Uniting Drug and Delivery: Metal Oxide Hybrid Nanotherapeutics for Skin Wound Care
Abstract
:1. Introduction
2. Skin Wound Therapy and Clinical Needs
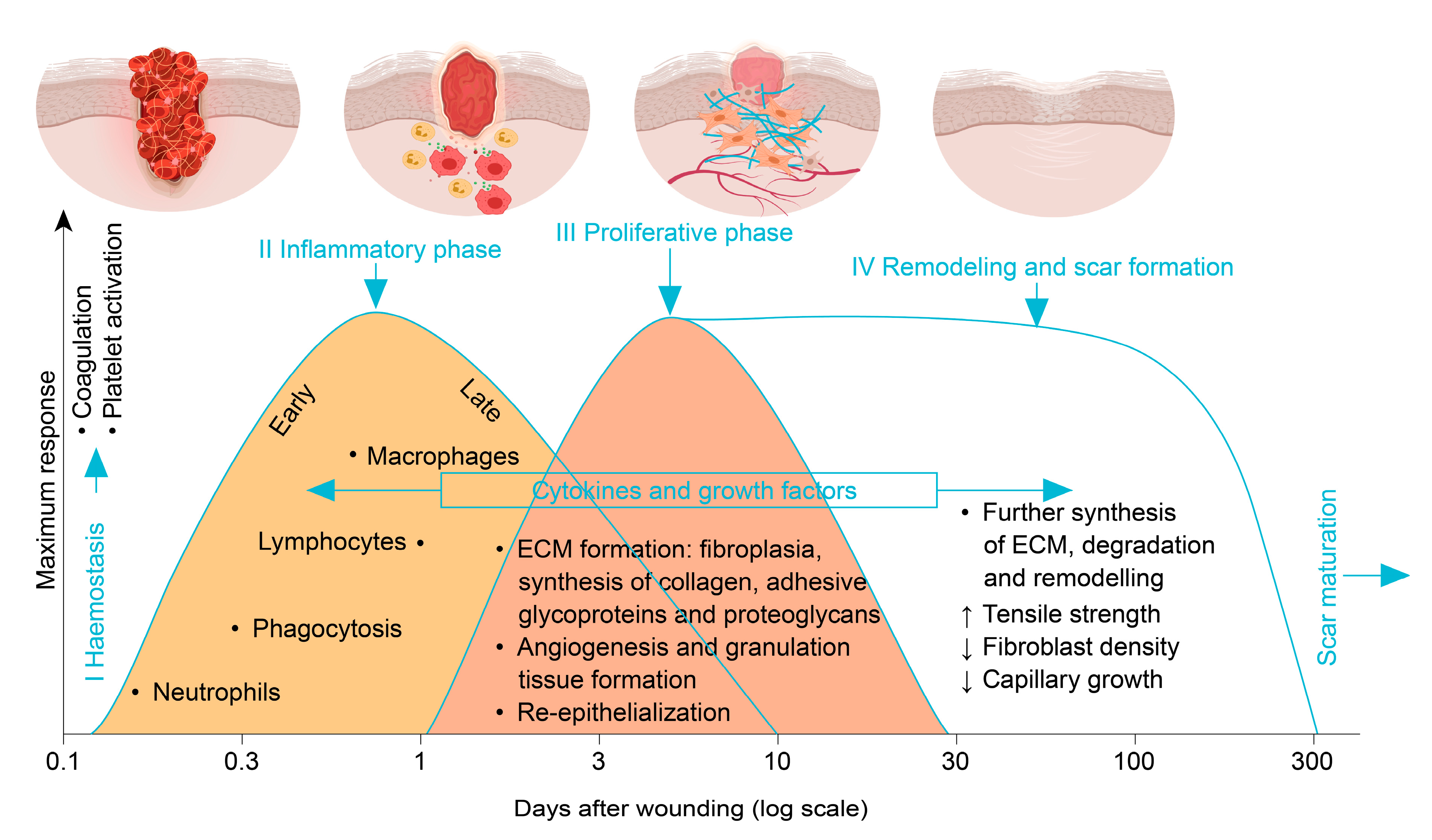
2.1. Phases of Wound Healing
2.2. Current Routine Measures for Skin Wound Management
2.3. Disease and Other Factors Affecting Skin Wound Healing
2.4. Clinically Used Measures to Support Healing of Problematic Wounds
2.5. Clinical Needs and Challenges
3. Emerging Skin Wound Care Materials
3.1. Bioengineered Tissues and Scaffolds
3.2. Bioinspired Materials for Wound Care
3.3. Metal and Metal Oxide Nanoparticles for Skin Wound Care
4. Metal Oxide Nanoparticle Hybrid Materials
4.1. Blends of Metal Oxides
4.2. Metal Oxide Nanoparticles Doped with Metal Ions
4.3. Inorganic Frameworks Decorated With Metal Oxide Nanoparticles
4.4. Core/shell and Janus-Shaped Hybrids
4.5. Combinations of the Above: Nano-Architected Hybrids
5. Disadvantages and Dangers
6. Conclusions and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [Green Version]
- Sarabahi, S. Recent advances in topical wound care. Indian J. Plast. Surg. 2012, 45, 379–387. [Google Scholar] [CrossRef]
- Shah, J.B. The History of Wound Care. J. Am. Coll. Certif. Wound Spec. 2012, 3, 65–66. [Google Scholar] [CrossRef] [Green Version]
- Basu, S.; Shukla, V. Complications of Wound Healing. In Measurements in Wound Healing; Mani, R., Romanelli, M., Shukla, V., Eds.; Springer: London, UK, 2012; pp. 109–144. ISBN 978-1-4471-2986-8. [Google Scholar]
- Guest, J.F.; Ayoub, N.; McIlwraith, T.; Uchegbu, I.; Gerrish, A.; Weidlich, D.; Vowden, K.; Vowden, P. Health economic burden that wounds impose on the National Health Service in the UK. BMJ Open 2015, 5, e009283. [Google Scholar] [CrossRef] [Green Version]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef] [Green Version]
- 2015 Edition | CMS. Available online: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/CMS-Statistics-Reference-Booklet/2015 (accessed on 16 April 2020).
- Gillespie, P.; Carter, L.; McIntosh, C.; Gethin, G. Estimating the health-care costs of wound care in Ireland. J. Wound Care 2019, 28, 324–330. [Google Scholar] [CrossRef]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [Green Version]
- Wound Care Market—Forecast to 2024 | Growing at a CAGR of 4.6% | MarketsandMarkets. Available online: https://www.marketsandmarkets.com/Market-Reports/wound-care-market-371.html (accessed on 6 March 2020).
- Jain, R.; Wairkar, S. Recent developments and clinical applications of surgical glues: An overview. Int. J. Biol. Macromol. 2019, 137, 95–106. [Google Scholar] [CrossRef]
- Savoji, H.; Godau, B.; Hassani, M.S.; Akbari, M. Skin Tissue Substitutes and Biomaterial Risk Assessment and Testing. Front. Bioeng. Biotechnol. 2018, 6, 86. [Google Scholar] [CrossRef]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. Biomedicine 2015, 5, 22. [Google Scholar] [CrossRef]
- Enoch, S.; Leaper, D.J. Basic science of wound healing. Surgery 2005, 23, 37–42. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2018, 99, 665–706. [Google Scholar] [CrossRef]
- Fife, C.E.; Carter, M.J.; Walker, D. Why is it so hard to do the right thing in wound care? Wound Repair Regen. 2010, 18, 154–158. [Google Scholar] [CrossRef]
- Franks, P.J.; Barker, J.; Collier, M.; Gethin, G.; Haesler, E.; Jawien, A.; Laeuchli, S.; Mosti, G.; Probst, S.; Weller, C. Management of Patients With Venous Leg Ulcers: Challenges and Current Best Practice. J. Wound Care 2016, 25, S1–S67. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, D.G.; Meyr, A.J. Risk factors for impaired wound healing and wound complications. In UpToDate; UpToDate: Waltham, MA, USA, 2020. [Google Scholar]
- Falanga, V. Wound healing and its impairment in the diabetic foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef]
- Anderson, K.; Hamm, R.L. Factors That Impair Wound Healing. J. Am. Coll. Clin. Wound Spec. 2012, 4, 84. [Google Scholar] [CrossRef] [Green Version]
- Leaper, D.J.; Schultz, G.; Carville, K.; Fletcher, J.; Swanson, T.; Drake, R. Extending the TIME concept: what have we learned in the past 10 years? Int. Wound J. 2012, 9, 1–19. [Google Scholar] [CrossRef]
- Martí-Carvajal, A.J.; Gluud, C.; Nicola, S.; Simancas-Racines, D.; Reveiz, L.; Oliva, P.; Cedeño-Taborda, J. Growth factors for treating diabetic foot ulcers. Cochrane Database Syst. Rev. 2015, 10, 1–115. [Google Scholar] [CrossRef]
- Catanzano, O.; Boateng, J. Local Delivery of Growth Factors Using Wound Dressings. In Therapeutic Dressings and Wound Healing Applications; Boateng, J., Ed.; Wiley: Hoboken, NJ, USA, 2020; pp. 291–314. ISBN 978-1-119-43326-2. [Google Scholar]
- Litwiniuk, M.; Grzela, T. Amniotic membrane: New concepts for an old dressing. Wound Repair Regen. 2014, 22, 451–456. [Google Scholar] [CrossRef]
- Dussoyer, M.; Michopoulou, A.; Rousselle, P. Decellularized Scaffolds for Skin Repair and Regeneration. Appl. Sci. 2020, 10, 3435. [Google Scholar] [CrossRef]
- Piaggesi, A.; Låuchli, S.; Bassetto, F.; Biedermann, T.; Marques, A.; Najafi, B.; Palla, I.; Scarpa, C.; Seimetz, D.; Triulzi, I.; et al. Advanced therapies in wound management: cell and tissue based therapies, physical and bio-physical therapies smart and IT based technologies. J. Wound Care 2018, 27, S1–S137. [Google Scholar] [CrossRef]
- Hoffman, T.; Khademhosseini, A.; Langer, R. Chasing the Paradigm: Clinical Translation of 25 Years of Tissue Engineering. Tissue Eng. Part A 2019, 25, 679–687. [Google Scholar] [CrossRef]
- O’Donnell, B.T.; Ives, C.J.; Mohiuddin, O.A.; Bunnell, B.A. Beyond the Present Constraints That Prevent a Wide Spread of Tissue Engineering and Regenerative Medicine Approaches. Front. Bioeng. Biotechnol. 2019, 7, 95. [Google Scholar] [CrossRef] [Green Version]
- Sundaram, S.; Siewert, J.; Balestrini, J.; Gard, A.; Boehm, K.; Wilcox, E.; Niklason, L. Tissue engineering and regenerative medicine. In Rossi’s Principles of Transfusion Medicine; Simon, T.L., McCullough, J., Snyder, E.L., Solheim, B.G., Strauss, R.G., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2016; pp. 488–504. ISBN 978-1-119-01302-0. [Google Scholar]
- Adibfar, A.; Retrouvey, H.; Padeanu, S.; Jeschke, M.G.; Shahrokhi, S. Current State of Selected Wound Regeneration Templates and Temporary Covers. Curr. Trauma Rep. 2019, 5, 79–89. [Google Scholar] [CrossRef]
- Kim, Y.S.; Smoak, M.M.; Melchiorri, A.J.; Mikos, A.G. An Overview of the Tissue Engineering Market in the United States from 2011 to 2018. Tissue Eng. Part A 2019, 25, 1–8. [Google Scholar] [CrossRef]
- King, N.M.P.; Coughlin, C.N.; Furth, M. Ethical Issues in Regenerative Medicine. Available online: https://ssrn.com/abstract=1380162 (accessed on 6 March 2020).
- Chan, S. Current and emerging global themes in the bioethics of regenerative medicine: the tangled web of stem cell translation. Regen. Med. 2017, 12, 839–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suarato, G.; Bertorelli, R.; Athanassiou, A. Borrowing From Nature: Biopolymers and Biocomposites as Smart Wound Care Materials. Front. Bioeng. Biotechnol. 2018, 6, 137. [Google Scholar] [CrossRef] [Green Version]
- Green, D.W.; Ben-Nissan, B.; Yoon, K.-S.; Milthorpe, B.; Jung, H.-S. Bioinspired materials for regenerative medicine: going beyond the human archetypes. J. Mater. Chem. B 2016, 4, 2396–2406. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Celiz, A.D.; Yang, J.; Yang, Q.; Wamala, I.; Whyte, W.; Seo, B.R.; Vasilyev, N.V.; Vlassak, J.J.; Suo, Z.; et al. Tough adhesives for diverse wet surfaces. Science 2017, 357, 378–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, K.; Senturk, B.; Matter, M.T.; Wu, X.; Herrmann, I.K.; Rottmar, M.; Toncelli, C. Mussel-Inspired Injectable Hydrogel Adhesive Formed under Mild Conditions Features Near-Native Tissue Properties. ACS Appl. Mater. Interfaces 2019, 11, 47707–47719. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, R.; Xu, T.; Ma, X.; Yao, Z.; Chi, B.; Xu, H. A mussel-inspired poly(γ-glutamic acid) tissue adhesive with high wet strength for wound closure. J. Mater. Chem. B 2017, 5, 5668–5678. [Google Scholar] [CrossRef]
- Sousa, M.P.; Neto, A.I.; Correia, T.R.; Miguel, S.P.; Matsusaki, M.; Correia, I.J.; Mano, J.F. Bioinspired multilayer membranes as potential adhesive patches for skin wound healing. Biomater. Sci. 2018, 6, 1962–1975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, J.; Wang, X.; Wang, E.; Li, T.; Chang, J.; Wu, C. Bioinspired multifunctional biomaterials with hierarchical microstructure for wound dressing. Acta Biomater. 2019, 100, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, B.P.; Messersmith, P.B. A reversible wet/dry adhesive inspired by mussels and geckos. Nature 2007, 448, 338–341. [Google Scholar] [CrossRef]
- Yuk, H.; Varela, C.E.; Nabzdyk, C.S.; Mao, X.; Padera, R.F.; Roche, E.T.; Zhao, X. Dry double-sided tape for adhesion of wet tissues and devices. Nature 2019, 575, 169–174. [Google Scholar] [CrossRef]
- Lee, Y.; Xu, C.; Sebastin, M.; Lee, A.; Holwell, N.; Xu, C.; Miranda Nieves, D.; Mu, L.; Langer, R.S.; Lin, C.; et al. Bioinspired Nanoparticulate Medical Glues for Minimally Invasive Tissue Repair. Adv. Healthcare Mater. 2015, 4, 2587–2596. [Google Scholar] [CrossRef] [Green Version]
- Blacklow, S.O.; Li, J.; Freedman, B.R.; Zeidi, M.; Chen, C.; Mooney, D.J. Bioinspired mechanically active adhesive dressings to accelerate wound closure. Sci. Adv. 2019, 5, eaaw3963. [Google Scholar] [CrossRef] [Green Version]
- Annabi, N.; Zhang, Y.-N.; Assmann, A.; Sani, E.S.; Cheng, G.; Lassaletta, A.D.; Vegh, A.; Dehghani, B.; Ruiz-Esparza, G.U.; Wang, X.; et al. Engineering a highly elastic human protein–based sealant for surgical applications. Sci. Transl. Med. 2017, 9, eaai7466. [Google Scholar] [CrossRef] [Green Version]
- Giner-Casares, J.J.; Henriksen-Lacey, M.; Coronado-Puchau, M.; Liz-Marzán, L.M. Inorganic nanoparticles for biomedicine: where materials scientists meet medical research. Mater. Today 2016, 19, 19–28. [Google Scholar] [CrossRef]
- Andreescu, S.; Ornatska, M.; Erlichman, J.S.; Estevez, A.; Leiter, J.C. Biomedical Applications of Metal Oxide Nanoparticles. In Fine Particles in Medicine and Pharmacy; Matijević, E., Ed.; Springer: Boston, MA, USA, 2012; pp. 57–100. ISBN 978-1-4614-0378-4. [Google Scholar]
- Issa, B.; Obaidat, I.M. Magnetic Nanoparticles as MRI Contrast Agents. Magn. Reson. Imaging 2019, 378, 40. [Google Scholar] [CrossRef] [Green Version]
- Gerken, L.R.H.; Keevend, K.; Zhang, Y.; Starsich, F.H.L.; Eberhardt, C.; Panzarasa, G.; Matter, M.T.; Wichser, A.; Boss, A.; Neels, A.; et al. Lanthanide-Doped Hafnia Nanoparticles for Multimodal Theranostics: Tailoring the Physicochemical Properties and Interactions with Biological Entities. ACS Appl. Mater. Interfaces 2018, 11, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, I.K.; Urner, M.; Koehler, F.M.; Hasler, M.; Roth-Z’Graggen, B.; Grass, R.N.; Ziegler, U.; Beck-Schimmer, B.; Stark, W.J. Blood Purification Using Functionalized Core/Shell Nanomagnets. Small 2010, 6, 1388–1392. [Google Scholar] [CrossRef] [PubMed]
- Anthis, A.H.C.; Matter, M.T.; Keevend, K.; Gerken, L.R.H.; Scheibler, S.; Doswald, S.; Gogos, A.; Herrmann, I.K. Tailoring the Colloidal Stability, Magnetic Separability, and Cytocompatibility of High-Capacity Magnetic Anion Exchangers. ACS Appl. Mater. Interfaces 2019, 11, 48341–48351. [Google Scholar] [CrossRef] [PubMed]
- Bechet, D.; Couleaud, P.; Frochot, C.; Viriot, M.-L.; Guillemin, F.; Barberi-Heyob, M. Nanoparticles as vehicles for delivery of photodynamic therapy agents. Trends Biotechnol. 2008, 26, 612–621. [Google Scholar] [CrossRef]
- Nethi, S.K.; Das, S.; Patra, C.R.; Mukherjee, S. Recent advances in inorganic nanomaterials for wound-healing applications. Biomater. Sci. 2019, 7, 2652–2674. [Google Scholar] [CrossRef]
- Urie, R.; Ghosh, D.; Ridha, I.; Rege, K. Inorganic Nanomaterials for Soft Tissue Repair and Regeneration. Annu. Rev. Biomed. Eng. 2018, 20, 353–374. [Google Scholar] [CrossRef]
- Silva, M.M.P.; de Aguiar, M.I.F.; Rodrigues, A.B.; Miranda, M.D.C.; Araújo, M.Â.M.; Rolim, I.L.T.P.; Souza, A.M.A.; Silva, M.M.P.; de Aguiar, M.I.F.; Rodrigues, A.B.; et al. The use of nanoparticles in wound treatment: a systematic review. Revista da Escola de Enfermagem da USP 2017, 51, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Miller, K.P.; Wang, L.; Benicewicz, B.C.; Decho, A.W. Inorganic nanoparticles engineered to attack bacteria. Chem. Soc. Rev. 2015, 44, 7787–7807. [Google Scholar] [CrossRef]
- Peña-Parás, L.; Sánchez-Fernández, J.A.; Vidaltamayo, R. Nanoclays for Biomedical Applications. In Handbook of Ecomaterials; Martínez, L.M.T., Kharissova, O.V., Kharisov, B.I., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–19. ISBN 978-3-319-48281-1. [Google Scholar]
- Oueslati, M.H.; Tahar, L.B.; Harrath, A.H. Catalytic, antioxidant and anticancer activities of gold nanoparticles synthesized by kaempferol glucoside from Lotus leguminosae. Arab. J. Chem. 2020, 13, 3112–3122. [Google Scholar] [CrossRef]
- Agarwal, H.; Nakara, A.; Shanmugam, V.K. Anti-inflammatory mechanism of various metal and metal oxide nanoparticles synthesized using plant extracts: A review. Biomed. Pharmacother. 2019, 109, 2561–2572. [Google Scholar] [CrossRef]
- Shibuya, S.; Ozawa, Y.; Watanabe, K.; Izuo, N.; Toda, T.; Yokote, K.; Shimizu, T. Palladium and Platinum Nanoparticles Attenuate Aging-Like Skin Atrophy via Antioxidant Activity in Mice. PLoS ONE 2014, 9, e109288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihai, M.M.; Dima, M.B.; Dima, B.; Holban, A.M. Nanomaterials for Wound Healing and Infection Control. Materials 2019, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihai, M.M.; Preda, M.; Lungu, I.; Gestal, M.C.; Popa, M.I.; Holban, A.M. Nanocoatings for Chronic Wound Repair—Modulation of Microbial Colonization and Biofilm Formation. Int. J. Mol. Sci. 2018, 19, 1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paladini, F.; Pollini, M. Antimicrobial Silver Nanoparticles for Wound Healing Application: Progress and Future Trends. Materials 2019, 12, 2540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boateng, J.; Catanzano, O. Silver and Silver Nanoparticle-Based Antimicrobial Dressings. In Therapeutic Dressings and Wound Healing Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 157–184. ISBN 978-1-119-43331-6. [Google Scholar]
- Chernousova, S.; Epple, M. Silver as Antibacterial Agent: Ion, Nanoparticle, and Metal. Angew. Chem. Int. Ed. 2013, 52, 1636–1653. [Google Scholar] [CrossRef]
- Chhabra, H.; Deshpande, R.; Kanitkar, M.; Jaiswal, A.; Kale, V.P.; Bellare, J.R. A nano zinc oxide doped electrospun scaffold improves wound healing in a rodent model. RSC Adv. 2016, 6, 1428–1439. [Google Scholar] [CrossRef]
- Reed, K.; Cormack, A.; Kulkarni, A.; Mayton, M.; Sayle, D.; Klaessig, F.; Stadler, B. Exploring the properties and applications of nanoceria: is there still plenty of room at the bottom? Environ. Sci. Nano 2014, 1, 390–405. [Google Scholar] [CrossRef] [Green Version]
- Chigurupati, S.; Mughal, M.R.; Okun, E.; Das, S.; Kumar, A.; McCaffery, M.; Seal, S.; Mattson, M.P. Effects of cerium oxide nanoparticles on the growth of keratinocytes, fibroblasts and vascular endothelial cells in cutaneous wound healing. Biomaterials 2013, 34, 2194–2201. [Google Scholar] [CrossRef] [Green Version]
- Rose, S.; Prevoteau, A.; Elzière, P.; Hourdet, D.; Marcellan, A.; Leibler, L. Nanoparticle solutions as adhesives for gels and biological tissues. Nature 2014, 505, 382–385. [Google Scholar] [CrossRef]
- Meddahi-Pellé, A.; Legrand, A.; Marcellan, A.; Louedec, L.; Letourneur, D.; Leibler, L. Organ Repair, Hemostasis, and In Vivo Bonding of Medical Devices by Aqueous Solutions of Nanoparticles. Angew. Chem. Int. Ed. 2014, 53, 6369–6373. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-H.; Kim, H.; Choi, Y.; Lee, D.S.; Kim, J.; Yi, G.-R. Colloidal Mesoporous Silica Nanoparticles as Strong Adhesives for Hydrogels and Biological Tissues. ACS Appl. Mater. Interfaces 2017, 9, 31469–31477. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Fernández, T.; Dobrovolskaia, M.; Camacho, T.; González-Fernández, Á.; Simón-Vázquez, R. Interference of Metal Oxide Nanoparticles with Coagulation Cascade and Interaction with Blood Components. Part. Part. Syst. Character 2019, 36, 1800547. [Google Scholar] [CrossRef]
- Barui, A.K.; Nethi, S.K.; Haque, S.; Basuthakur, P.; Patra, C.R. Recent Development of Metal Nanoparticles for Angiogenesis Study and Their Therapeutic Applications. ACS Appl. Bio Mater. 2019, 2, 5492–5511. [Google Scholar] [CrossRef]
- Kargozar, S.; Baino, F.; Hamzehlou, S.; Hamblin, M.R.; Mozafari, M. Nanotechnology for angiogenesis: opportunities and challenges. Chem. Soc. Rev. 2020, 49, 5008–5057. [Google Scholar] [CrossRef] [PubMed]
- Sanaeimehr, Z.; Javadi, I.; Namvar, F. Antiangiogenic and antiapoptotic effects of green-synthesized zinc oxide nanoparticles using Sargassum muticum algae extraction. Cancer Nanotechnol. 2018, 9. [Google Scholar] [CrossRef]
- Kurtjak, M.; Aničić, N.; Vukomanovicć, M. Inorganic Nanoparticles: Innovative Tools for Antimicrobial Agents. In Antibacterial Agents; Kumavath, R.N., Ed.; IntechOpen: London, UK, 2017; ISBN 978-953-51-3199-1. [Google Scholar]
- Hench, L.L.; Jones, J.R. Bioactive Glasses: Frontiers and Challenges. Front. Bioeng. Biotechnol. 2015, 3, 194. [Google Scholar] [CrossRef] [Green Version]
- Hench, L.L. The story of Bioglass. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Miguez-Pacheco, V.; Hench, L.L.; Boccaccini, A.R. Bioactive glasses beyond bone and teeth: Emerging applications in contact with soft tissues. Acta Biomater. 2015, 13, 1–15. [Google Scholar] [CrossRef]
- Pourshahrestani, S.; Kadri, N.A.; Zeimaran, E.; Towler, M.R. Well-ordered mesoporous silica and bioactive glasses: promise for improved hemostasis. Biomater. Sci. 2019, 7, 31–50. [Google Scholar] [CrossRef]
- Ostomel, T.A.; Shi, Q.; Tsung, C.-K.; Liang, H.; Stucky, G.D. Spherical Bioactive Glass with Enhanced Rates of Hydroxyapatite Deposition and Hemostatic Activity. Small 2006, 2, 1261–1265. [Google Scholar] [CrossRef]
- Jebahi, S.; Oudadesse, H.; Jardak, N.; Khayat, I.; Keskes, H.; Khabir, A.; Rebai, T.; El Feki, H.; El Feki, A. Biological therapy of strontium-substituted bioglass for soft tissue wound-healing: Responses to oxidative stress in ovariectomised rats. Ann. Pharm. Fr. 2013, 71, 234–242. [Google Scholar] [CrossRef] [Green Version]
- Naseri, S.; Lepry, W.C.; Nazhat, S.N. Bioactive glasses in wound healing: hope or hype? J. Mater. Chem. B 2017, 5, 6167–6174. [Google Scholar] [CrossRef]
- Kargozar, S.; Mozafari, M.; Hamzehlou, S.; Baino, F. Using Bioactive Glasses in the Management of Burns. Front. Bioeng. Biotechnol. 2019, 7, 62. [Google Scholar] [CrossRef]
- Neščáková, Z.; Zheng, K.; Liverani, L.; Nawaz, Q.; Galusková, D.; Kaňková, H.; Michálek, M.; Galusek, D.; Boccaccini, A.R. Multifunctional zinc ion doped sol—Gel derived mesoporous bioactive glass nanoparticles for biomedical applications. Bioact. Mater. 2019, 4, 312–321. [Google Scholar] [CrossRef]
- Kantipudi, S.; Sunkara, J.R.; Rallabhandi, M.; Thonangi, C.V.; Cholla, R.D.; Kollu, P.; Parvathaneni, M.K.; Pammi, S.V.N. Enhanced wound healing activity of Ag-ZnO composite NPs in Wistar Albino rats. IET Nanobiotechnol. 2018, 12, 473–478. [Google Scholar] [CrossRef]
- Malka, E.; Perelshtein, I.; Lipovsky, A.; Shalom, Y.; Naparstek, L.; Perkas, N.; Patick, T.; Lubart, R.; Nitzan, Y.; Banin, E.; et al. Eradication of Multi-Drug Resistant Bacteria by a Novel Zn-doped CuO Nanocomposite. Small 2013, 9, 4069–4076. [Google Scholar] [CrossRef]
- Oves, M.; Arshad, M.; Khan, M.S.; Ahmed, A.S.; Azam, A.; Ismail, I.M.I. Anti-microbial activity of cobalt doped zinc oxide nanoparticles: Targeting water borne bacteria. J. Saudi. Chem. Soc. 2015, 19, 581–588. [Google Scholar] [CrossRef] [Green Version]
- Rekha, K.; Nirmala, M.; Nair, M.G.; Anukaliani, A. Structural, optical, photocatalytic and antibacterial activity of zinc oxide and manganese doped zinc oxide nanoparticles. Phys. B Condens. Matter 2010, 405, 3180–3185. [Google Scholar] [CrossRef]
- Guo, B.-L.; Han, P.; Guo, L.-C.; Cao, Y.-Q.; Li, A.-D.; Kong, J.-Z.; Zhai, H.-F.; Wu, D. The Antibacterial Activity of Ta-doped ZnO Nanoparticles. Nanoscale Res. Lett. 2015, 10, 336. [Google Scholar] [CrossRef] [Green Version]
- Nethi, S.K.; Rico-Oller, B.; Rodríguez-Diéguez, A.; Gómez-Ruiz, S.; Patra, C.R. Design, synthesis and characterization of doped-titanium oxide nanomaterials with environmental and angiogenic applications. Sci. Total Environ. 2017, 599–600, 1263–1274. [Google Scholar] [CrossRef]
- Dhanasekar, M.; Jenefer, V.; Nambiar, R.B.; Babu, S.G.; Selvam, S.P.; Neppolian, B.; Bhat, S.V. Ambient light antimicrobial activity of reduced graphene oxide supported metal doped TiO2 nanoparticles and their PVA based polymer nanocomposite films. Mater. Res. Bull. 2018, 97, 238–243. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.Y.; Song, S.Y.; Go, S.; Sohn, H.S.; Baik, S.; Soh, M.; Kim, K.; Kim, D.; Kim, H.-C.; et al. Synergistic Oxygen Generation and Reactive Oxygen Species Scavenging by Manganese Ferrite/Ceria Co-decorated Nanoparticles for Rheumatoid Arthritis Treatment. ACS Nano 2019, 13, 3206–3217. [Google Scholar] [CrossRef]
- Wu, H.; Li, F.; Wang, S.; Lu, J.; Li, J.; Du, Y.; Sun, X.; Chen, X.; Gao, J.; Ling, D. Ceria nanocrystals decorated mesoporous silica nanoparticle based ROS-scavenging tissue adhesive for highly efficient regenerative wound healing. Biomaterials 2018, 151, 66–77. [Google Scholar] [CrossRef]
- Jin, C.; Liu, X.; Tan, L.; Cui, Z.; Yang, X.; Zheng, Y.; Yeung, K.W.K.; Chu, P.K.; Wu, S. Ag/AgBr-loaded mesoporous silica for rapid sterilization and promotion of wound healing. Biomater. Sci. 2018, 6, 1735–1744. [Google Scholar] [CrossRef]
- Munusamy, P.; Sanghavi, S.; Varga, T.; Suntharampillai, T. Silica supported ceria nanoparticles: A hybrid nanostructure to increase stability and surface reactivity of nano-crystalline ceria. RSC Adv. 2014, 4, 8421–8430. [Google Scholar] [CrossRef]
- Shin, K.; Choi, J.W.; Ko, G.; Baik, S.; Kim, D.; Park, O.K.; Lee, K.; Cho, H.R.; Han, S.I.; Lee, S.H.; et al. Multifunctional nanoparticles as a tissue adhesive and an injectable marker for image-guided procedures. Nature Commun. 2017, 8, 15807. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Sun, W.; Yang, W.; Gao, S.; Sun, C.; Li, Q. Mesoporous silica-protected silver nanoparticle disinfectant with controlled Ag+ ion release, efficient magnetic separation, and effective antibacterial activity. Nanoscale Adv. 2019, 1, 840–848. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Xu, J.; Wang, Y.; Chen, C.; Gu, H.; Chai, Y.; Wang, Y. Silver nanoparticles-decorated and mesoporous silica coated single-walled carbon nanotubes with an enhanced antibacterial activity for killing drug-resistant bacteria. Nano Res. 2020, 13, 389–400. [Google Scholar] [CrossRef]
- He, L.; Huang, G.; Liu, H.; Sang, C.; Liu, X.; Chen, T. Highly bioactive zeolitic imidazolate framework-8-capped nanotherapeutics for efficient reversal of reperfusion-induced injury in ischemic stroke. Sci. Adv. 2020, 6, eaay9751. [Google Scholar] [CrossRef] [Green Version]
- Matter, M.T.; Starsich, F.; Galli, M.; Hilber, M.; Schlegel, A.A.; Bertazzo, S.; Pratsinis, S.E.; Herrmann, I.K. Developing a tissue glue by engineering the adhesive and hemostatic properties of metal oxide nanoparticles. Nanoscale 2017, 9, 8418–8426. [Google Scholar] [CrossRef] [Green Version]
- Mädler, L.; Kammler, H.K.; Mueller, R.; Pratsinis, S.E. Controlled synthesis of nanostructured particles by flame spray pyrolysis. J. Aerosol Sci. 2002, 33, 369–389. [Google Scholar] [CrossRef]
- Matter, M.T.; Furer, L.A.; Starsich, F.H.L.; Fortunato, G.; Pratsinis, S.E.; Herrmann, I.K. Engineering the Bioactivity of Flame-Made Ceria and Ceria/Bioglass Hybrid Nanoparticles. ACS Appl. Mater. Interfaces 2018. [Google Scholar] [CrossRef] [PubMed]
- Marie, P.J.; Ammann, P.; Boivin, G.; Rey, C. Mechanisms of action and therapeutic potential of strontium in bone. Calcif. Tissue Int. 2001, 69, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Shahnazari, M.; Sharkey, N.A.; Fosmire, G.J.; Leach, R.M. Effects of Strontium on Bone Strength, Density, Volume, and Microarchitecture in Laying Hens. J. Bone Miner. Res. 2006, 21, 1696–1703. [Google Scholar] [CrossRef]
- Brandão-Neto, J.; Stefan, V.; Mendonça, B.B.; Bloise, W.; Castro, A.V.B. The essential role of zinc in growth. Nutr. Res. 1995, 15, 335–358. [Google Scholar] [CrossRef]
- Yamaguchi, M. Role of zinc in bone formation and bone resorption. J. Trace Elem. Exp. Med. 1998, 11, 119–135. [Google Scholar] [CrossRef]
- Lese, I.; Graf, D.A.; Tsai, C.; Taddeo, A.; Matter, M.T.; Constantinescu, M.A.; Herrmann, I.K.; Olariu, R. Bioactive nanoparticle-based formulations increase survival area of perforator flaps in a rat model. PLoS ONE 2018, 13, e0207802. [Google Scholar] [CrossRef]
- Food and Drug Administration. Guidance for Industry: Safety of Nanomaterials in Cosmetic Products. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-safety-nanomaterials-cosmetic-products (accessed on 6 August 2020).
- Fytianos, G.; Rahdar, A.; Kyzas, G.Z. Nanomaterials in Cosmetics: Recent Updates. Nanomaterials 2020, 10, 979. [Google Scholar] [CrossRef]
- Albanese, A.; Tang, P.S.; Chan, W.C.W. The Effect of Nanoparticle Size, Shape, and Surface Chemistry on Biological Systems. Ann. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Stark, W.J. Nanoparticles in Biological Systems. Angew. Chem. Int. Ed. 2011, 50, 1242–1258. [Google Scholar] [CrossRef]
- Home | Nanotechnology Characterization Lab (NCL). Available online: https://ncl.cancer.gov/ (accessed on 6 August 2020).
- EUNCL | Nanomedicine Characterisation Laboratory. Available online: http://www.euncl.eu/ (accessed on 6 August 2020).
- Yan, L.; Zhao, F.; Wang, J.; Zu, Y.; Gu, Z.; Zhao, Y. A Safe-by-Design Strategy towards Safer Nanomaterials in Nanomedicines. Adv. Mater. 2019, 31, 1805391. [Google Scholar] [CrossRef]
- Rösslein, M.; Liptrott, N.J.; Owen, A.; Boisseau, P.; Wick, P.; Herrmann, I.K. Sound understanding of environmental, health and safety, clinical, and market aspects is imperative to clinical translation of nanomedicines. Nanotoxicology 2017, 11, 147–149. [Google Scholar] [CrossRef] [Green Version]
- Mârza, S.M.; Magyari, K.; Bogdan, S.; Moldovan, M.; Peştean, C.; Nagy, A.; Tăbăran, F.; Licarete, E.; Suarasan, S.; Dreanca, A.; et al. Skin wound regeneration with bioactive glass-gold nanoparticles ointment. Biomed. Mater. 2019, 14, 025011. [Google Scholar] [CrossRef]
- Herrmann, I.K.; Beck-Schimmer, B.; Schumacher, C.M.; Gschwind, S.; Kaech, A.; Ziegler, U.; Clavien, P.-A.; Günther, D.; Stark, W.J.; Graf, R.; et al. In vivo risk evaluation of carbon-coated iron carbide nanoparticles based on short- and long-term exposure scenarios. Nanomedicine 2016, 11, 783–796. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J.A. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Liu, H.H.; Ji, Z.; Chang, C.H.; Xia, T.; Nel, A.E.; Cohen, Y. Evaluation of Toxicity Ranking for Metal Oxide Nanoparticles via an in Vitro Dosimetry Model. ACS Nano 2015, 9, 9303–9313. [Google Scholar] [CrossRef]
- Matusiewicz, H. Potential release of in vivo trace metals from metallic medical implants in the human body: From ions to nanoparticles—A systematic analytical review. Acta Biomater. 2014, 10, 2379–2403. [Google Scholar] [CrossRef]
- Sharifi, S.; Behzadi, S.; Laurent, S.; Laird Forrest, M.; Stroeve, P.; Mahmoudi, M. Toxicity of nanomaterials. Chem. Soc. Rev. 2012, 41, 2323–2343. [Google Scholar] [CrossRef]
- Soenen, S.J.; Rivera-Gil, P.; Montenegro, J.-M.; Parak, W.J.; De Smedt, S.C.; Braeckmans, K. Cellular toxicity of inorganic nanoparticles: Common aspects and guidelines for improved nanotoxicity evaluation. Nano Today 2011, 6, 446–465. [Google Scholar] [CrossRef]
- Matter, M.T.; Li, J.-H.; Lese, I.; Schreiner, C.; Bernard, L.; Scholder, O.; Hubeli, J.; Keevend, K.; Tsolaki, E.; Bertero, E.; et al. Multiscale Analysis of Metal Oxide Nanoparticles in Tissue: Insights into Biodistribution and Biotransformation. Adv. Sci. 2020, 7, 2000912. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matter, M.T.; Probst, S.; Läuchli, S.; Herrmann, I.K. Uniting Drug and Delivery: Metal Oxide Hybrid Nanotherapeutics for Skin Wound Care. Pharmaceutics 2020, 12, 780. https://doi.org/10.3390/pharmaceutics12080780
Matter MT, Probst S, Läuchli S, Herrmann IK. Uniting Drug and Delivery: Metal Oxide Hybrid Nanotherapeutics for Skin Wound Care. Pharmaceutics. 2020; 12(8):780. https://doi.org/10.3390/pharmaceutics12080780
Chicago/Turabian StyleMatter, Martin T., Sebastian Probst, Severin Läuchli, and Inge K. Herrmann. 2020. "Uniting Drug and Delivery: Metal Oxide Hybrid Nanotherapeutics for Skin Wound Care" Pharmaceutics 12, no. 8: 780. https://doi.org/10.3390/pharmaceutics12080780
APA StyleMatter, M. T., Probst, S., Läuchli, S., & Herrmann, I. K. (2020). Uniting Drug and Delivery: Metal Oxide Hybrid Nanotherapeutics for Skin Wound Care. Pharmaceutics, 12(8), 780. https://doi.org/10.3390/pharmaceutics12080780






