blot
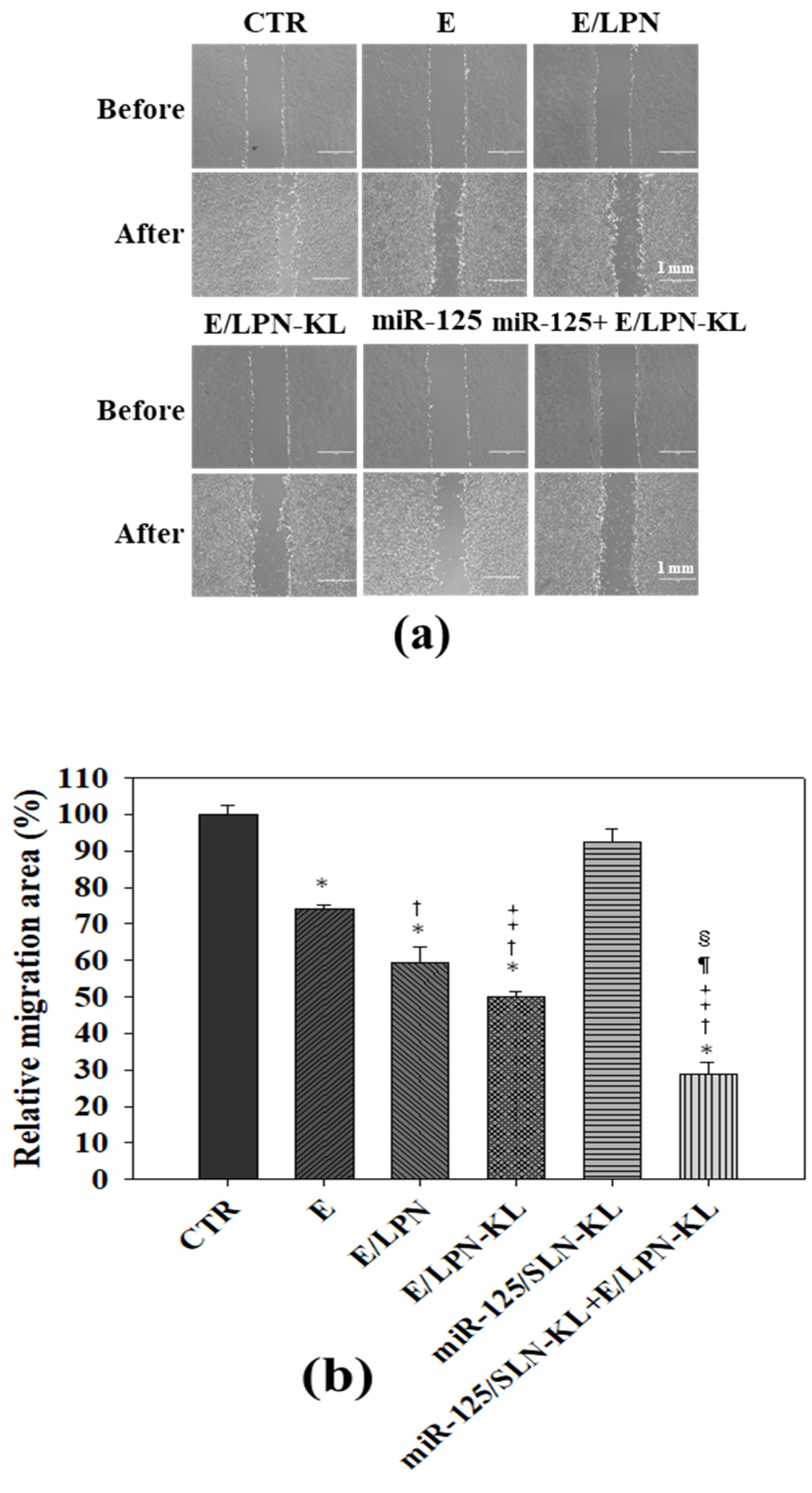
3.8. Reduced Migration of SAS Cells Treated with E- and miR-125-Loaded Formulations
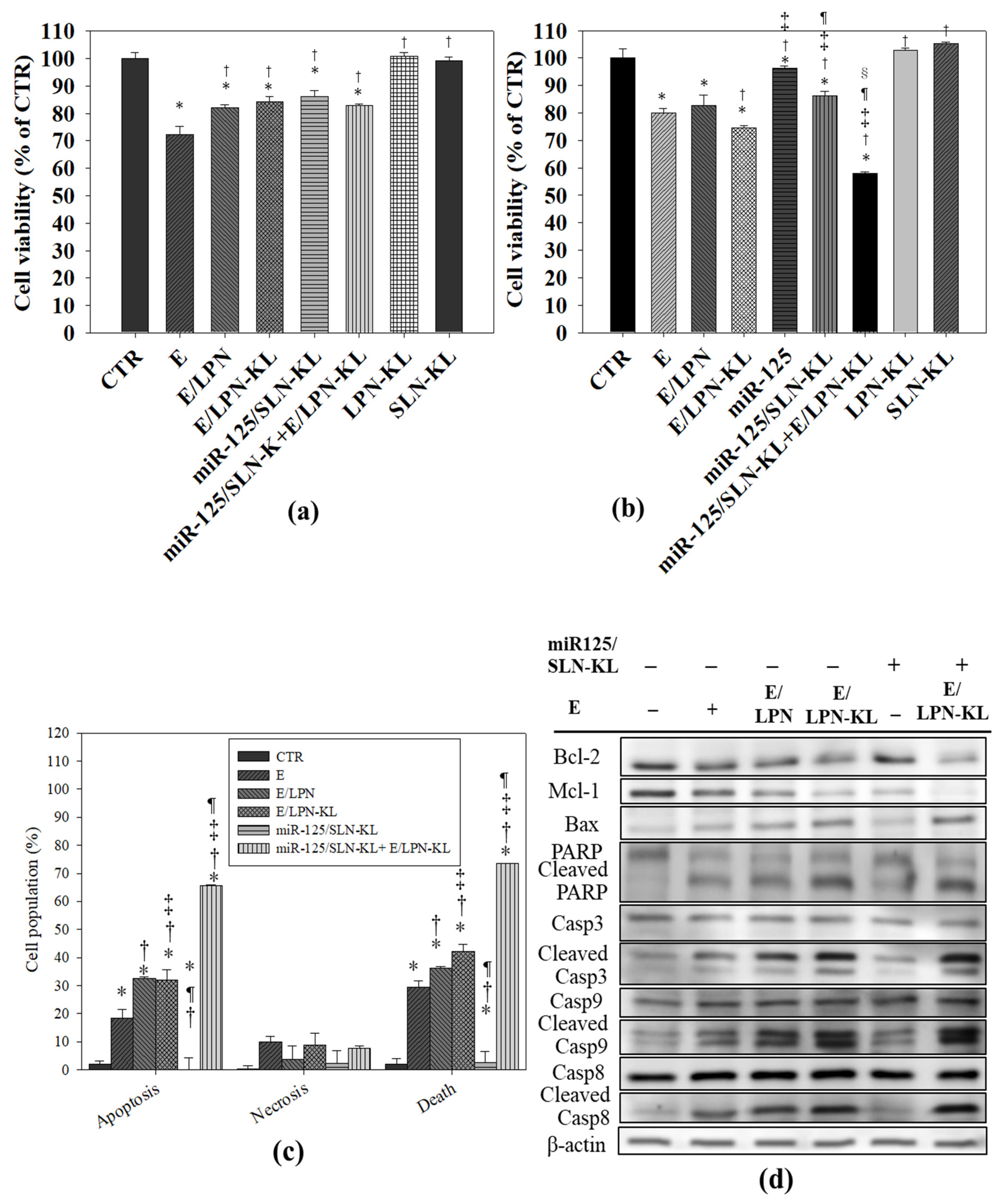
3.9. Cytotoxicity of E in Various Formulations on NOK and SAS Cells
3.10. Apoptotic Effect of E- and miR-125-Loaded Formulations on SAS Cells
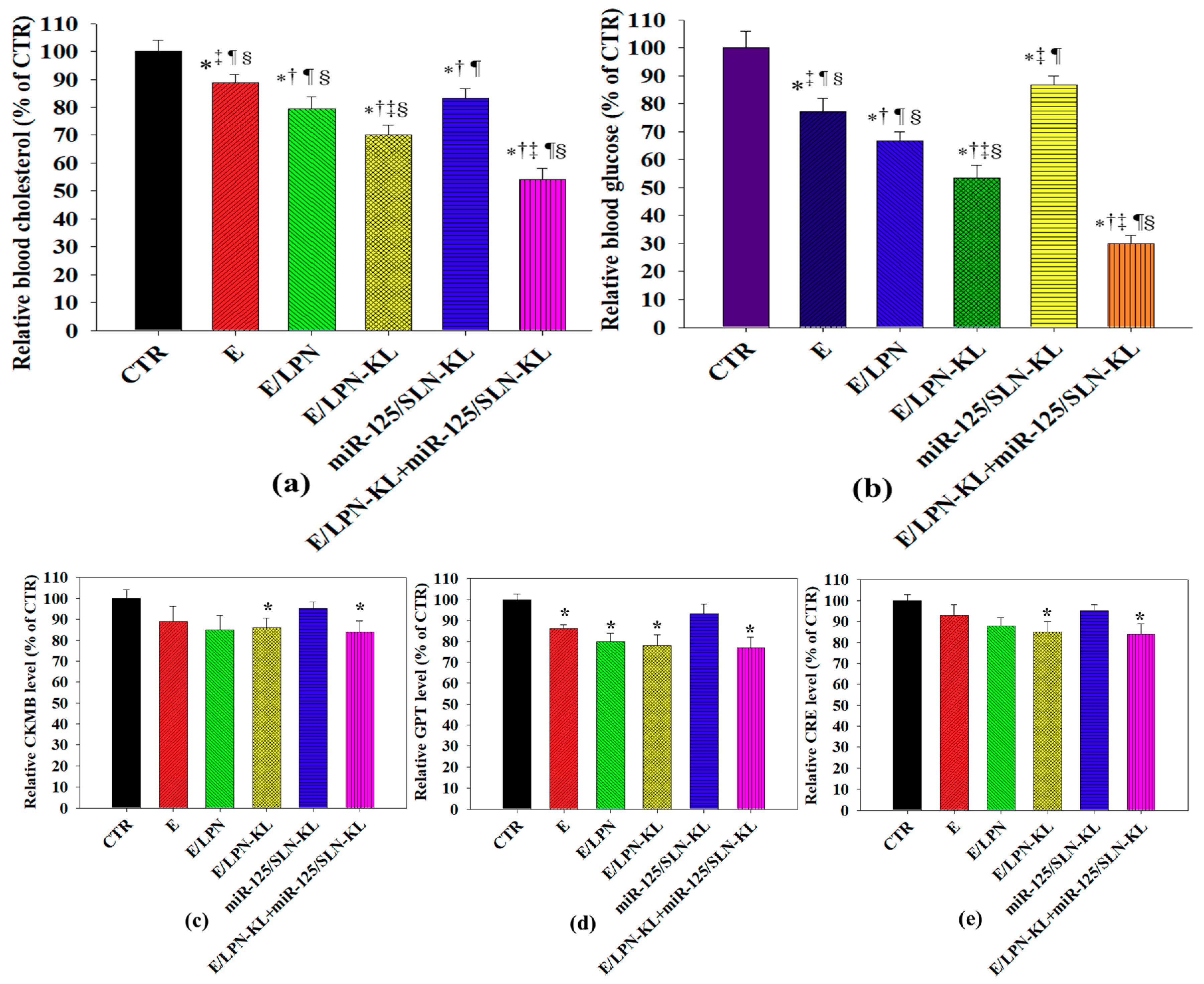
3.11. Serum Cholesterol and Glucose Levels In Vivo
3.12. In Vivo Biosafety Evaluation
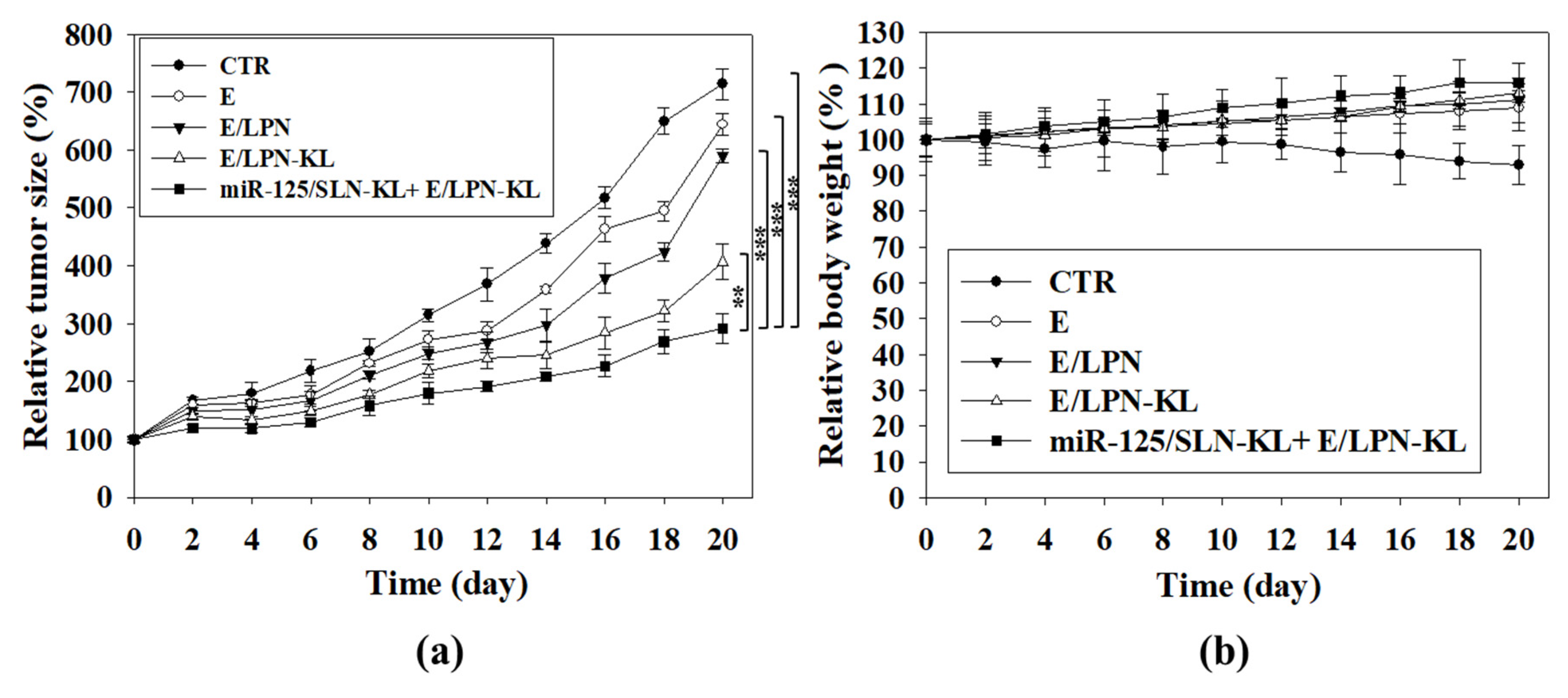
3.13. In Vivo Antitumor Efficacy and Body Weight Studies on SAS Tumor-Bearing Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kao, Y.Y.; Chou, C.H.; Yeh, L.Y.; Chen, Y.F.; Chang, K.W.; Liu, C.J.; Fan Chiang, C.Y.; Lin, S.C. MicroRNA miR-31 targets SIRT3 to disrupt mitochondrial activity and increase oxidative stress in oral carcinoma. Cancer Lett. 2019, 456, 40–48. [Google Scholar] [CrossRef]
- Li, S.; Wu, Y.; Ding, Y.; Yu, M.; Ai, Z. CerS6 regulates cisplatin resistance in oral squamous cell carcinoma by altering mitochondrial fission and autophagy. J. Cell Physiol. 2018, 233, 9416–9425. [Google Scholar] [CrossRef]
- Wen, R.; Banik, B.; Pathak, R.K.; Kumar, A.; Kolishetti, N.; Dhar, S. Nanotechnology inspired tools for mitochondrial dysfunction related diseases. Adv. Drug Deliv. Rev. 2016, 99, 52–69. [Google Scholar] [CrossRef]
- Zhao, Q.; Ye, M.; Yang, W.; Wang, M.; Li, M.; Gu, C.; Zhao, L.; Zhang, Z.; Han, W.; Fan, W.; et al. Effect of Mst1 on Endometriosis Apoptosis and Migration: Role of Drp1-Related Mitochondrial Fission and Parkin-Required Mitophagy. Cell Physiol. Biochem. 2018, 45, 1172–1190. [Google Scholar] [CrossRef]
- McWilliams, T.G.; Muqit, M.M. PINK1 and Parkin: emerging themes in mitochondrial homeostasis. Curr. Opin. Cell Biol. 2017, 45, 83–91. [Google Scholar] [CrossRef]
- Shefa, U.; Jeong, N.Y.; Song, I.O.; Chung, H.J.; Kim, D.; Jung, J.; Huh, Y. Mitophagy links oxidative stress conditions and neurodegenerative diseases. Neural Regen Res. 2019, 14, 749–756. [Google Scholar]
- Zhao, C.; He, R.; Shen, M.; Zhu, F.; Wang, M.; Liu, Y.; Chen, H.; Li, X.; Qin, R. PINK1/Parkin-Mediated Mitophagy Regulation by Reactive Oxygen Species Alleviates Rocaglamide A-Induced Apoptosis in Pancreatic Cancer Cells. Front. Pharm. 2019, 10, 968. [Google Scholar] [CrossRef]
- Yao, N.; Wang, C.; Hu, N.; Li, Y.; Liu, M.; Lei, Y.; Chen, M.; Chen, L.; Chen, C.; Lan, P.; et al. Inhibition of PINK1/Parkin-dependent mitophagy sensitizes multidrug-resistant cancer cells to B5G1, a new betulinic acid analog. Cell Death Dis. 2019, 10, 232. [Google Scholar] [CrossRef]
- Huang, C.Y.; Kuo, W.W.; Ho, T.J.; Chiang, S.F.; Pai, P.Y.; Lin, J.Y.; Lin, D.Y.; Kuo, C.H.; Huang, C.Y. Rab9-dependent autophagy is required for the IGF-IIR triggering mitophagy to eliminate damaged mitochondria. J. Cell Physiol. 2018, 233, 7080–7091. [Google Scholar] [CrossRef]
- He, L.; Gu, K. Tanshinone IIA regulates colorectal cancer apoptosis via attenuation of Parkin-mediated mitophagy by suppressing AMPK/Skp2 pathways. Mol. Med. Rep. 2018, 18, 1692–1703. [Google Scholar] [CrossRef]
- Di Rita, A.; Peschiaroli, A.; Pasquale, D.; Strobbe, D.; Hu, Z.; Gruber, J.; Nygaard, M.; Lambrughi, M.; Melino, G.; Papaleo, E.; et al. HUWE1 E3 ligase promotes PINK1/PARKIN-independent mitophagy by regulating AMBRA1 activation via IKKalpha. Nat. Commun. 2018, 9, 3755. [Google Scholar] [CrossRef]
- Yan, C.; Li, T.S. Dual Role of Mitophagy in Cancer Drug Resistance. Anticancer Res. 2018, 38, 617–621. [Google Scholar]
- Wang, J.; Gao, S.; Wang, S.; Xu, Z.; Wei, L. Zinc oxide nanoparticles induce toxicity in CAL 27 oral cancer cell lines by activating PINK1/Parkin-mediated mitophagy. Int. J. Nanomed. 2018, 13, 3441–3450. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, S.; Henning, S.M.; Lee, R.; Hsu, M.; Grojean, E.; Pisegna, R.; Ly, A.; Heber, D.; Li, Z. Cholesterol-lowering effects of dietary pomegranate extract and inulin in mice fed an obesogenic diet. J. Nutr. Biochem. 2018, 52, 62–69. [Google Scholar] [CrossRef]
- Les, F.; Arbones-Mainar, J.M.; Valero, M.S.; Lopez, V. Pomegranate polyphenols and urolithin A inhibit alpha-glucosidase, dipeptidyl peptidase-4, lipase, triglyceride accumulation and adipogenesis related genes in 3T3-L1 adipocyte-like cells. J. Ethnopharmacol. 2018, 220, 67–74. [Google Scholar] [CrossRef]
- Panchal, S.K.; Ward, L.; Brown, L. Ellagic acid attenuates high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. Eur. J. Nutr. 2013, 52, 559–568. [Google Scholar] [CrossRef]
- Liu, M.P.; Liao, M.; Dai, C.; Chen, J.F.; Yang, C.J.; Liu, M.; Chen, Z.G.; Yao, M.C. Sanguisorba officinalis L synergistically enhanced 5-fluorouracil cytotoxicity in colorectal cancer cells by promoting a reactive oxygen species-mediated, mitochondria-caspase-dependent apoptotic pathway. Sci. Rep. 2016, 6, 34245. [Google Scholar] [CrossRef]
- Shi, C.; Cai, Y.; Li, Y.; Li, Y.; Hu, N.; Ma, S.; Hu, S.; Zhu, P.; Wang, W.; Zhou, H. Yap promotes hepatocellular carcinoma metastasis and mobilization via governing cofilin/F-actin/lamellipodium axis by regulation of JNK/Bnip3/SERCA/CaMKII pathways. Redox Biol. 2018, 14, 59–71. [Google Scholar] [CrossRef]
- Wu, Q.B.; Chen, J.; Zhu, J.W.; Yin, X.; You, H.Y.; Lin, Y.R.; Zhu, H.Q. MicroRNA125 inhibits RKO colorectal cancer cell growth by targeting VEGF. Int. J. Mol. Med. 2018, 42, 665–673. [Google Scholar]
- Ma, J.; Fan, Y.; Feng, T.; Chen, F.; Xu, Z.; Li, S.; Lin, Q.; He, X.; Shi, W.; Liu, Y.; et al. HOTAIR regulates HK2 expression by binding endogenous miR-125 and miR-143 in oesophageal squamous cell carcinoma progression. Oncotarget 2017, 8, 86410–86422. [Google Scholar] [CrossRef]
- Giroud, M.; Pisani, D.F.; Karbiener, M.; Barquissau, V.; Ghandour, R.A.; Tews, D.; Fischer-Posovszky, P.; Chambard, J.C.; Knippschild, U.; Niemi, T.; et al. miR-125b affects mitochondrial biogenesis and impairs brite adipocyte formation and function. Mol. Metab. 2016, 5, 615–625. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, D.-Y.; Huang, L. In vivo delivery of miRNAs for cancer therapy: challenges and strategies. Adv. Drug Deliv. Rev. 2015, 81, 128–141. [Google Scholar] [CrossRef]
- Dhingra, A.; Jayas, R.; Afshar, P.; Guberman, M.; Maddaford, G.; Gerstein, J.; Lieberman, B.; Nepon, H.; Margulets, V.; Dhingra, R.; et al. Ellagic acid antagonizes Bnip3-mediated mitochondrial injury and necrotic cell death of cardiac myocytes. Free Radic. Biol. Med. 2017, 112, 411–422. [Google Scholar] [CrossRef]
- Hong, S.T.; Lin, H.; Wang, C.S.; Chang, C.H.; Lin, A.M.; Yang, J.C.; Lo, Y.L. Improving the anticancer effect of afatinib and microRNA by using lipid polymeric nanoparticles conjugated with dual pH-responsive and targeting peptides. J. Nanobiotechnol. 2019, 17, 89. [Google Scholar] [CrossRef]
- Du, J.B.; Song, Y.F.; Ye, W.L.; Cheng, Y.; Cui, H.; Liu, D.Z.; Liu, M.; Zhang, B.L.; Zhou, S.Y. PEG-detachable lipid-polymer hybrid nanoparticle for delivery of chemotherapy drugs to cancer cells. Anticancer Drugs 2014, 25, 751–766. [Google Scholar] [CrossRef]
- Ashizawa, A.T.; Cortes, J. Liposomal delivery of nucleic acid-based anticancer therapeutics: BP-100-1.01. Expert Opin. Drug Deliv. 2015, 12, 1107–1120. [Google Scholar] [CrossRef]
- Juang, V.; Chang, C.H.; Wang, C.S.; Wang, H.E.; Lo, Y.L. pH-Responsive PEG-Shedding and Targeting Peptide-Modified Nanoparticles for Dual-Delivery of Irinotecan and microRNA to Enhance Tumor-Specific Therapy. Small 2019, 15, e1903296. [Google Scholar] [CrossRef]
- Han, C.Y.; Yue, L.L.; Tai, L.Y.; Zhou, L.; Li, X.Y.; Xing, G.H.; Yang, X.G.; Sun, M.S.; Pan, W.S. A novel small peptide as an epidermal growth factor receptor targeting ligand for nanodelivery in vitro. Int. J. Nanomed. 2013, 8, 1541–1549. [Google Scholar]
- Jiang, L.; Li, L.; He, X.; Yi, Q.; He, B.; Cao, J.; Pan, W.; Gu, Z. Overcoming drug-resistant lung cancer by paclitaxel loaded dual-functional liposomes with mitochondria targeting and pH-response. Biomaterials 2015, 52, 126–139. [Google Scholar] [CrossRef]
- Kim, J.Y.; Han, J.H.; Park, G.; Seo, Y.W.; Yun, C.W.; Lee, B.C.; Bae, J.; Moon, A.R.; Kim, T.H. Necrosis-inducing peptide has the beneficial effect on killing tumor cells through neuropilin (NRP-1) targeting. Oncotarget 2016, 7, 32449–32461. [Google Scholar] [CrossRef]
- Meschenmoser, K.; Kim, Y.; Franken, S.; Nowak, M.; Feldmann, G.; Bendas, G.; Wolfgarten, M.; Messmer, D.; Schmidt-Wolf, I.G. Targeting cancer with a bi-functional peptide: in vitro and in vivo results. In Vivo 2013, 27, 431–442. [Google Scholar]
- He, C.; Jiang, S.; Jin, H.; Chen, S.; Lin, G.; Yao, H.; Wang, X.; Mi, P.; Ji, Z.; Lin, Y.; et al. Mitochondrial electron transport chain identified as a novel molecular target of SPIO nanoparticles mediated cancer-specific cytotoxicity. Biomaterials 2016, 83, 102–114. [Google Scholar] [CrossRef]
- Zhang, D.M.; Shu, C.; Chen, J.J.; Sodani, K.; Wang, J.; Bhatnagar, J.; Lan, P.; Ruan, Z.X.; Xiao, Z.J.; Ambudkar, S.V.; et al. BBA, a Derivative of 23-Hydroxybetulinic Acid, Potently Reverses ABCB1-Mediated Drug Resistance in Vitro and in Vivo. Mol. Pharm. 2012, 9, 3147–3159. [Google Scholar] [CrossRef]
- Tang, Q.Q.; Otto, T.C.; Lane, M.D. CCAAT/enhancer-binding protein beta is required for mitotic clonal expansion during adipogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 850–855. [Google Scholar] [CrossRef]
- Domori, A.; Sunahara, A.; Tateno, M.; Miyama, T.S.; Setoguchi, A.; Endo, Y. The clinical utility of two human portable blood glucose meters in canine and feline practice. Vet. Clin. Pathol. 2014, 43, 55–62. [Google Scholar] [CrossRef]
- Ma, D. Enhancing endosomal escape for nanoparticle mediated siRNA delivery. Nanoscale 2014, 6, 6415–6425. [Google Scholar] [CrossRef]
- Wood Dos Santos, T.; Cristina Pereira, Q.; Teixeira, L.; Gambero, A.; A Villena, J.; Lima Ribeiro, M. Effects of Polyphenols on Thermogenesis and Mitochondrial Biogenesis. Int. J. Mol. Sci 2018, 19, 2757. [Google Scholar] [CrossRef]
- He, W.; Zhang, A.; Qi, L.; Na, C.; Jiang, R.; Fan, Z.; Chen, J. FOXO1, a Potential Therapeutic Target, Regulates Autophagic Flux, Oxidative Stress, Mitochondrial Dysfunction, and Apoptosis in Human Cholangiocarcinoma QBC939 Cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 45, 1506–1514. [Google Scholar] [CrossRef]
- Hohnholt, M.C.; Andersen, V.H.; Bak, L.K.; Waagepetersen, H.S. Glucose, Lactate and Glutamine but not Glutamate Support Depolarization-Induced Increased Respiration in Isolated Nerve Terminals. Neurochem. Res. 2017, 42, 191–201. [Google Scholar] [CrossRef]
- Li, L.; Chen, Q.; Yu, Y.; Chen, H.; Lu, M.; Huang, Y.; Li, P.; Chang, H. RKI-1447 suppresses colorectal carcinoma cell growth via disrupting cellular bioenergetics and mitochondrial dynamics. J. Cell Physiol. 2020, 235, 254–266. [Google Scholar] [CrossRef]
- Abdelkader, N.F.; Elyamany, M.; Gad, A.M.; Assaf, N.; Fawzy, H.M.; Elesawy, W.H. Ellagic acid attenuates liver toxicity induced by valproic acid in rats. J. Pharm. Sci. 2020, 143, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.; Lee, H.; Park, J.; Kim, S.H.; Park, J. Targeting Cancer Metabolism - Revisiting the Warburg Effects. Toxicol. Res. 2016, 32, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Bruno, B.J.; Rabenau, M.; Lim, C.S. Delivery of drugs and macromolecules to the mitochondria for cancer therapy. J. Control. Release 2016, 240, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Sorice, A.; Siano, F.; Capone, F.; Guerriero, E.; Picariello, G.; Budillon, A.; Ciliberto, G.; Paolucci, M.; Costantini, S.; Volpe, M.G. Potential Anticancer Effects of Polyphenols from Chestnut Shell Extracts: Modulation of Cell Growth, and Cytokinomic and Metabolomic Profiles. Molecules 2016, 21, 1411. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Wei, Y.Y.; Yang, C.C.; Liu, C.J.; Yeh, L.Y.; Chou, C.H.; Chang, K.W.; Lin, S.C. miR-125b suppresses oral oncogenicity by targeting the anti-oxidative gene PRXL2A. Redox Biol. 2019, 22, 101140. [Google Scholar] [CrossRef]
- Jiang, J.X.; Gao, S.; Pan, Y.Z.; Yu, C.; Sun, C.Y. Overexpression of microRNA-125b sensitizes human hepatocellular carcinoma cells to 5-fluorouracil through inhibition of glycolysis by targeting hexokinase II. Mol. Med. Rep. 2014, 10, 995–1002. [Google Scholar] [CrossRef]
- Li, Y.; Kasim, V.; Yan, X.; Li, L.; Meliala, I.T.S.; Huang, C.; Li, Z.; Lei, K.; Song, G.; Zheng, X.; et al. Yin Yang 1 facilitates hepatocellular carcinoma cell lipid metabolism and tumor progression by inhibiting PGC-1β-induced fatty acid oxidation. Theranostics 2019, 9, 7599–7615. [Google Scholar] [CrossRef]
- Cheng, K.T.; Wang, Y.S.; Chou, H.C.; Chang, C.C.; Lee, C.K.; Juan, S.H. Kinsenoside-mediated lipolysis through an AMPK-dependent pathway in C3H10T1/2 adipocytes: Roles of AMPK and PPARalpha in the lipolytic effect of kinsenoside. Phytomedicine 2015, 22, 641–647. [Google Scholar] [CrossRef]
- Ruan, W.; Li, J.; Xu, Y.; Wang, Y.; Zhao, F.; Yang, X.; Jiang, H.; Zhang, L.; Saavedra, J.M.; Shi, L.; et al. MALAT1 Up-Regulator Polydatin Protects Brain Microvascular Integrity and Ameliorates Stroke through C/EBPbeta/MALAT1/CREB/PGC-1alpha/PPARgamma Pathway. Cell Mol. Neurobiol. 2019, 39, 265–286. [Google Scholar] [CrossRef]
- Woo, M.S.; Choi, H.S.; Seo, M.J.; Jeon, H.J.; Lee, B.Y. Ellagic acid suppresses lipid accumulation by suppressing early adipogenic events and cell cycle arrest. Phytother. Res. 2015, 29, 398–406. [Google Scholar] [CrossRef]
- Lv, O.; Wang, L.; Li, J.; Ma, Q.; Zhao, W. Effects of pomegranate peel polyphenols on lipid accumulation and cholesterol metabolic transformation in L-02 human hepatic cells via the PPARgamma-ABCA1/CYP7A1 pathway. Food Funct. 2016, 7, 4976–4983. [Google Scholar] [CrossRef] [PubMed]
- Piccinin, E.; Peres, C.; Bellafante, E.; Ducheix, S.; Pinto, C.; Villani, G.; Moschetta, A. Hepatic peroxisome proliferator-activated receptor γ coactivator 1β drives mitochondrial and anabolic signatures that contribute to hepatocellular carcinoma progression in mice. Hepatology 2018, 67, 884–898. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Cao, J.; Yao, S. Matrine promotes liver cancer cell apoptosis by inhibiting mitophagy and PINK1/Parkin pathways. Cell Stress Chaperones 2018, 23, 1295–1309. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shi, X.; Wei, S.; Ma, D.; Oyinlade, O.; Lv, S.Q.; Ying, M.; Zhang, Y.A.; Claypool, S.M.; Watkins, P.; et al. Kruppel-like factor 4 (KLF4) induces mitochondrial fusion and increases spare respiratory capacity of human glioblastoma cells. J. Biol. Chem. 2018, 293, 6544–6555. [Google Scholar] [CrossRef] [PubMed]
- Tasaki, M.; Umemura, T.; Maeda, M.; Ishii, Y.; Okamura, T.; Inoue, T.; Kuroiwa, Y.; Hirose, M.; Nishikawa, A. Safety assessment of ellagic acid, a food additive, in a subchronic toxicity study using F344 rats. Food Chem. Toxicol. 2008, 46, 1119–1124. [Google Scholar] [CrossRef]

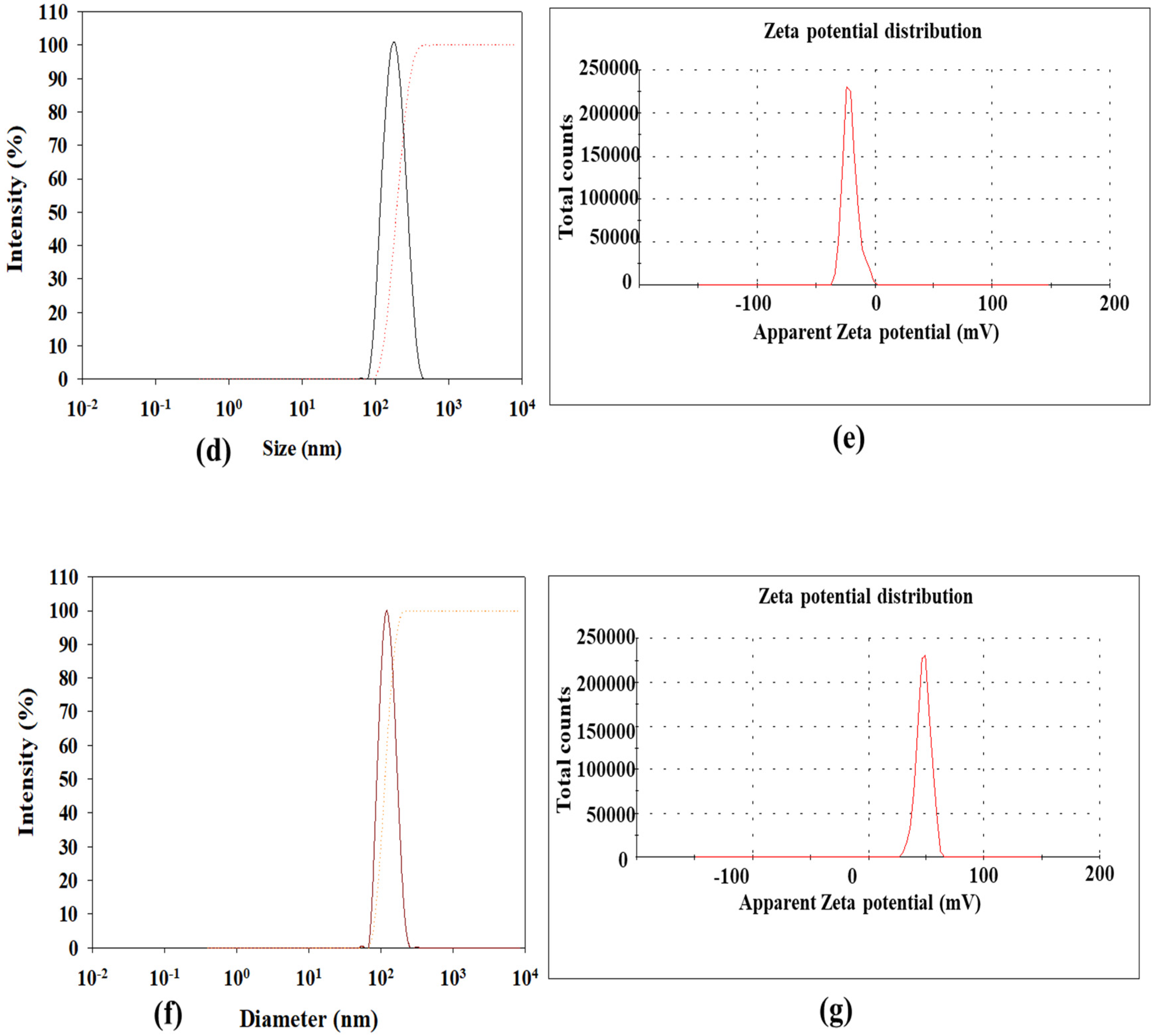

| Formulation | Particle Size (nm) | PdIa | Zeta Potential (mV) | EEb (%) |
|---|---|---|---|---|
| E/LPN-KL | 195.23 ± 5.88 | 0.24 ± 0.08 | −18.73 ± 2.29 | 85.53 ± 1.35 |
| miR-125/SLN-KL | 158.67 ± 3.69 | 0.20 ± 0.06 | 46.47 ± 1.22 | 86.28 ± 1.56 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo, Y.-L.; Wang, C.-S.; Chen, Y.-C.; Wang, T.-Y.; Chang, Y.-H.; Chen, C.-J.; Yang, C.-P. Mitochondrion-Directed Nanoparticles Loaded with a Natural Compound and a microRNA for Promoting Cancer Cell Death via the Modulation of Tumor Metabolism and Mitochondrial Dynamics. Pharmaceutics 2020, 12, 756. https://doi.org/10.3390/pharmaceutics12080756
Lo Y-L, Wang C-S, Chen Y-C, Wang T-Y, Chang Y-H, Chen C-J, Yang C-P. Mitochondrion-Directed Nanoparticles Loaded with a Natural Compound and a microRNA for Promoting Cancer Cell Death via the Modulation of Tumor Metabolism and Mitochondrial Dynamics. Pharmaceutics. 2020; 12(8):756. https://doi.org/10.3390/pharmaceutics12080756
Chicago/Turabian StyleLo, Yu-Li, Chen-Shen Wang, Yen-Chun Chen, Tse-Yuan Wang, Yih-Hsin Chang, Chun-Jung Chen, and Ching-Ping Yang. 2020. "Mitochondrion-Directed Nanoparticles Loaded with a Natural Compound and a microRNA for Promoting Cancer Cell Death via the Modulation of Tumor Metabolism and Mitochondrial Dynamics" Pharmaceutics 12, no. 8: 756. https://doi.org/10.3390/pharmaceutics12080756
APA StyleLo, Y.-L., Wang, C.-S., Chen, Y.-C., Wang, T.-Y., Chang, Y.-H., Chen, C.-J., & Yang, C.-P. (2020). Mitochondrion-Directed Nanoparticles Loaded with a Natural Compound and a microRNA for Promoting Cancer Cell Death via the Modulation of Tumor Metabolism and Mitochondrial Dynamics. Pharmaceutics, 12(8), 756. https://doi.org/10.3390/pharmaceutics12080756